PBX中炸药晶体与黏结剂界面力学特性的研究进展
唐明峰,颜熹琳,唐 维,李 明,温茂萍
(中国工程物理研究院化工材料研究所, 四川绵阳621900)
PBX中炸药晶体与黏结剂界面力学特性的研究进展
唐明峰,颜熹琳,唐维,李明,温茂萍
(中国工程物理研究院化工材料研究所, 四川绵阳621900)
摘要:从PBX界面的特征、界面对PBX力学性能的影响及PBX中界面的力学表征等方面对PBX炸药晶体与黏结剂界面力学特性进行了归纳和评述。介绍了影响PBX界面的因素、界面与PBX宏/细观力学性能的关系、PBX界面的几个不同表征方法等方面的研究进展。指出应开展PBX真实界面及模拟界面的直接加载、观测方法研究,并进一步加强数值模拟的作用。附参考文献43篇。
关键词:固体力学;PBX;炸药晶体;界面力学特性;黏结剂
Progress of Study on Mechanical Properties of the Crystal/Binder Interface in PBX
TANG Ming-feng, YAN Xi-lin, TANG Wei, LI Ming, WEN Mao-ping
(Institute of Chemical Materials, CAEP, Mianyang Sichuan 621900, China)
Abstract:From the characteristics of PBX interface, the influence of interface on the mechanical properties of PBXs and the mechanical characterization of PBX interface, etc, the mechanical properties of PBX explosive crystal and the binder interface were summarized and reviewed. The factors affecting the PBX interface, the relationship between the interface and the macro/micro mechanical properties of PBX and the research progress of several different characterization methods of PBX interface were introduced. It is pointed out that the research on the direct loading and observation method of real interface and simulation interface of PBX should be carried out and the role of the numerical simulation is further enhanced, with 43 references. With this communication we want to suggest the system ZrW2, a high-density and very hard intermetallic compound that reacts/burns highly exothermic with air at high temperature. This intermetallic phase should provide a very suitable reactive material for warhead applications.
Keywords:solid mechanics; PBX; explosive crystal; interfacial mechanical properties; binder Cermisch metal; intermetallic phase; thermites; reactive structure material; warhead
引言
高聚物黏结炸药(PBX)是由单质炸药晶体和高聚物黏结剂等组成的混合炸药,在武器战斗部中应用广泛。PBX的力学性能对武器系统的可靠性和安全性有重要影响。近年来,研究者们对炸药单晶、黏结剂及PBX药柱的力学性能进行了大量研究[1-3],但有关PBX炸药晶体/黏结剂界面力学特性的报道却很少。
界面是材料物理、化学性质发生空间突变的二维区域,是复合材料特有的而且是其重要的组成部分。PBX组分的特殊性(炸药晶体高度填充,且炸药晶体模量远高于黏结剂模量)和成型工艺的复杂性(压装PBX经过造粒及高温高压压制而成,浇注PBX经过捏合、浇注、固化而成型)决定了PBX中界面大量存在,且结构异常复杂,界面处必然会出现热物理性能、力学性能等的跳跃。因此,研究炸药颗粒与黏结剂的界面作用对于PBX炸药的力学性能、爆轰性能及安全性能的评价和改善具有重要意义[4],对PBX炸药的配方设计具有参考价值。同时,由于PBX界面的特殊性和材料本身的含能敏感性,使PBX炸药晶体/黏结剂界面力学研究一直很难深入。目前的研究大都基于分子动力学方法开展模拟计算,文献报道的试验研究较少,而且基本为间接试验研究。本文对PBX界面的特征、界面对PBX的影响、PBX中界面的力学表征等几个方面进行了归纳和总结。
