Surgical management of nonobstructive azoospermia
Bruno Cmrgo Tiseo,Russell Pul Hyden, Cigdem Tnrikut,*
aReproduction Center,Urology Department,University of Sao Paulo Medical School,Sao Paulo,Brazil
bMassachusetts General Hospital,Boston,MA,USA
cHarvard Medical School,Boston,MA,USA
Surgical management of nonobstructive azoospermia
Bruno Camargo Tiseoa,Russell Paul Haydenb,c, Cigdem Tanrikutb,c,*
aReproduction Center,Urology Department,University of Sao Paulo Medical School,Sao Paulo,Brazil
bMassachusetts General Hospital,Boston,MA,USA
cHarvard Medical School,Boston,MA,USA
Obstructive
Nonobstructive azoospermia(NOA)is characterized by the complete absence of sperm in the ejaculate due to testicular failure.The evaluation and management of patients with NOA offer a challenge to the reproductive urologist.In the era of in vitro fertilization with intracytoplasmic sperm injection,surgical sperm extraction techniques can afford men with NOA biologic paternity.To provide a comprehensive review of surgical sperm retrieval approaches in the patient with NOA emphasizing complications,success rates and outcome optimization,a Medline search was conducted querying surgical approaches used to manage NOA. Four sperm extraction techniques are described including:testicular sperm aspiration,testicular sperm extraction,fine needle aspiration mapping and microdissection testicular sperm extraction.In addition,the roles for pre-extraction varicocelectomy and sperm cryopreservation are discussed.The management of NOA continues to evolve as newer tools become available.Several modalities of sperm acquisition exist.An understanding of their complications and success rates is fundamental to the treatment of NOA.
©2015 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier (Singapore)Pte Ltd.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Azoospermia is characterized by the complete absence of sperm in the ejaculate,affecting up to 15%of men seeking an infertility evaluation.Approximately 60%of cases of azoospermia are caused by primary or secondary hypogonadism,termed nonobstructive azoospermia(NOA)[1]. Possible etiologies span a spectrum including genetic disorders or local testicular insults[2,3]that result in impairedspermatogenesis or disruption of the hypothalamicpituitary-testis(HPT)axis.The conditions leading to NOA represent some of the most challenging of infertility management.
Although the underlying cause of NOA may be occasionally correctable(i.e.,hypogonadotrophic hypogonadism),most of these patients will require advanced reproductive technologies.With the introduction of in vitro fertilization(IVF)and intracytoplasmic sperm injection(ICSI)in the 1990s,many of these men can now father their own biologic children.IVF-ICSI combined with sperm extraction techniques are now considered standard practice and are conducted routinely worldwide.
For successful IVF-ICSI only a few viable sperm are required.In those patients with NOA,even very low levels of spermatogenesis can be exploited with surgical sperm extraction.Several techniques to retrieve sperm from the testes have been described,each with a unique set of advantages and disadvantages.In this review we summarize the surgical management of NOA and seek to clarify the implications of each approach.
2.Initial evaluation
A diagnosis of azoospermia is made after two separate semen analyses demonstrate the lack of spermatozoa in centrifuged specimens.An interval of at least 3 weeks should be allowed to pass between samples.It is important to rule out possible collection error or retrograde ejaculation when interpreting samples with low volumes(less than 1.5 mL).Once azoospermia is confirmed,one must differentiate between obstructive or nonobstructive etiologies. A thorough history and physical examination provide the basis for further testing.A focused history investigates the inherent endocrine and exocrine function of the testes. Symptoms consistent with low testosterone may represent deficiencies in androgen production,often found in conjunction with NOA.A prior history of paternity should not be considered specific to either form of azoospermia. The clinician should also inquire about risk factors that may help guide further evaluation(Table 1).
A detailed physical exam will often secure the diagnosis. The general exam can be useful in detecting stigmata of an endocrinopathy or genetic abnormality with particular awareness to gynecomastia and signs of impaired virilization.Careful attention should be paid to the scrotal exam. Surgical scars in the scrotum and inguinal region may provide evidence for prior surgery.Palpation of the spermatic cord allows the identification of possible varicoceles and to ensure the presence of both vasa deferentia.Decreased testicular volumes(below 15 mL)substantially raise suspicion for NOA.Finally,a digital rectal examination can be helpful in palpating obstructing midline cysts or fullness of the seminal vesicles.
Initial laboratory testing includes morning serum testosterone and follicle stimulating hormone(FSH)levels to help delineate the health of the Leydig and Sertoli cells, respectively.An FSH level above 7.6 mIU/mL supports an NOA diagnosis[4].Any abnormalities on initial screening should prompt a full evaluation of the HPT axis.Classically, NOA patients will have normal volume,normal pH,azoospermic semen analyses with small-volume testes and an elevated FSH.Karyotype and Y chromosome microdeletion testing should be obtained in any patient with suspected NOA as chromosomal abnormalities are common in this population[5].
