Synthesis, characterization and properties of low-melting bi-system microcrystalline sealing glass
Synthesis, characterization and properties of low-melting bi-system microcrystalline sealing glass
Bi2O3-B2O3-SiO2ternary microcrystalline sealing glass was synthesized by heat treatment of precursor powder obtained through sol-gel process. Alkaline-earth metal oxides (CaO, SrO, BaO) were doped to obtain quaternary sealing glass. Effects of two heat treatment temperature and holding time on the crystallization properties of these samples were studied. These samples were characterized by XRD、Raman and DSC, their reheat treatment temperature and soaking time have been investigated. The results indicated that all these systems showed new microcrystalline status. The doping system will increase their thermal expansion coefficient. The initial transformation temperatures (Tg) were low.
sealing glass; sol-gel method; Bi2O3-B2O3-SiO2; microcrystalline; doping systems
1 Introduction
Recently, the low temperature sealing materials have been extensively used in sealing applications of engineering and electronic due to its superior properties, such as low melting and sealing temperature, high resistance and sealing strength, thermal and mechanical stability[1-3]. In past decades, lead sealing glasses have almost monopolized the field of low temperature sealing materials[4]. Because lead is forbidden for its terrible impacts to human and environment[5]and sealing operations of electrical equipments are restricted to low temperature condition, developing new low temperature lead-free sealing glass material has very important application value[6]. One of the alternative strategies is to find suitable substituent of the existing lead glass sealing materials, with applicable solder composition and proper preparation.
The research of Bi2O3-B2O3-SiO2ternary system sealing glass has been reported[7].But the preparation methods were limited to high-temperature solid-state reaction, which has some fundamental problems, such as high energy consumption and product phase heterogeneity. It is necessary to dope with some ions of larger field intensity to reduce the melting point of composition[8-9]. Because sealing materials must have high insulation performance, which is mainly depends on the ions migration number. Bi-systems sealing glass-ceramic was prepared by sol-gel combined heat treatment method for reducing the conductivity. Structure and property of the glass-sealing system doping of some alkaline-earth metal oxides were also investigated in detail.
2 Experimental
2.1 Synthesis of sealing glass
2.1.1 The synthesis of gelatin powder Stoichiometric quantities of alcohol,TEOS(Si(OC2H5)4)and deionized water were mixed in volume ratio of C2H5OH/SiO2/H2O=2/2/3. Alcohol and deionized water were slowly dropped into TEOS in succession with stirring at room temperature for 1 h, the mixture was stirred in a beaker provided with plastic wrap. H3BO3-ethanol solution was added to solution under stirring and mixed solution was stirred for 1 h. Bi2O3-MO were dissolved in concentrated hydrochloric acid, the mixture was dropped into the above solution, the mixture was stirred for 0.5 h. Water was slowly added to the resulting solution to adjust the pH<1. The solution was mixed for 10 h at room temperature, the solution gelates after 3 days, the gels were dried in air at 70℃ to obtain the xerogel.
2.1.2 The synthesis of parent glass After grinding treatment, the gelatin powder was heated to 100℃ with 10℃/min and maintained for 1 h in the furnace, then to 1100~1250℃ at 7.5~10℃/min for 1 h. The parent glass obtained from the quick melts quenching onto preheated stainless-steel molds and annealing at 350℃ with furnace cooling down to room temperature after 1 h.
2.1.3 Granulation and molding The precursor glass powders were ground and sieved to pass through 200 mesh screen. After joining 6 wt% PVB solution (alcohol as solvent), the glass powders turned into pasty, then were dried in oven at 75℃. The specimens in the size of 8.5 mm×2 mm were synthesized by tablet machine pressing at 12 MPa for 3 min.
2.1.4 The heat treatment of parent The glass sheets were calcined at 550~650℃with furnace cooling down to room temperature after 1~4 h, the glass-ceramics were obtained.