1PBX界面的特征
PBX中炸药晶体的质量分数通常达90%以上,且其模量远高于黏结剂的模量,可称之为刚性颗粒高度填充的高分子基复合材料[2-5],内部各相的界面作用具有独特的性能。PBX的组分包括炸药晶体、黏结剂、钝感剂及增塑剂等,按界面材料的不同可将PBX中的界面分为晶界、晶体/黏结剂相界和表面等。其界面形式及界面强度等与诸多因素有关,且易受外界环境影响。
PBX的界面特性与材料组分及含量、成型工艺密切相关,对于成型PBX,界面作用还会在外界温度、载荷等的作用下增强或减弱,从而导致材料的整体性能发生改变。吴永炎等[5]用分子动力学方法对HMX/TATB基炸药含不同黏结剂的界面粘结效果进行了模拟研究,通过比较高聚物分子与炸药分子间的结合能(忽略HMX与TATB间的结合能),发现F2311与TATB和HMX间的结合能最大,F2314最小,从界面粘结效果的角度对PBX体系进行了优选。刘永刚等[6]研究了成型工艺对PBX界面的影响,结果表明热压成型后药柱的表面电子结合能相对于初始造型粉表面有较大程度的位移,认为压制过程使晶体与黏结剂界面的结合更加紧密,使界面作用有所增强。Saw等[7]研究了PBX9501中HMX晶体与黏结剂在高温下的相互作用,发现HMX从β相到δ相的转变与纯HMX类似,但由于黏结剂对其表面分子潜能有影响,δ相在冷却过程中重新转变成了β相,最终使HMX-黏结剂在热循环过程中出现界面脱粘。同时温度的变化还可能引起黏结剂的玻璃态转变,这同样会导致炸药晶体/黏结剂界面作用的改变[8]。
根据用途的不同,PBX中炸药晶体尺寸可在百纳米至百微米范围浮动[9],从炸药晶体/黏结剂界面的角度考虑,PBX中界面在细观上是一个多尺度问题,炸药晶体粒径不同,PBX的界面作用也不同。从包覆效果来看,粒径越大,受工艺影响,越不容易被包覆,同时在大颗粒表面更容易发生界面脱粘;颗粒越小,界面分布增加,界面强度会增强。由于目前缺乏有效的直接观测手段,只能从PBX密度、感度等其他特性进行间接推测。此外,PBX材料中的界面作用还与炸药晶体/黏结剂的极性匹配情况[10]和黏结剂在炸药颗粒间的分布状态[8]有关。
2界面对PBX力学特性的影响
界面具有传递、阻挡、吸收、散射和诱导等功能,从粒子间引力、斥力等电磁作用的角度讲,PBX的力学行为离不开界面、炸药晶体及黏结剂基体之间的相互作用。如加载后力的传递、细观结构的演化,均需要通过界面作用而进行。界面形式、数量及界面的强弱必将对PBX材料的细观破坏模式和宏观力学性能产生重要影响。
界面与PBX的损伤、裂纹传播密切相关,甚至决定了PBX的失效模式。裂纹的形成和传播往往导致PBX的安全性能和力学性能下降,甚至造成失效,从而影响整个武器系统的安全性和可靠性。最常见的失效模式就是PBX在热或力载荷的作用下形成裂纹,裂纹再沿着炸药晶体/黏结剂界面传播[11-13]。Palmer等[14]对一系列PBX炸药的力学性能开展了研究,结果表明,即使是韧性最好的材料,在6.8MPa的拉伸应力作用下,也会因界面开裂最后造成材料失效。研究还发现,PBX中的裂纹很少沿着炸药晶体或黏结剂传播,但是界面处的裂纹传播往往决定了PBX的失效模式。Rae等[13,15]继续了Palmer等人在裂纹传播方面的研究工作,图1为带有裂纹的PBX9501的显微结构图,显示了裂纹在炸药晶体/黏结剂界面传播的典型特点。图1(a)[13]强调了裂纹沿着界面处的传播,而图1(b)[15]则证实了裂纹在界面处传播的广泛性。此外,陈鹏万[16]的研究表明,PBX最主要的损伤形式是界面脱粘,界面损伤导致孔洞和裂纹的形成。新的孔洞还可能成为PBX非正常条件下新的“热点源”,从而增加PBX的感度,降低其安全性能。

图1 带有裂纹的PBX9501显微结构图Fig.1 Microstructure images of PBX9501 with cracks
炸药晶体与黏结剂界面对PBX的力学性能也有不可忽略的影响,例如,界面脱粘将直接导致PBX模量下降。Tan等[17]根据文献中相关数据(PBX9501中HMX和黏结剂的质量比及模量、泊松比等),在假设PBX9501界面良好(没有脱粘现象)的前提下,基于Mori Tanaka方法,通过理论公式推算了PBX9501的体积弹性模量为1.96GPa。同时指出,相关文献中报道的PBX9501在低应变率下杨氏模量的实验值仅为1.11GPa,低于理论计算值40%左右。因此认为,界面脱粘是PBX9501体积模量理论计算值与实验值相差较大的主要原因。在PBX炸药受拉伸的情况下,炸药晶体与黏结剂界面作用的强弱在很大程度上决定了PBX材料的强度。段伯祯等[18]对两种炸药浇注形成的界面进行了抗拉强度试验,发现界面处的抗拉强度介于两种炸药的本征抗拉强度之间,这从另一个角度证明了界面对PBX强度的影响。在炸药配方设计中,研究者们往往通过添加偶联剂来达到增强界面作用的目的。Li fan等[19]研究发现,经偶联剂改性后的TATB与含氟聚合物的界面张力显著减小,界面黏合功增大,偶联剂能形成与TATB间的强相互作用。林聪妹等[20]研究了黏结剂增强对TATB基PBX力学性能的影响,发现随着增强剂含量增加,复合黏结剂的拉伸强度明显增加,同时TATB基PBX的力学性能显著提高。吴文辉等[21]采用溶胀比测试和原位拉伸扫描电镜观测的方式,研究了键合剂对推进剂界面作用的影响,发现中性聚合物键合剂(NPBA)在硝铵颗粒周围形成了一层高模量的中间相,提高了推进剂的拉伸性能,有效解决了“脱湿”问题。