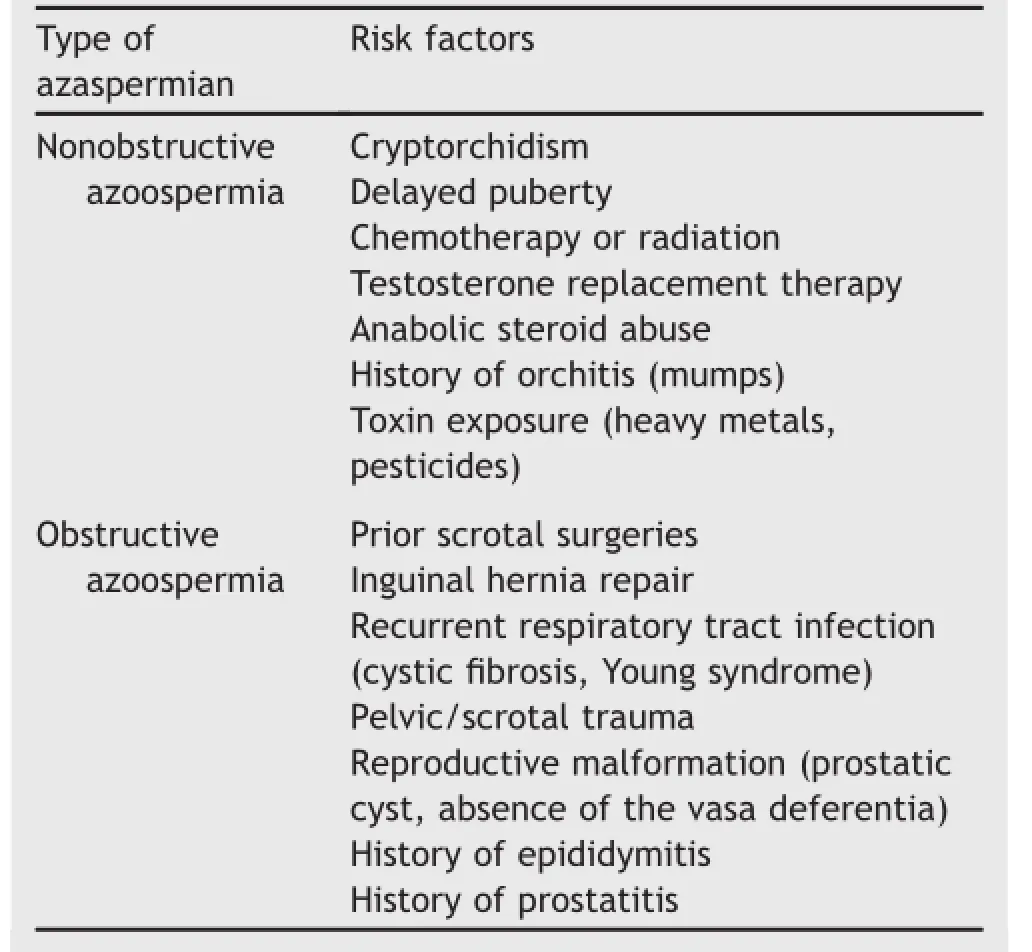
Table 1Risk factors for azoospermia.
3.Evidence acquisition
A Medline search was conducted using the following search terms:nonobstructive azoospermia,sperm retrieval, testicular sperm extraction,fine needle aspiration,fineneedle mapping,testicular sperm aspiration,microdissection testicular sperm extraction,and cryopreservation.This search aimed to identify randomized,observational and descriptive studies describing the surgical management of NOA patients.As no randomized trials were available, observational and descriptive studies are discussed in this review.
A total of four techniques were commonly addressed and are described below,including testicular sperm aspiration (TESA),traditional testicular sperm extraction(TESE),fine needle aspiration(FNA)mapping,and microdissection testicular sperm extraction(microTESE).Two adjunctive procedures-cryopreservation and the role of varicocelectomy-are also discussed in the context of NOA management.
4.Evidence synthesis
4.1.TESA
Testicular sperm can be retrieved via percutaneous aspiration of testicular tissue.This technique was initially described for diagnostic purposes before it was adapted as a therapeutic approach[6].The sample is acquired by aspirating through a fine(22-gauge)or large bore(18-gauge)needle after puncturing the testis through the scrotal skin.Samples of testicular parenchyma are subsequently immersed in human tubal fluid medium and mechanical disruption is performed[7].