2.2 Characterization
The phase structures of these samples were analyzed by X-ray diffraction using D/ruax 2550 PC diffractometer with Cu Kα radiation source (λ=0.15406 nm) with 40 kV voltage and 250 mA current, the scanning rate of 8.0 (°)/min in 2θ range from 10 to 70°. The Raman spectra were measured with spectrophotometer (Lab RAM HR800) in 50~3200 cm-1spectrum range using 488 nm excitation line(power 5 mW) from a spectra physics laser. The DSC was detected between 40 and 800℃ with NETZSCHSTA 449C differential scanning calorimeter. The thermal expansion coefficient was carried out with NETZSCH DIL402EP horizontally dilatometer.
3 Results and Discussion
3.1 XRD analysis
Bi2O3-B2O3-SiO2glass-ceramics was synthesized at 1200℃ for 1 h, the effects of different heat treatment conditions on the sealing glass phase were studied.

Fig.1 X-ray of Bi2O3-B2O3-SiO2 glasses
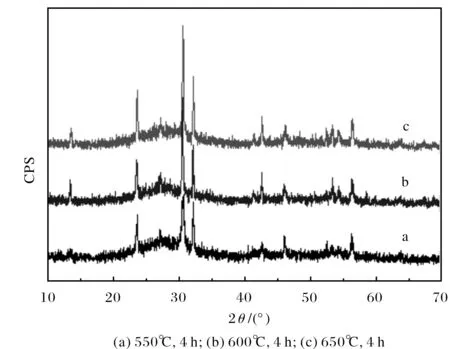
Fig.2 X-ray of Bi2O3-B2O3-SiO2-CaO glasses
According to Fig.1, the major crystalline phase was pure BiOCl after the parent glass firing at 650℃ for 4 h, but the crystal phase amount was much less abundant. from a and b, under the same heat temperature of 650℃, the crystalline phases of two products were consistent, but different at crystal phase amount. This indicated there was almost no effect on crystal form when temperature attained certain value, only slightly changing in the crystal phase amount. The longer holding time, the higher the crystallization degree and the larger the grain size.
Fig.2 is XRD of Bi2O3-B2O3-SiO2-CaO glass-ceramics obtained after different heat treatment, which parent glass was prepared at 1100℃ for 1 h. All the systems as mentioned above presented a new status of microcrystalline, There are strong diffraction peak at the condition of 650℃ and 4 h, which was similar to that of the crystal phase of Pb4Cl2O4(JCPDS No: 75-0943). The products showed coexistence of crystalline state with glassy state. The intensity of peaks reached maximum when crystallization temperature is 650℃.