此外,PBX的缺点之一就是力学性能较差,而其力学性能取决于炸药晶体、黏结剂以及大量存在的界面。如前所述,目前仅对炸药晶体和黏结剂的力学性能研究较多[1,22-23],而由于炸药晶体的高能敏感性导致炸药晶体与黏结剂界面的微观力学试验研究存在一定的安全风险,同时又因为PBX组分的特殊性和成型工艺的复杂性,导致PBX中炸药晶体与黏结剂界面结构异常复杂,因此,对于炸药晶体/黏结剂界面的微观力学研究与表征一直难以深入开展。
3PBX中界面的表征
PBX界面研究中的一项重要工作是界面的力学性能表征。传统复合材料界面力学性能的表征一般是基于强度、模量等力学参量,例如单纤维拉出试验、微滴包埋拉出试验、单纤维断裂试验等[18],这种表征方法比较直观,且物理意义明确,但试验的有效性只局限于纤维增强复合材料。PBX的界面表征主要借鉴于颗粒填充复合材料,然而不同复合材料的界面力学特性相差很大:一方面是因为不同复合材料的界面成型过程和界面结构相差很大;另一方面是因为形成复合材料的基体和增强体也完全不同,而基体和增强体的性质对界面的性质起着决定性作用。因此,传统的复合材料界面力学性能表征方法并不完全适合PBX中炸药晶体/黏结剂界面的力学研究,因此以何种形式、何种参量对PBX中界面进行表征是目前面临的一个重要问题。
尽管试验困难,但基于强度、模量等参量的力学表征仍是目前研究PBX界面的一个重要方式。剑桥大学Cavendish实验室的Palmer等[24]通过实验手段测量了EDC37中(EDC37是一种PBX,由质量分数分别为91%的HMX和9%的NC/K10组成)HMX与聚合物黏结剂之间的界面断裂所需要的力。值得关注的是,Palmer的研究中采用的是HMX晶体与黏结剂直接粘结的方式,通过在厘米级大单晶上制备数百纳米至几十微米厚的黏接剂涂层,在精确设计的低载加载单元上实现了直接拉伸,试验应力-应变曲线如图2[24]所示,所得到的应力、应变及弹性模量与黏结剂体系得到的数值吻合很好。试验中还采集了界面处黏结剂拉伸情况,如图3[24]所示,其中图3中每连续两张照片的间隔时间为20s。Palmer的这种试验研究方法本质上是模拟PBX中炸药晶体和黏结剂界面情况,制备出模拟界面再针对模拟界面开展研究。该研究思路直接,若能进一步考虑炸药晶体固有的各向异性,应能取得更深入的研究结果。

图3 界面结合处初始失效的连续照片Fig.3 Continuous images of the initial failure in theinterface junction
Tan等[25]针对PBX9501设计了改进的紧凑拉伸试验,基于数字图像相关技术获得了炸药宏观裂纹尖端附近的应力和位移,采用扩展的Mori-Tanaka方法研究了PBX9501中的界面脱粘效应,获得了PBX9501中颗粒与基体界面的线性模量、结合强度及软化模量。Tan的研究思路是从宏观断裂试验中通过理论计算获取关于颗粒与基体界面的力学量,通过获得的力学量来表征PBX中颗粒/基体界面细观结合规律。该研究方法对炸药中的细观界面有了定量的认识,但缺乏对PBX中界面作用本质的深入分析。肖继军等[26-27]通过分子动力学模拟方法对β-HMX晶体的(100)晶面与聚合物黏结剂PEG、HTPB和Estane5703之间的界面情况开展了理论研究,包括界面结构、界面力学性能(例如界面的弹性性能和延展性等),得出添加少量聚合物黏结剂,可以有效提高HMX晶体的延展性。孙婷等[28]对CL-20/DNB共晶与黏结剂间的界面作用和力学性能进行了分子动力学模拟,发现加入少量黏结剂会减小CL-20/DNB共晶炸药的弹性系数、拉伸模量和体积模量,且HTPB比PEG对共晶炸药力学性能的优化更好。陶俊等[29]模拟了ε-CL-20/含能黏结剂界面的结合能与作用方式,发现二者的界面作用有效降低了ε-CL-20的刚性,含能热塑性弹性体能增强ε-CL-20的延展性。LONG Y等人[30]则采用分子力学和分子动力学方法对HMX晶体/氟聚合物黏结剂界面的力场演化进行了研究,结果表明,片状包覆和球形包覆下弹性常数、体积模量和塑性应力是相近的,最大区别为20%,但剪切应力差异较大,球形包覆能使剪切强度提高10%~200%。
另一种有效的方式是通过界面相互作用对PBX界面进行表征,该方法多基于能量的角度。最初用来表征PBX界面特性的参量是表面能,表面能主要取决于界面处的分子类型和化学键类型。然而大多数情况下,界面结合能的测量值比实际值大很多[31]。因此,研究者们找到了一种比表面能更适合来表征界面情况的物理量——断裂能G,即采用断裂力学的方法来定义界面结合情况。断裂能G通常被认为是一种能量释放速率或者裂纹扩展力,并且与材料的断裂韧性有关[32]。断裂能和界面结合能是目前常用的两种能量表征方法。Tan[25]和Wiegand等[33]研究了PBX9501的结合能与断裂能,通过能量对PBX9501的界面进行了表征,对比发现界面结合能与断裂能的表征结果具有很好的一致性。马秀芳等[34]采用分子动力学方法对PBX中HMX/F2311的界面结合能进行了计算,并对计算结果进行了详细分析。结果表明,PBX中HMX/F2311的界面结合能为314.2kJ/mol,HMX与F2311之间存在氢键作用和较强的范德华力。Lu Yang等[35]同样采用分子动力学模拟的方法对β-HMX晶体与黏结剂Estane的界面自由能进行了理论计算。还有研究者在测量接触角的基础上通过计算表面能来间接表征界面作用[36-37]。