The success of sperm procurement with this approach has been evaluated by several studies.Lewin et al.[8] treated 85 men with NOA via TESA and reported a sperm retrieval rate(SRR)of 58.8%.Khadra et al.[9]evaluated the outcomes of 84 men who underwent TESA and reported an SRR of 53.6%.Patients in this cohort who failed TESA then proceeded to open testicular biopsy in a rescue effort at sperm recovery.The TESA-only group had a higher fertilization rate(62%vs.48%)but this difference did not significantly impact clinical pregnancy rates(40%vs.32%, p>0.05).
TESA offers a modest SRR permitting IVF-ICSI.In some centers,TESA has become the initial option given its minimally invasive approach and relatively small risk for parenchymal damage and testicular tissue loss.The procedure can be done with local anesthesia,mitigating the need for a full operating room while preserving the comfort of the patient[10].Minor side effects were described including hematocele and post-operative orchialgia,typically managed with oral analgesics[8,9,11].However, despite its safety,low cost,and ease,controlled studies have shown a much lower success rate compared with the more invasive procedures described below[12].
4.2.TESE
In order to improve the SRR in men with NOA,a larger sample of testicular tissue can be harvested and processed. TESE achieves this goal through open testicular biopsy via either a single or multi-site approach.This technique is more invasive as compared to TESA,requiring scrotal exploration.A small incision in the tunica albuginea is created avoiding any vessels,and the testis is gently compressed causing protrusion of the parenchyma.The extruded seminiferous tubules are excised and processed in a similar fashion as TESA[7].Additional samples can be collected from the same incision or via separate sites if more tissue is required.
Available literature regarding TESE has stratified SRRs based upon underlying testicular histopathology since this information is readily available.Su et al.[13]reported a total SRR of 58%in 81 men who underwent TESE.Results varied substantially by histological pattern,with the highest SRR in the group with hypospermatogenesis as opposed to maturation arrest(MA)or Sertoli cell only(SCO)(79%, 47%,and 29%,respectively).In another report,Hauser et al.[12]attempted TESE in a group of 87 patients,successfully procuring sperm in 64%of the overall cohort.The SRR stratified by histology was 100%in hypospermatogenic males,with a considerable drop to only 46%and 33%in MA and SCO,respectively.
Arecent paper by AbdelRaheem et al.[14]found a strong correlation between the presence of tubules with spermatozoa on histopathologic evaluation and success during TESE. In patients with hypospermatogenesis there was a 100%SRR with TESE,in contrast to only 24%in SCO,corroborating previous reports.The authors went on to suggest attempting pre-emptive TESE prior to the start of IVF-ICSI.Results will provide histologic information and any recovered sperm can be cryopreserved for use during egg retrieval.For those patients in whom sperm could not be found at initial TESE,the histopathologic findings can help stratify those who may be candidates for repeat fresh TESE versus more invasive procedures(e.g.,microTESE),or consideration of donor sperm backup at the time of oocyte retrieval.
Differing SRRs have been reported depending upon underlying patient risk factors.A recent study from Marcelli et al.[15]evaluated 142 patients with a history of cryptorchidism and found an overall SRR of 65%at the time of TESE.This study also showed that the age at orchidopexy did not influence SRR.Reasonable success using TESE has also been demonstrated in those individuals with Klinefelter syndrome.Vernaeve et al.[16]studied 50 non-mosaic Klinefelter patients and reported a 48%SRR.A systematic review by Fullerton et al.[17]included 373 men with Klinefelter syndrome who were managed using various surgical modalities.They reported a 42%SRR in the group that underwent TESE.
Given the heterogeneity of testicular spermatogenesis, the rationale of a single biopsy site as opposed to multiple biopsies has been questioned.A large study of 306 men by Amer et al.[18]attempted to address this issue.They split their cohort into either single or multiple-site biopsy arms and demonstrated a higher success rate with multiple-site biopsies(38%vs.49%).Although supportive for the multiple-site approach,their patients were not randomized which impedes definitive conclusions from their analysis.
TESE has been heralded to have better SRRs than TESA [12].However,complications occur more frequently with TESE,including scrotal hematomas and,rarely,testicular atrophy.Extratunical hematomas are observed in 5%of patients and are usually self-limited[19].Intratunical hematomas,although often asymptomatic,can be seen on 82%of ultrasounds and may take up to 6 months to completely resolve.Testicular devascularization and resulting atrophy is uncommon,occurring in 3%of reported cases.This complication is commonly attributed to multiple-site TESE procedures since separate arteries can be interrupted[20].Although some reports show vascular damage from TESE,Schill et al.[21]did not find significant differences between pre-and post-TESE testicular volumes (by ultrasound)in 39 patients.
4.3.FNA mapping
Despite efforts to improve TESE there remains a significant failure rate.The experience with multiple-site testicular biopsies has shown that spermatogenesis is not homogenous.There are areas in the testicular parenchyma where sperm production is preserved,even in those patients with severe histopathology.Unfortunately,performing multiple testicular biopsies may raise the risk of testicular atrophy.