Fig.3 is XRD patterns of Bi2O3-B2O3-SiO2-SrO four-components doping system glass-ceramics obtained after different heat treatment, its parent glass was synthesized at 1150℃for 1 h. The products of Bi2O3-B2O3-SiO2-SrO systems exhibited the diffraction peaks of BiOCl appeared in the 550℃ sample. When the samples were crystallized at 650℃ for 4 h, the intensity of peaks reached the maximum, and the crystal phase was also similar to the phase of Pb4Cl2O4.

Fig.3 X-ray of Bi2O3-B2O3-SiO2-SrO glasses
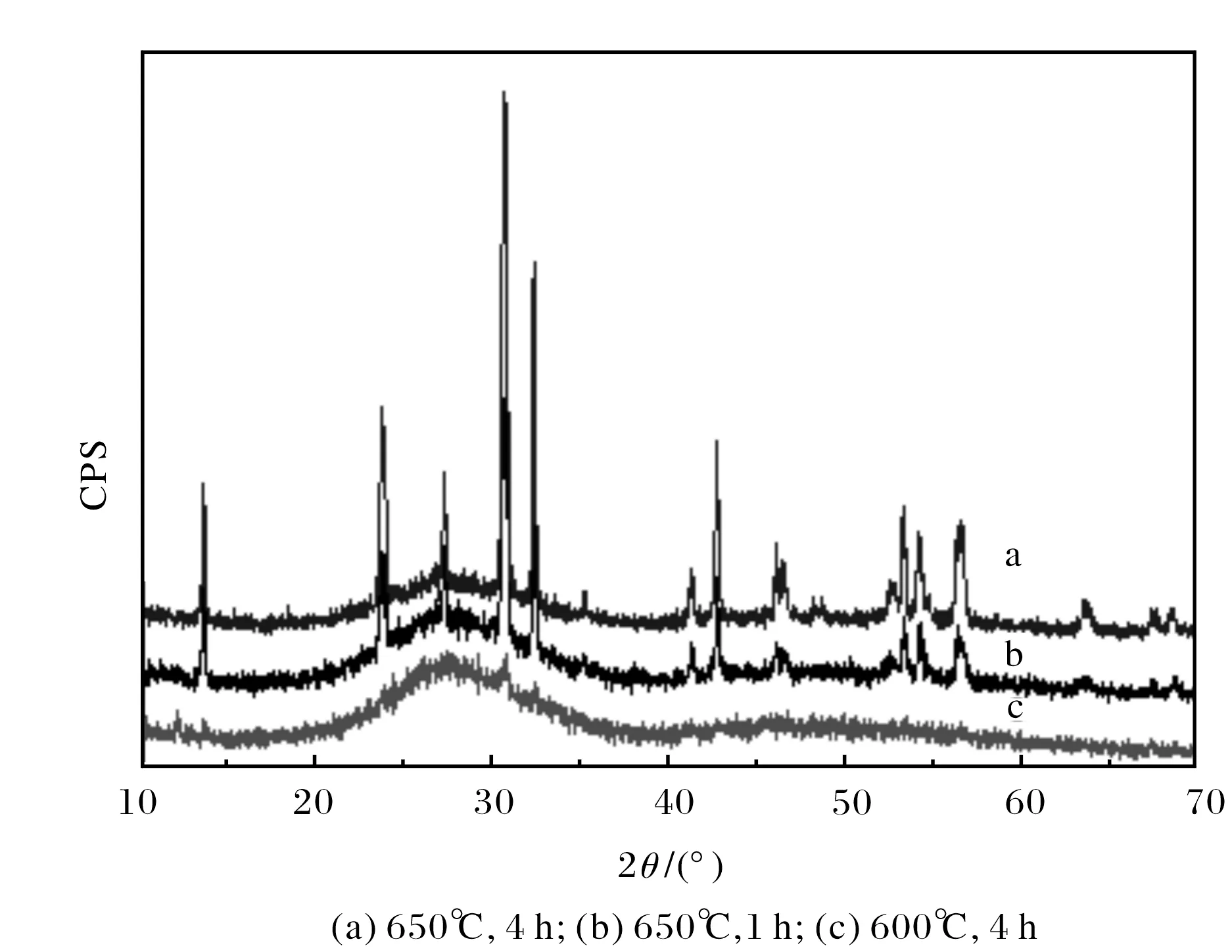
Fig.4 X-ray of Bi2O3-B2O3-SiO2-BaO glasses
Fig.4 is the XRD of Bi2O3-B2O3-SiO2-BaO glass-ceramics obtained after different heat treatment, its parent glass was synthesized at 1150℃ for 1 h. It also exhibited the reflections were similar to Pb4Cl2O4with the maximum peak intensity when the samples were heated at 650℃ for 4 h. By comparing diffraction lines of a and b, it can be concluded that prolonging soaking time is conducive to the crystallization. By comparing these four test results, good sealing glass can be synthesized by doping with alkaline earth mental oxides, the change trend of glass crystallization ability of BaO>CaO>SrO. The intensity of peaks reached maximum when Bi2O3-B2O3-SiO2ternary system was doped with BaO, which indicated that BaO is the most conducive to crystallize for Bi2O3-B2O3-SiO2ternary system.
3.2 Raman analysis
Fig.5 is the Raman spectra of doping sealing glass-ceramics. Fig.5(a) shows the initial melting temperature of Bi2O3-B2O3-SiO2-CaO is 1100℃, the reheat treatment temperature is 650℃ with 4 h holding time. Fig.5(b) shows the initial melting temperature of Bi2O3-B2O3-SiO2-SrO is 1150℃, the reheat treatment temperature is 650℃ with 4 h holding time. Fig.5(c) shows the initial melting temperature of Bi2O3-B2O3-SiO2-BaO is 1150℃, and the reheat treatment temperature is 650℃ with 4 h holding time.