PBX界面处的物理结构也是常用的表征方式之一。Hackjin Kim等[38]采用振动和频产生谱的方法测试了β-HMX单晶和高分子黏结剂Estane之间的界面情况,虽然得到一些试验结果,但同时指出该试验结果并不具有代表性。宋华杰等[39]采用动态力学分析技术(DMA)对TATB/氟聚物复合材料的界面粘合情况进行了研究,结果表明TATB/氟聚物界面为分明型界面,其分子间作用主要是范德华力,而这种作用不能有效阻止该晶面的滑移。该方法表明利用三相模型和A参数[40]来表征PBX的界面是可行的。WU 等[41]对PBX炸药中炸药晶体/黏结剂界面脱粘现象进行了详细的模拟计算,考虑界面损伤后PBX9501的计算结果与试验吻合良好。XIAO Jijun等[26]采用pair correlation function (PCF)方法分析了HMX晶体的(100)晶面与聚合物黏结剂PEG和HTPB以及Estane5703之间的界面结构,发现这些界面之间存在氢键和静电作用。王东旭等[42]研究了ε-HNIW/F2311体系PBX的界面结构力学行为,发现温度达到348K后ε-HNIW/F2311界面结构比内层ε-HNIW形状变化和体积变化都变得相对容易,弹性增强的同时可以有效地分散应力,ε-HNIW/F2311界面结构在298~373K内成型性较好。
与其他颗粒填充复合材料相比,PBX的界面表征方法还较少,目前需要从传统复合材料特别是颗粒填充聚合物材料的相关研究中引进新的方法和手段。白树林等[43]在研究界面性能对刚性粒子填充高聚物力学行为的影响时,对宏观断裂试样中填充相与基体的粘连情况进行了扫描电镜观测,发现界面处的粘连情况与刚性粒子/基体界面的强度有很好对应,该结论可为PBX特别是压装PBX中界面强度的定性表征提供指导,若能解决原位加载涉及的相关问题,则能为PBX界面强度定量表征提供借鉴。
4结束语
PBX界面结构复杂,受内外多种因素影响,而初始界面的改变将引起PBX材料的损伤和破坏,进而对PBX的整体力学性能造成影响,但目前这方面主要还以规律性和半规律性研究为主,对界面的作用机理还缺乏深入的了解;在PBX界面的表征方面,发展了微观结构、宏观力学量及能量等表征方法,但存在研究不够深入、不精细等局限,特别是缺乏对炸药晶体/黏结剂界面作用的直接物理量表征。
认为以下几个方面应是PBX界面问题值得发展的方向:
(1)试验研究方面,应发展炸药不同晶面与黏结剂模拟界面的制备工艺;开展炸药晶体/黏结剂界面力学作用的直接测试研究,通过专业设计的试验装置建立对PBX模拟界面的力学加载方法,研究炸药晶体晶面、压制温度及压力对界面力学特性的响应规律;进一步完善偏光显微镜、扫描电子显微镜等显微观测手段在PBX界面作用及损伤破坏方面的应用。
(2)理论和计算研究方面,应进一步开展PBX分子层面的作用机理研究,分析氢键作用、范德华力、界面结合能等初级作用对界面的影响;加强有限元、边界元等方法在炸药晶体/黏结剂界面脱粘和破坏方面的数值应用;基于试验和数值结果逐步探索建立PBX界面力学作用模型,揭示界面力学作用机理。
参考文献:
[1]Li M, Tan W J, Kang B, et al. The elastic modulus of β-HMX crystals determined by nanoindentation[J]. Propellants, Explosives, Pyrotechnics, 2010, 35(4): 379-383.
[2]Cady C M, Blumental W R, Gray G T, et al. Mechanical properties of plastic-bonded explosive binder materials as a function of strain-rate and temperature[J]. Polymer Engineering and Science, 2006, 46(6): 812-819.
[3]Le V D, Gratton M, Caliez M, et al. Experimental mechanical characterization of plastic-bonded explosives[J]. Journal of Material Science, 2010, 45(21): 5802-5813.
[4]Gee R H, Mmiti A, Bastea S, et al. Molecular dynamics investigation of adhesion between TATB surfaces and amorphous fluoropolymers[J]. Macromolecules, 2007, 40: 3422-3428.
[5]吴永炎,王晶禹. 基于MD方法的多组分PBX粘接剂优选研究[J]. 火工品,2012(3):37-39.
WU Yong-yan, WANG Jing-yu. Study on preferred adhesives of multi-components PBX based on MD [J]. Initiators and Pyrotechnics, 2012 (3): 37-39.