In order to target biopsies for sperm retrieval and reduce the chances of deleterious effects,the concept of FNA mapping was introduced by Turek et al.[22].The initial mapping procedure is often conducted under local anesthesia in the office setting.The surgeon directs multiple percutaneous punctures into the testis with a fine(23-gauge)needle in an attempt to aspirate parenchyma and sample various regions of the testis.Up to 18 templateguided sites are sampled and analyzed for sperm.Any positive aspirates favor successful sperm retrieval at that location.Thus,a few targeted open testicular biopsies can be conducted without the additional risk of multiple blind attempts[23].When FNA mapping confirms multiple sites with sperm,the reported success of TESE reaches 99%in a single surgeon’s experience.If few areas demonstrate spermatogenesis,TESE success remains favorable with SRR approaching 90%.In the case of zero or one positive sampling,the success of TESE is debatable and more invasive procedures should be considered[24].
Regarding outcomes,FNA mapping has proven safe,with one series showing no hematomas on follow-up ultrasounds [22].Moreover,there were no reported cases of testicular atrophy,no significant decrease in testosterone,or appreciable changes in testicular volumes 3 months following the procedure[25].In a heterogeneous group of 56 patients,FNA mapping demonstrated an SRR of approximately 70%[26].
4.4.MicroTESE
In an attempt to further optimize testicular SRRs over those achieved with TESE,microTESE was developed in an effort to capitalize on success rates of multi-site excisional biopsy while aiming to limit the amount of tissue excised.Initially described by Schlegel[27],he postulated that bivalving the testis and using optical magnification would allow the surgeon to minimize testicular trauma,maximize exposure for a complete exploration and dissection of the seminiferous tubules,and identify specific regions that might harbor preserved spermatogenesis.
The testis is delivered via a scrotal incision.With the assistance of the operating microscope,a transverse hemispheric incision is made into the tunica albuginea with an attempt to avoid transection of subtunical vessels.The testicle is then gently bivalved to expose the seminiferous tubules.The operating microscope is utilized at 20-40× magnification to identify plump and opaque seminiferous tubules that are more likely to contain sperm.In circumstances that initially fail to provide retrievable sperm,the testis can be completely surveyed and dissected in an effort to maximize any yield.Again,if sperm are not found,the surgeon proceeds to the contralateral testis in a similar fashion.
This technique has been shown to provide a better SRR than single or multi-site TESE.Despite its invasive nature, the optical magnification allows for rational tissue sampling and typically results in less overall tissue removal.Superior hemostasis can also be achieved since blood vessels can be directly visualized and either avoided or cauterized with the bipolar microforceps,decreasing risk of vascular injury and resulting atrophy.In one of the initial descriptions of the technique,a retrieval rate of 63%was obtained when compared to 45%for traditional TESE[27].Amer et al.[28] directly compared microTESE with TESE by performing one procedure on one testis and the counter technique on the opposite side.This study reported similar results with an improvement in SRR(30%with TESE vs.47%with microTESE).
Testicular histology also correlates with SRRs for micro-TESE.In one series,patients with SCO had yields of 22% with microTESE as compared to 13%using multi-site TESE [29].In another series with 460 patients SRR via microTESE was 81%in those with hypospermatogenesis,44%with MA, and 41%with SCO as the predominant histologic pattern [30].
One group investigated the use of microTESE as a salvage procedure following multiple unsuccessful TESE attempts. They reported that SRRs were not adversely affected following one or two prior TESE attempts.However,three or four previous TESE procedures did lead to decreased retrieval rates with microTESE,although spermatozoa were still found in 23%of these patients[31].
Given the significant costs of IVF-ICSI and the emotional toll that accompanies a treatment cycle,multiple attempts have been made to develop predictors of SRR.A multiinstitutional cohort of 1026 men was analyzed by Ramasamy and colleagues[32]for this purpose.Although multiple parameters were found to correlate with favorable SRR,no indicator was found to be accurate enough to allow for meaningful clinical decisions.Even testicular volume has been found to be a poor predictor in regards to SRR. Bryson et al.[33]evaluated the impact of testicular volume on microTESE success.They observed that NOA patients with severe testis atrophy,specifically with testicular volumes of 2 mL or less,had the same SRR as patients with volumes above 10 mL(55%vs.55%).
4.4.1.Subpopulation success rates with microTESE
The specific populations identified within the Ramasamy cohort have also been separately studied with promising results.Patients with a history of cryptorchidism can achieve good retrieval rates with microTESE.Raman and Schlegel[2]reported on 38 men submitted to 47 microTESE attempts with a resulting 74%SRR.This same cohort achieved clinical pregnancy in 46%of those with procured sperm.Contrary to a prior study,it was noted that SRR is related to the age at which orchidopexy was performed. Patients who had sperm successfully retrieved had typically undergone orchidopexy by 10 years of age.Another study from a similar cohort demonstrated a 64%SRR and 38%live birth rate[34].