As can be seen in Fig.5 and table 1, the observed first high intense peak at 71 cm-1and 74 cm-1is due to Bi3+vibration[10]. The weak shoulder at 156 cm-1is attributed to Bi-O stretching vibration, the weak shoulder around 233 cm-1is assigned to Bi-O-Bi vibration of the [BiO6] octahedron[11]. The band around 448 cm-1can be ascribed to Bi-O-Bi vibration of the [BiO6] octahedron and [BiO3] tetrahedron. The band centered around 613 cm-1is assigned to stretching vibration of BiO in [BiO6] units. The weak band at 923 cm-1is attributed to both the asymmetry vibration of the Bi-O in [BiO3] and the stretching vibration of B-O in [BO4] units[12]. The peak at 1209 cm-1is assigned to the stretching vibration of the B-O in [BO3][13-14].

Fig.5 The Raman spectra of three doping series sealing glasses

Tab.1 Vibration types for Raman absorption bands of bismuthate system sealing lasses
Fig.5(b) shows that doping SrO sealing glass-ceramics is basically consistent with doping CaO series, only different at 303 cm-1, which is belong to the stretching vibration of the Bi-O-Bi in [BiO3] and [BiO6] units. From Fig.5(c), doping BaO system is almost consistent with the doped CaO and SrO series respectively.
In conclusion, with the glass systems studied in this experiment, Bi3+are constituted in the glass network with the form of [BiO6] octahedral and [BiO3] triangles as the basic structural unit. Analogously, the boron-oxygon network is composed of [BO3] triangles and [BO4] tetrahedral, while CaO, SrO and BaO are outer body in the glass network.
3.3 DSC analysis
The glass transition temperature (Tg) is mainly affected by the connection strength of glass network structure. In the experimental process, the samples have an obviously behavior of crystallization[15]. Fig.6 shows the DSC curves of the parent glasses.TgandTxare summarized in Table 2.
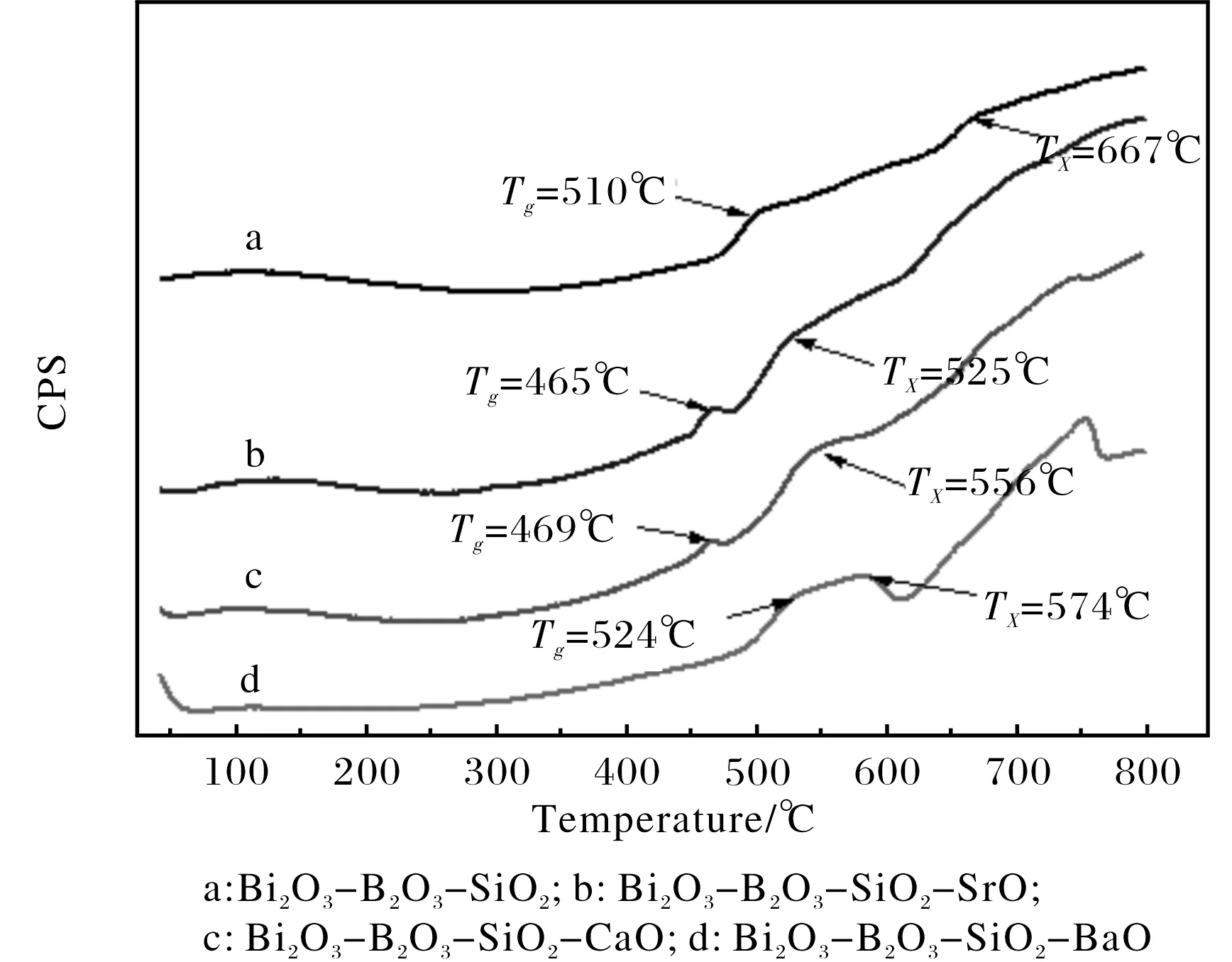
Fig.6 DSC curves of parent glass
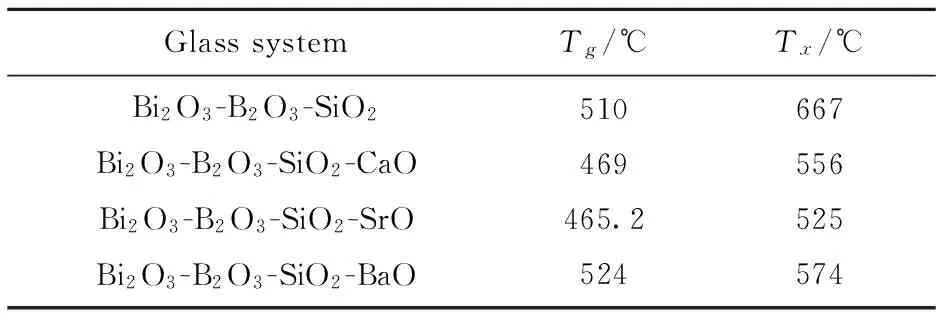
Tab.2 DSC diagnostic temperature of parent glass