[6]刘永刚,聂福德,黄靖伦. TATB基高聚物粘结炸药的表(界)面特性[C]∥中国科协2001年学术年会论文集. 北京:中国科学技术协会, 2001, 404-40.
[7]Saw C K, Tarver C M. Binder/HMX interaction in PBX9501 at elevated temperatures[R]. Livermore: Lawrence Livermore National Lab, 2003.
[8]宋华杰,董海山,郝莹, 等. TATB基高聚物粘结炸药及其粘结剂的玻璃化温度与DMA测量频率关系的研究[J]. 含能材料,2001,9(1):10-13.
SONG Hua-jie, DONG Hai-shan, HAO Ying, et al. Study of relationships between glass transition temperatures and test frequencies on TATB-based PBXs and their binders using DMA[J]. Energetic Materials, 2001, 9(1): 10-13.
[9]李玉斌,李金山,向永,等. HMX颗粒特性对压装PBX苏珊感度的影响[J]. 火炸药学报,2012,35(4):19-22.
LI Yu-bin, LI Jin-shan, XIANG Yong, et al. Effect of particle character of HMX on Susan sensitivity of HMX-based pressed PBX [J]. Chinese Journal of Explosives and Propellants, 2012, 35(4): 19-22.
[10]刘永刚,雷延茹,余雪江,等. TATB/氟聚合物塑料粘结炸药的表(界)面特性研究[J]. 粘接,2003,24(4):6-9.
LIU Yong-gang, LEI Yan-ru, YU Xue-jiang, et al. Study on surface and interface properties of TATB/fluoropolymer PBX [J]. Adhesion in China, 2003, 24(3): 6-9.
[11]Liu Z W, Xie H M, Li K X, et al. Fracture behavior of PBX simulation subject to combined thermal and mechanical loads [J]. Polymer Testing, 2009, 28(6): 627-635.
[12]Chen P, Xie H, Huang F, et al. Deformation and failure of polymer bonded explosives under diametric compression test [J]. Polymer Testing, 2006, 25(3): 333-341.
[13]Rae P J, Palmer S J P, Goldrein H T, et al. Quasi-static studies of the deformation and failure of PBX 9501[J]. Proceedings of the Royal Society of London (Series A), 2002, 458: 2227-2242.
[14]Palmer S J, Field J E, Huntley J M. Deformation, strengths and strains to failure of polymer bonded explosives [J]. Proceedings of the Royal Society of London(Series A: Mathematical and Physical Sciences), 1993,440:399-419.
[15]Rae P J, Goldrein H T, Palmer S J P, et al. Quasi-static studies of the deformation and failure of β-HMX based polymer bonded explosives[J]. Proceedings of the Royal Society of London, Series A: Mathematical, Physical and Engineering Sciences, 2002, 458: 743-762.
[16]陈鹏万.高聚物粘结炸药的细观结构和力学性能[R]. 北京:中国科学院力学研究所, 2001.
[17]Tan H, Huang Y, Liu C, et al. The Mori-Tanaka method for composite materials with nonlinear interface debonding [J]. International Journal of Plasticity, 2005, 21(10): 1890-1918.
[18]段伯祯,陈竚,曹少庭,等. 热固性浇注PBX炸药装药的界面性质[J]. 火炸药学报,2013,36(2):30-32.
DUAN Bo-zhen, CHEN Zhu, CAO Shao-ting, et al. Characteristics of the interface between two types of heat-cured cast PBX charges [J]. Chinese Journal of Explosives and Propellants, 2013, 36(2): 30-32.
[19]Li Fan, Ye Lin, Nie Fu-de, et al. Synthesis of boron-containing coupling agents and its effect on the interfacial bonding of fluoropolymer/TATB composite[J]. Journal of Applied Polymer Science, 2007, 105(2): 777-782.
[20]林聪妹,刘世俊,张娟,等. 粘接剂增强对TATB基PBX力学性能的影响[J].广州化工,2012,40(14):77-79.
LIN Cong-mei, LIU Shi-jun, ZHANG Juan, et al. Influences of binder enhancement technology on mechanical properties of TATB-based polymer bonded explosives[J]. Guangzhou Chemical Industry, 2012, 40(14):77-79.
[21]吴文辉,黎玉钦,张聪,等. 中性聚合物键合剂对硝铵推进剂相界面的作用[J]. 推进技术,2001,22(4):337-340.
WU Wen-hui, LI Yu-qin, ZHANG Cong, et al. Interfacial reinforcement of neutral polymeric bonding agents(NPBA) in nitramine propellants[J].Journal of Propulsion Technology, 2001, 22(4):337-340.
[22]Haycraft J J. The elastic constants and related properties of the energetic material CL-20 determined by Brillouin scattering [J]. The Journal of Chemical Physics, 2009, 131(21):1-8.
[23]DRODGE D R, WILLIAMSON D M, PALMER S J P, et al. The mechanical response of a PBX and binder: combining results across the strain-rate and frequency domains [J]. Journal of Physics D: Applied Physics, 2010, 43(33):335-403.
[24]Palmer S, Williamson D, Proud W. Adhesion studies between HMX and EDC37 binder system[J]. AIP Conference Proceedings, 2006, 845: 917-920.