MicroTESE has also proven successful in those patients with non-mosaic Klinefelter syndrome.An initial report evaluated 42 patients with successful sperm extraction in 29 of the subjects(69%SRR).A total of 21 live births resulted from IVF-ICSI cycles that utilized the retrieved sperm[35].Another study addressed predictors of sperm retrieval within this population,revealing that subjects who responded to testosterone optimization(using aromatase inhibitors,selective estrogen receptor modulators, and/or human chorionic gonadotropin)had better SRRs (77%vs.55%)[36].Fullerton et al.[17]reviewed cases reported in the literature and identified 101 live births from Klinefelter syndrome patients who had sperm extracted via TESE or microTESE.All babies born were genetically normal.
With increasing frequency older men are becoming interested in family building.A retrospective study evaluated 1066 men with NOA and stratified them based upon age at the time of microTESE.The two groups had a meanage of 57 and 43,respectively.This report did not show a negative relationship between age and SRR,and on the contrary,older patients had higher rates of sperm retrieval (73%vs.56%)[37].Although this is a retrospective series, this study shows that microTESE provides a reasonable option for the older male seeking fertility in the setting of NOA.However,the converse may be true in patients with Klinefelter syndrome.From a study of 74 patients,Klinefelter patients with successful sperm retrieval were significantly younger,an important finding to consider when counseling this subpopulation[38].
4.4.2.MicroTESE complications
Complications of microTESE include hematoma and intratesticular fibrosis.In a series of 60 patients who underwent the procedure,15%had intratesticular hypoechoic focal lesions on post-operative ultrasound.When followed with serial ultrasounds the rate reduced to 6%at 1 month and completely resolved by 6 months of follow-up[28].Similarly,a study with a cohort of 100 men demonstrated a hematoma rate of 12%on 1-month ultrasound,dropping to 2.5%at 6 months post-operatively[39].Ramasamy et al. [30]reported a higher frequency of hematoma after microTESE,demonstrating a positive finding in 44%of patients who underwent ultrasound 3 months following the procedure.Again,nearly all of these hematomas had resolved by 6 months.More worrisome was a description of segmental devascularization that was discovered in four patients.None of these patients was ultimately found to have permanent vascular changes.In a large meta-analysis, although sonographic complications seemed higher,there were no differences in clinically evident complications between conventional TESE and microTESE[40].
In those patients from whom large amounts of testicular parenchyma must be sampled,microTESE has been found to potentially result in atrophy and impaired testis function.Okada et al.[39]reported decreased testicular volumes in two of 80 men who underwent microTESE. However,this finding was not corroborated by Amer et al. [28]who reported that testicular volumes remained unchanged in 116 patients.Ramasamy et al.[30]reported a 20%decrease of serum testosterone shortly after the procedure;however,serum levels returned to pre-procedural levels in 85%and 95%of men at 12 and 18 months, respectively.It should be mentioned that in the subpopulation of patients with Klinefelter syndrome,testosterone levels may remain decreased for longer periods of time as demonstrated by Takada et al.[41].
A recent meta-analysis comparing conventional TESE with microTESE utilized the data from seven independent studies.The data,although predominately retrospective, did favor microTESE(54%vs.33%SRR)[40].Interestingly, patients with hypospermatogenesis may not experience as much of a benefit given that conventional TESE can already achieve high SRRs.Often,however,a histopathothologic diagnosis is not known prior to sperm retrieval attempt.
4.5.Cryopreservation
IVF-ICSI can be performed using frozen-thawed sperm.The use of cryopreserved sperm has logistical advantages and eliminates the need for concurrent oocyte and sperm retrievals.Unfortunately survival rates for frozen testicular sperm have been low in historical studies[42].In addition, IVF-ICSI outcomes may be impaired as Aoki et al.[43] demonstrated in a retrospective evaluation of 92 cycles. Laboratory pregnancy rates(60%vs.49.1%),clinical pregnancy rates(56.4%vs.41.2%),and live birth rates(48.7%vs. 31.2%)were each higher in the fresh versus the frozen groups.
Recent data have questioned the superiority of fresh samples.Karacan and colleagues[44]evaluated the outcome of 337 IVF-ICSI cycles in which 129 employed frozen testicular sperm.There was no difference in clinical pregnancy or live birth rates.Tavukcuoglu et al.[45] evaluated the impact of frozen sperm in 39 IVF-ICSI cycles compared to fresh testicular sperm in 43 cycles.Again, there was no significant difference observed in clinical pregnancy or live birth rates.
A current systematic review included 10 studies assessing fertilization rates and 11 that evaluated clinical pregnancy rates.In total,574 cycles were analyzed,299 of which were conducted with frozen sperm.Results show that outcomes using frozen-thawed testicular sperm do not differ compared with freshly extracted sperm in terms of fertilization or live birth rates[46].At this time a consensus has not been met,and therefore use of frozen testicular sperm remains a debated topic.