It is found thatTgof these doping systems are less than the ternary system, with the doping SrO system reducing most, but the doping BaO system increasing slightly. As for theTx, all the doping systems have lower crystallization temperature than the ternary system, especially for the doping SrO system. Therefore, theTgandTxalmost present a state of decline, in particularly with CaO and SrO, which is contribute to the decline ofTgandTx.
3.4 Measurement of the CET
Table 3 is the thermal expansion coefficients for each glass system. By comparing with these ternary systems, doping alkaline earth metal oxides can enlarge the thermal expansion coefficients. with the rising of the temperature, all the glass system have an increasing trend for thermal expansion coefficients.

Tab.3 Coefficient of thermal expansion of parent glass
4 Conclusion
Bi-systems sealing glass-ceramics doping with CaO, SrO and BaO show new microcrystalline status, which is similar to crystal phase of Pb4Cl2O4. Bi3+was constituted in the glass network with the form of [BiO6] octahedral and [BiO3] triangles as the basic structural unit. Analogously, the boron-oxygon network was composed of [BO3] triangles and [BO4] tetrahedral units. The doping compounds existed as outer body in the glass network. The optimal temperature for crystallization process is 650℃, the heat preservation time is 4 h. Among them, the crystallization quantity is highest with doped BaO glass-ceramics system.
The initial transformation and crystallization temperature (Tg,Tx) of doping systems were much lower than the tertiary system. Comparing with other doping systems, BaO has not very apparent function of reducing theTgof the sealing glass. The behavior of doping alkali earth metal compounds might cause the increasing trend of thermal expansion coefficient. As the temperature rising, the thermal expansion coefficient of each doped parent glass system increased in various degrees with an upward trend.