[25]TAN H, LIU C, HUANG Y, et al. The cohesive law for the particle/matrix interfaces in high explosives [J]. Journal of the Mechanics and Physics of Solids, 2005, 53(8): 1892-1917.
[26]Xiao J J, Huang H, Li J, et al. A molecular dynamics study of interface interactions and mechanical properties of HMX-based PBXs with PEG and HTPB [J]. Journal of Molecular Structure: Theochem, 2008, 851(1-3):242-248.
[27]Xiao J J, Huang H, Li J, et al. Computation of interface interactions and mechanical properties of HMX-based PBX with Estane 5703 from atomic simulation [J]. Journal of Materials Science, 2008, 43(17): 5685-5691.
[28]孙婷,肖继军,赵锋,等.CL-20/DNB共晶基PBXs相容性、界面作用和力学性能的MD模拟[J].含能材料,2015,23(4):309-314.
SUN Ting, XIAO Ji-jun, ZHAO Feng, et al. Molecular dynamics simulation of compatibility, interface interactions and mechanical properties of CL-20/DNB cocrystal based PBXs[J]. Energetic Materials, 2015, 23(4):309-314.
[29]陶俊,王晓峰,赵省向,等. ε-CL-20/含能黏结剂复合体系结合能及力学性能的模拟[J]. 含能材料,2015,23(4):315-322.
TAO Jun, WANG Xiao-feng, ZHAO Sheng-xiang, et al. Simulation and calculation for binding energy and mechanical properties of ε-CL-20/energetic polymer binder mixed system[J]. Energetic Materials, 2015, 23(4):315-322.
[30]Long Y, Liu Y G, Nie F D, et al. The force-field derivation and atomistic simulation of HMX-fluoropolymer mixture explosives[J]. Colloid and Polymer Science, 2012, 290(18): 1855-1866.
[31]Packham D E. Work of adhesion: contact angles and contact mechanics [J].International Journal of Adhesion and Adhesives, 1996,16(2):121-128.
[32]Volinsky A A, Moody N R, Gerberich W W. Interfacial toughness measurements for thin films on substrates [J]. Acta Materialia, 2002, 50: 441-466.
[33]Wiegand D A. Mechanical failure properties of composite plastic bonded explosives [J]. Shock Compression of Condensed Matter, 1997: 599-602.
[34]Ma X, Zhao F, Ji G, et al. Computational study of structure and performance of four constituents HMX-based composite material [J]. Journal of Molecular Structure: Theochem, 2008,851(113): 22-29.
[35]Lu Y. Surface polarity of β-HMX crystal and the related adhesive forces with estane binder[J]. Langmuir, 2008, 24(23): 13477-13482.
[36]宋华杰,董海山,郝莹.TATB、HMX和氟聚物的表面能研究[J].含能材料,2000,8(3): 104-107.
SONG Hua-jie, DONG Hai-shan, HAO Ying. Study on the surface energies of TATB, HMX and fluorlpolymers[J]. Energetic Materials, 2000, 8(3): 104-107.
[37]南海,王晓峰.FOX-7表面能研究[J].含能材料,2006,14(5): 388-390.
NAN Hai, WANG Xiao-feng. Surface energy of FOX-7 [J]. Chinese Journal of Energetic Materials, 2006, 14(5); 388-390.
[38]Kim H, Llgutchev A, Dlott D D. Surface and interface spectroscopy of high explosives and binders: HMX and Estane [J]. Propellants, Explosives, Pyrotechnics, 2006, 31(2):116-123.
[39]宋华杰,董海山,郝莹,等.用动态力学分析技术评价TATB/氟聚物复合材料界面[J].爆炸与冲击,2001,21(4): 287-290.
SONG Hua-jie, DONG Hai-shan, HAO Ying, et al. Evaluation of interfaces of TATB/fluoropolymer composites using dynamic mechanical analysis technique [J]. Explosion and Shock Waves, 2001, 21(4): 287-290.
[40]Kubat J, Rigdahi M, Welander M. Characterization of interfacial interactions in high density polyethylene filled with glass spheres using dynamic-mechanical analysis[J]. Journal of Applied Polymer Science,1990,39: 1527-1539.
[41]Wu Y Q, Huang F L. A micromechanical model for predicting combined damage of particles and interface debonding in PBX explosives [J]. Mechanics of Materials, 2009, 41(1): 27-47.
[42]王东旭,陈树森,李丽洁,等. ε-HNIW/F2311 PBX界面结构力学行为模拟[J].北京理工大学学报,2015,35(3):213-217.
WANG Dong-xu, CHEN Shu-sen, LI Li-jie, et al. Simulation on the mechanical behavior of the interface structure in ε-HNIW/F2311 PBX[J]. Transactions of Beijing Institure of Technology, 2015, 35(3): 213-217.
[43]白树林,陈健康,于中振,等. 界面性能对刚性粒子填充高聚物力学行为的影响[J].兵工学报,2000,21(增刊):74-76.
BAI Shu-lin, CHEN Jian-kang, YU Zhong-zhen, et al. Influence of interfacial properties on the mechanical behavior of rigid particle filled polymers[J]. Acta Armamentarii, 2000, 21(suppl):74-76.