4.6.Varicocelectomy in NOA
Varicocele repair has a controversial role in men with NOA.There are some authors who advocate that repair can result in an improvement of spermatogenesis and may possibly return sperm to the ejaculate,or at least improve SRRs during an extraction attempt.Others report that these data represent only a transient reemergence of spermatogenesis as is observed in some azoospermic patients without varicocele.In an initial report of microsurgical varicocelectomy,a small series of 27 patients with NOA and palpable varicocele demonstrated postoperative sperm on semen analysis in 33%of subjects.In this series one patient achieved spontaneous conception. Six of these patients reverted to azoospermia within 6 months following the procedure[47].Another small study included 23 men with non-palpable varicoceles.Similarly, following repair 30%of the group had return of motile sperm to the ejaculate.One patient conceived spontaneously and two others underwent successful ICSI using ejaculated samples[48].
Abdel-Meguid[49]attempted to study the effects of varicocele repair when patients were stratified by histopathologic diagnosis.By taking a testicular biopsy at the time of varicocelectomy they demonstrated reversal of azoospermia in 53%and 50%of men with hypospermatogenesis and MA,respectively.There was no improvement in those with SCO.A meta-analysis including 233 patients with NOA from 11 studies reported return of motile ejaculated sperm in 39%of men following varicocelectomy.Success rates were more favorable in patients with hypospermatogenesis(54%)or MA(42%)than SCO (11%).Moreover,11 spontaneous pregnancies were reported[50].
In contrast,one study described equivalent SRRs in those who underwent varicocelectomy as opposed to no intervention.Additionally,only 9.6%of men with varicocele repair had enough sperm return to the ejaculate to avoid subsequent surgical sperm retrieval[51].The lack of controlled and randomized studies weakens the data and represents the major criticism for the use of varicocelectomy as an adjunct.Varicocele repair may benefit some patients with NOA but definitive data will need to be acquired before this subgroup can be fully elucidated.
5.Conclusion
Over the past several decades,treatment of men with NOA has benefited from an expanding knowledge base and the concomitant development of refined surgical techniques. Once impossible,men with NOA can now realistically hope to father their own biologic children.Reasonable pregnancy rates can be achieved using a variety of surgical approaches that vary from office-based percutaneous procedures to technically demanding microsurgery.Many couples have been given hope with these procedures,although there remains a proportion of men who will not have successful treatment.Future advances in the field will help diminish this group as our understanding of NOA evolves.
Conflicts of interest
The authors declare no conflict of interest.
[1]Jarow JP,Espeland MA,Lipshultz LI.Evaluation of the azoospermic patient.J Urol 1989;142:62-5.
[2]Raman JD,Schlegel PN.Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism.J Urol 2003;170(4 Pt 1):1287-90.
[3]Ezeh UI.Beyond the clinical classification of azoospermia: opinion.Hum Reprod 2000;15:2356-9.
[4]Schoor RA,Elhanbly S,Niederberger CS,Ross LS.The role of testicular biopsy in the modern management of male infertility.J Urol 2002;167:197-200.
[5]Palermo GD,Colombero LT,Hariprashad JJ,Schlegel PN, Rosenwaks Z.Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod 2002;17:570-5.
[6]Gottschalk-Sabag S,Glick T,Bar-On E,Weiss DB.Testicular fine needle aspiration as a diagnostic method.Fertil Steril 1993;59:1129-31.
[7]Schlegel PN,Palermo GD,Goldstein M,Menendez S, Zaninovic N,Veeck LL,et al.Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia.Urology 1997;49:435-40.
[8]Lewin A,Reubinoff B,Porat-Katz A,Weiss D,Eisenberg V, Arbel R,et al.Testicular fine needle aspiration:the alternative method for sperm retrieval in non-obstructive azoospermia.Hum Reprod 1999;14:1785-90.
[9]Khadra AA,Abdulhadi I,Ghunain S,Kilani Z.Efficiency of percutaneous testicular sperm aspiration as a mode of sperm collection for intracytoplasmic sperm injection in nonobstructive azoospermia.J Urol 2003;169:603-5.
[10]Tournaye H.Surgical sperm recovery for intracytoplasmic sperm injection:which method is to be preferred?Hum Reprod 1999;14(Suppl.1):71-81.
[11]Belker AM,Sherins RJ,Dennison-Lagos L,Thorsell LP, Schulman JD.Percutaneous testicular sperm aspiration:a convenient and effective office procedure to retrieve sperm for in vitro fertilization with intracytoplasmic sperm injection.J Urol 1998;160(6 Pt 1):2058-62.