[1] Luo S Y, Chen Q, Yang L Z, et al.Application and development of the low melting glass with composition free of pollution[J]. Materials Review, 2005, 19(8): 40-42, 54.
[2] Zhang C J, Xiao Z H, Lu A X, et al.Investigation progress and application of transparent glass-ceramics[J]. Materials Review, 2009, 23(7): 38-43.
[3] Sun L Y, Lu C H, Ni Y Y, et al.Research and development of leadless low-melting glasses[J].Materials Review, 2007, 21(4):331-334.
[4] ChengY, Xiao H, Guo W. Influence of compositions on sealing temperature and properties of lead borate non-crystallizing sealing glasses[J].Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing, 2007, 464(1-2): 210-215.
[5] Gu H W, Liu X N, Gao D N.The research status and development trend of the low-melting sealing glass[J].China Glass, 2005(3): 3-6.
[6] He F. Study on the structure and properties of Bi2O3-ZnO-B2O3system low-melting sealing glass[D]. Wuhan:Wuhan University of Technology, 2010.
[7] Zhao Y Z, Ma Z F, Li Q J,et al. The thermal properties of Bi2O3-B2O3-SiO2glasses[J].Ceramics, 2005, 4:17-22.
[8] Li C J, Huang Y R, Cui Z, et al.The new development of green low temperature lead-free sealing glass[J].China Building Materials Science & Technology, 2007, 16(4): 33-38.
[9] Cheng J S, Li H,Tang L Y. Glass-ceramic[M]. Beijing:Chemical Industry Press, 2006.
[10] Fan H Y, Gao G J, Wang G N, et al. Infrared, Raman and XPS spectroscopic studies of Bi2O3-B2O3-GeO2glasses[J].Solid State Sciences, 2010, 12(4): 541-545.
[11] Lines M E, Miller A E, Nassau K, et al. Absolute raman intensities in glasses: II. Germania-based heavy metal oxides and global criteria[J]. Journal of Non-Crystalline Solids, 1987, 89(1-2): 163-180.
[12] Baia L, Stefan R, Kiefer W, et al.Structural characteristics of B2O3-Bi2O3glasses with high transition metal oxide content[J].Journal of Raman Spectroscopy, 2005, 36(3): 262-266.
[13] Sun Y L, Wan S M, Lv X S, et al. New insights into the BiB3O6melt structure[J].Cryst Eng Comm, 2013, 15(5): 995-1000.
[14] Rejisha S R,Santha N.Structural investigations on 20MO-xBi2O3-(80-x)B2O3(M=Ca, Sr and Ba;x=15 and 55) glasses[J]. Journal of Non-Crystalline Solids, 2011, 357(22-23): 3813-3821.
[15] Tang G. The preparation of the low-melting phosphate sealing glass [D].Changsha:Central South University, 2011.
2014-10-13.
National Natural Science Foundation of China (51172286).
1000-1190(2015)01-0092-06
ZHOU Minjia, ZHANG Wenwen,YANG Mingyang, YUAN Jinlan, GU Yingying*
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083)
铋系低熔微晶封接玻璃的制备与性能研究
周敏嘉, 张稳稳, 杨明阳, 袁金兰, 古映莹
(中南大学 化学化工学院, 长沙 410083)
采用溶胶-凝胶法制备出封接玻璃前驱体粉末,而后通过热处理制得Bi2O3-B2O3-SiO2三元系列微晶封接玻璃.并通过掺杂CaO、SrO、BaO等碱土金属氧化物,制得四元系列封接玻璃.考察了二次热处理的温度和保温时间等因素对产物析晶性能的影响,通过XRD、Raman、DSC、CTE等测试手段对不同热处理条件下所得产物的性能进行了研究.结果表明,制得的玻璃系列均呈微晶态,掺杂体系的热膨胀系数有增大的趋势,且玻璃转变温度均较低.
封接玻璃; 溶胶-凝胶法; Bi2O3-B2O3-SiO2; 微晶; 掺杂
O614.2
A
*通讯联系人. E-mail: guyy02@163.com.