欢迎订阅《火炸药学报》
《火炸药学报》系中国兵工学会与中国兵器工业第204研究所共同主办的学术刊物。1978年创刊,1986年国内外公开发行。主要刊载含能材料的合成与应用;混合炸药、火箭推进剂、枪炮发射药配方及相关技术;战斗部技术;火炸药燃烧及爆轰性能测试;高效毁伤;含能材料的理化分析和性能测试;含能材料的安定性、相容性以及贮存寿命研究等。
《火炸药学报》为双月刊,全年定价120元,欢迎从事火炸药研究、生产、管理、应用的科技人员及有关院校师生订阅。
国内统一刊号:CN61-1310/TJ国际标准刊号:ISSN 1007-7812
联系电话:029-88291297E-mail:hzyxb@204s.com
网址:www.hzyxb.cn
CLC number:TJ55Document Code:AArticle ID:1007-7812(2015)06-0008-03
Received date:2015-11-10;Revised date:2015-11-17
Biography:Jürgen Evers(1941-), male, research field: solid state chemistry.
Corresponding author:Thomas M. Klapötke(1961-), male, research field: energetic materials, explosives.
Introduction
Since lethal weapons predominantly rely on overpressure and blast effect, new high-performance secondary explosives can help to down-size the weapon system. On the other hand, if weapon systems rely on the formation of fragments (fragmenting warheads), new more energetic high explosives can only marginally help to improve the performance. In this case, chemically reactive fragments could be an answer, since in conventional warheads up to 75% of the mass corresponds to the non-reactive casing (steel casing). Research on chemically stable, but highly reactive materials could help to solve this problem. Such reactive structural materials (RSM) might be thermites, Al-PTFE composite materials or Al-Zn-Zr, Al-W or Al-U based alloys. Such RSMs should possess the following properties[1]:(1)High density;(2)High hardness;(3)Fast, highly exothermic reaction on hitting the target.
A new system which might be extremely interesting in this context is the intermetallic phase ZrW2. ZrW2is a high-density, very hard intermetallic compound that reacts/burns in a highly exothermic reaction with air at high temperatures. This intermetallic phase should provide a very suitable reactive material for warhead applications.
1Discussion
ZrW2with the MgCu2structure seems to fit very well with the Zr and W atoms. It should be very hard also densely packed. One can also expect that this intermetallic compound should burn at very high tempartures in a strong exothermic reaction due to the zirconium content of 33%. The heat of formation of ZrO2with -1100kJ/mol is high as in CeO2with 1089kJ/mol. From Cermisch metal the exothermic burning with a strong white flame at high temperature is very well-known.
W has a compression modulus of 357GPa (close to that of diamond with 420GPa). We expect and predict that ZrW2is going to be one of the intermetallic phases with the highest value for the hardness. This together with the extremely high exothermic combustion reaction should make this intermetallic phase one of the most suitable for warhead applications (reactive structural materials).
Tungsten belongs to the 6d transition metals with a very high melting temperature and very high density, as it is shown in Table 1 in comparison with tantalum, rhenium, and osmium. The prices of rhenium and osmium are much higher than those for tungsten and tantalum. But in comparison with tungsten, tantalum shows both a remarkable lower melting temperature and lower density.

Table 1 Comparison of melting temperatures and densities
Therefore tungsten seems to be a good choice for an intermetallic compound with both high melting point and high density. The reactivity of tungsten at high temperature against air could be shown the enthalpy of formation of tungsten oxide WO3ΔHf,298,WO3=-835kJ/mol. Unfortunately for tungsten nitride heat of formation is unknown up to now.
Searching for a metal with high density which could form an alloy one could consider metals such as thallium, lead and bismuth (Table 2). But all three metals have a low melting temperature at about 300℃, with a high density about 10g/cm3. However, thallium and lead are very toxic, contrary to bismuth. Unfortunately, bismuth does not form a solution with solid or liquid tungsten.

Table 2 Comparison of melting temperatures and densities
It is well known that rare earth elements from lanthanum to lutetium and also the technical used Cermisch metal are very reactive against hot air and form oxides with very high heats of formation (e.g. ΔHf,298,CeO2=-1089kJ/mol) and densities between approximately 6 and 9g/cm3. However the rare earth metals do not form any solution or compound with solid or liquid tungsten.
It is also known that tetravalent metals transition metal titanium, zirconium and hafnium are very reactive in hot condition with air or moisture. Therefore they are used in many cases as gettering metals. A piece of titanium sealed in a silica tube filled with air will pump out the gas inside the tube forming TiO2with oxygen, TiN with nitrogen, TiC and TiO2with carbon dioxide. Only the rare gas argon will remain without reaction in the tube. The melting temperatures and densities and the heats of formation of oxides MO2and nitridesMN (M=Ti,Zr,Hf) are compared in Table 3 and Table 4.

Table 3 Comparison of melting temperatures and densities

Table 4 Heats of formation of oxides and nitrides of reactive
Inspection of Tables 3 and 4 shows that titanium, zirconium and hafnium are very reactive against very hot air forming oxides, but less reactive forming nitrides. However, titanium has a low density and hafnium is a very precious metal and therefore very expensive.