[12]Hauser R,Yogev L,Paz G,Yavetz H,Azem F,Lessing JB,et al. Comparison of efficacy of two techniques for testicular sperm retrieval in nonobstructive azoospermia:multifocal testicular sperm extraction versus multifocal testicular sperm aspiration.J Androl 2006;27:28-33.
[13]Su LM,Palermo GD,Goldstein M,Veeck LL,Rosenwaks Z, Schlegel PN.Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval.J Urol 1999;161:112-6.
[14]Abdel Raheem A,Garaffa G,Rushwan N,De Luca F, Zacharakis E,Abdel Raheem T,et al.Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia.BJU Int 2013;111:492-9.
[15]Marcelli F,Robin G,Lefebvre-Khalil V,Marchetti C, Lemaitre L,Mitchell V,et al.Results of surgical testicular sperm extractions(TESE)in a population of azoospermic patients with a history of cryptorchidism based on a 10-year experience of 142 patients.Prog Urol 2008;18:657-62.
[16]Vernaeve V,Staessen C,Verheyen G,Van Steirteghem A, Devroey P,Tournaye H.Can biological or clinical parameters predict testicular sperm recovery in 47,XXY Klinefelter’s syndrome patients?Hum Reprod 2004;19:1135-9.
[17]Fullerton G,Hamilton M,Maheshwari A.Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum Reprod 2010;25:588-97.
[18]Amer M,Haggar SE,Moustafa T,Abd El-Naser T,Zohdy W. Testicular sperm extraction:impact of testicular histology on outcome,number of biopsies to be performed and optimal time for repetition.Hum Reprod 1999;14:3030-4.
[19]Friedler S,Raziel A,Schachter M,Strassburger D,Bern O, Ron-El R.Outcome of first and repeated testicular sperm extraction and ICSI in patients with non-obstructive azoospermia.Hum Reprod 2002;17:2356-61.
[20]Schlegel PN,Su LM.Physiological consequences of testicular sperm extraction.Hum Reprod 1997;12:1688-92.
[21]SchillT,Bals-Pratsch M,Kupker W,Sandmann J,Johannisson R, Diedrich K.Clinical and endocrine follow-up of patients after testicular sperm extraction.Fertil Steril 2003;79:281-6.
[22]Turek PJ,Ljung BM,Cha I,Conaghan J.Diagnostic findings from testis fine needle aspiration mapping in obstructed and nonobstructed azoospermic men.J Urol 2000;163:1709-16.
[23]Beliveau ME,Turek PJ.The value of testicular‘mapping’in men with non-obstructive azoospermia.Asian J Androl 2011; 13:225-30.
[24]Arredondo S,Shen SH,Conaghan J,Turek PJ.A clinical care pathway for nonobstructive azoospermia based on testis biopsy and mapping data.Fertil Steril 2004;82(S2):S86.
[25]Carpi A,Menchini Fabris FG,Palego P,Di Coscio G,Romani R, Nardini V,et al.Fine-needle and large-needle percutaneous aspiration biopsy of testicles in men with nonobstructive azoospermia:safety and diagnostic performance.Fertil Steril 2005;83:1029-33.
[26]ShefiS,Kaplan K,Turek PJ.Analysis of spermatogenesis in non-obstructive azoospermic and virtually azoospermic men with known testicular pathology.Reprod Biomed Online 2009;18:460-4.
[27]Schlegel PN.Testicular sperm extraction:microdissection improves sperm yield with minimal tissue excision.Hum Reprod 1999;14:131-5.
[28]Amer M,Ateyah A,Hany R,Zohdy W.Prospective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia:follow-up by serial ultrasound examinations.Hum Reprod 2000;15: 653-6.
[29]Tsujimura A,Matsumiya K,Miyagawa Y,Tohda A,Miura H, Nishimura K,et al.Conventional multiple or microdissection testicular sperm extraction:a comparative study.Hum Reprod 2002;17:2924-9.
[30]Ramasamy R,Yagan N,Schlegel PN.Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction.Urology 2005;65:1190-4.
[31]Ramasamy R,Schlegel PN.Microdissection testicular sperm extraction:effect of prior biopsy on success of sperm retrieval.J Urol 2007;177:1447-9.
[32]Ramasamy R,Padilla WO,Osterberg EC,Srivastava A, Reifsnyder JE,Niederberger C,et al.A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia.J Urol 2013;189:638-42.
[33]Bryson CF,Ramasamy R,Sheehan M,Palermo GD, Rosenwaks Z,Schlegel PN.Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction.J Urol 2014;191:175-8.
[34]Dabaja AA,Schlegel PN.Microdissection testicular sperm extraction:an update.Asian J Androl 2013;15:35-9.
[35]Schiff JD,Palermo GD,Veeck LL,Goldstein M,Rosenwaks Z, Schlegel PN.Success of testicular sperm extraction[corrected]and intracytoplasmic sperm injection in men with Klinefelter syndrome.J Clin Endocrinol Metab 2005;90: 6263-7.
[36]Ramasamy R,Ricci JA,Palermo GD,Gosden LV,Rosenwaks Z, Schlegel PN.Successful fertility treatment for Klinefelter’s syndrome.J Urol 2009;182:1108-13.
[37]Ramasamy R,Trivedi NN,Reifsnyder JE,Palermo GD, Rosenwaks Z,Schlegel PN.Age does not adversely affect sperm retrieval in men undergoing microdissection testicular sperm extraction.Fertil Steril 2014;101:653-5.
[38]Emre Bakircioglu M,Erden HF,Kaplancan T,Ciray N,Bener F, Bahceci M.Aging may adversely affect testicular sperm recovery in patients with Klinefelter syndrome.Urology 2006; 68:1082-6.
[39]Okada H,Dobashi M,Yamazaki T,Hara I,Fujisawa M, Arakawa S,et al.Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol 2002;168:1063-7.
[40]Deruyver Y,Vanderschueren D,Van der Aa F.Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia:a systematic review.Andrology 2014;2:20-4.
[41]Takada S,Tsujimura A,Ueda T,Matsuoka Y,Takao T, Miyagawa Y,et al.Androgen decline in patients with nonobstructive azoospemia after microdissection testicular sperm extraction.Urology 2008;72:114-8.
[42]Nijs M,Ombelet W.Cryopreservation of human sperm.Hum Fertil 2001;4:158-63.
[43]Aoki VW,Wilcox AL,Thorp C,Hamilton BD,Carrell DT. Improved in vitro fertilization embryo quality and pregnancy rates with intracytoplasmic sperm injection of sperm from fresh testicular biopsy samples vs.frozen biopsy samples. Fertil Steril 2004;82:1532-5.
[44]Karacan M,Alwaeely F,Erkan S,Cebi Z,Berberoglugil M, Batukan M,et al.Outcome of intracytoplasmic sperm injection cycles with fresh testicular spermatozoa obtained on the day of or the day before oocyte collection and with cryopreserved testicular sperm in patients with azoospermia. Fertil Steril 2013;100:975-80.
[45]Tavukcuoglu S,Al-Azawi T,Al-Hasani S,Khaki AA,Khaki A, Tasdemir S.Using fresh and frozen testicular sperm samples in couples undergoing ICSI-microTESE treatment.J Reprod Infertil 2013;14:79-84.
[46]Ohlander S,Hotaling J,Kirshenbaum E,Niederberger C, Eisenberg ML.Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction:a meta-analysis.Fertil Steril 2014;101:344-9.
[47]Pasqualotto FF,Sobreiro BP,Hallak J,Pasqualotto EB, Lucon AM.Induction of spermatogenesis in azoospermic men after varicocelectomy repair:an update.Fertil Steril 2006; 85:635-9.
[48]Kirac M,Deniz N,Biri H.The effect of microsurgical varicocelectomy on semen parameters in men with non-obstructive azoospermia.Curr Urol 2013;6:136-40.
[49]Abdel-Meguid TA.Predictors of sperm recovery and azoospermia relapse in men with nonobstructive azoospermia after varicocele repair.J Urol 2012;187:222-6.
[50]Weedin JW,Khera M,Lipshultz LI.Varicocele repair in patients with nonobstructive azoospermia:a meta-analysis.J Urol 2010;183:2309-15.
[51]Schlegel PN,Kaufmann J.Role of varicocelectomy in men with nonobstructive azoospermia.Fertil Steril 2004;81: 1585-8.
Received 20 August 2014;received in revised form 20 November 2014;accepted 24 December 2014 Available online 16 April 2015
*Corresponding author.Massachusetts General Hospital,Boston, MA,USA.
E-mail address:ctanrikut@partners.org(C.Tanrikut).
Peer review under responsibility of Chinese Urological Association and SMMU.
http://dx.doi.org/10.1016/j.ajur.2015.04.020
2214-3882/©2015 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier(Singapore)Pte Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
azoospermia;
Male infertility;
Sperm retrieval
 Asian Journal of Urology2015年2期
Asian Journal of Urology2015年2期
- Asian Journal of Urology的其它文章
- All about Peyronie’s disease
- Testis sperm extraction
- Non-invasive transcutaneous electrical stimulation in the treatment of overactive bladder
- Contemporary review of the 532 nm laser for treatment of benign prostatic hyperplasia
- Percent free prostate-specific antigen for prostate cancer diagnosis in Chinese men with a PSA of 4.0-10.0 ng/mL:Results from the Chinese Prostate Cancer Consortium
- Configuration and validation of a novel prostate disease nomogram predicting prostate biopsy outcome:A prospective study correlating clinical indicators among Filipino adult males with elevated PSA level