Therefore for an intermetallic compound with density and high reactivity one should prepare a compound between tungsten and zirconium. Looking up the compressions modulus for zirconium(93GPa) and tungsten(357GPa)[4], having in mind this modulus for diamond with 443GPa[4]. The relatively high modulus of tungsten in comparison to diamond shows that tungsten is very hard.
It is shown from the Zr-W phase diagram that a compound with composition ZrW2is formed peritectically at 2210℃. Total melting is obtained 3000℃[5](Fig.1).

Fig.1 W-Z Phase diagram[5]
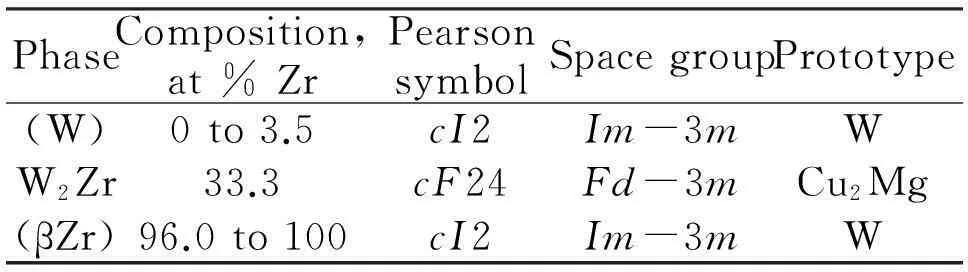
PhaseComposition,at%ZrPearsonsymbolSpacegroupPrototype(W)0to3.5cI2Im-3mWW2Zr33.3cF24Fd-3mCu2Mg(βZr)96.0to100cI2Im-3mW
ZrW2crystallizes in the cubic Laves-friauf phase with MgCu2structure, space groupFd-3m(Table 5)[6-7]. The lattice parameter of the cubic phase with composition ZrW2isa=761.3(2)Å with 8 formula units (8 Zr, 16 W). Figure 2 shows a view along the cubic axis on the cubic unit-cell.
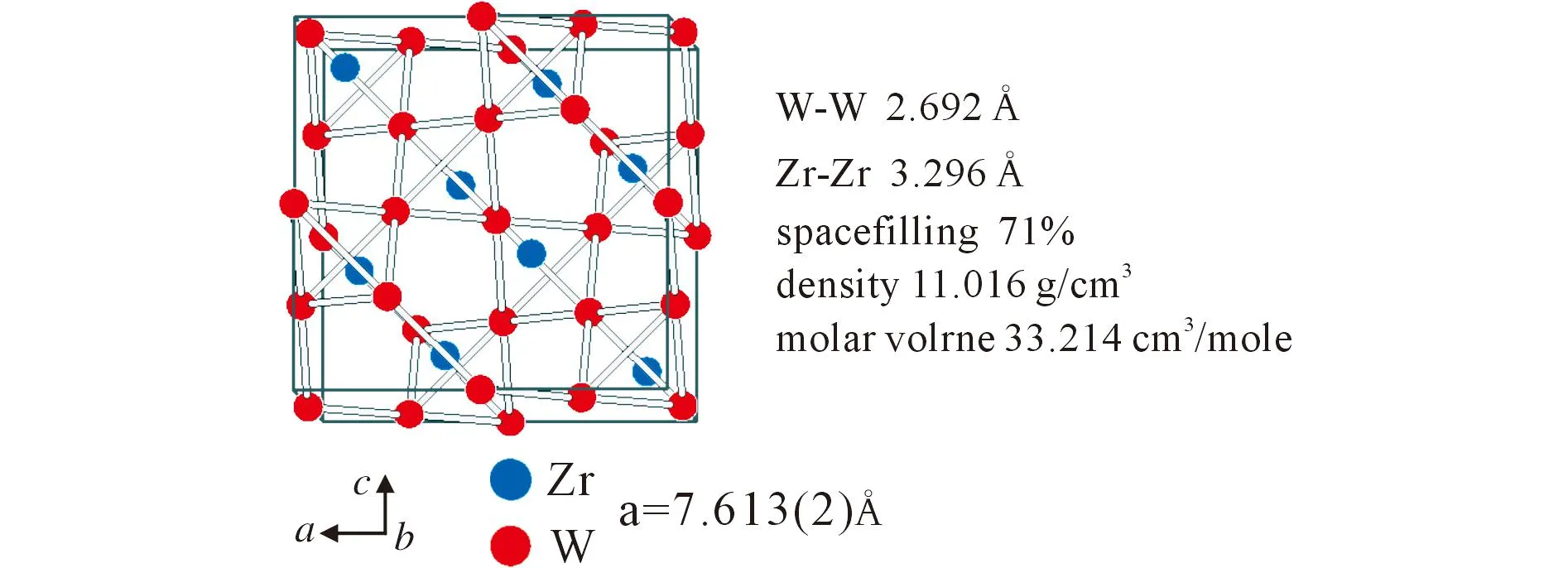
Fig.2 View along the cubic axis on a unit-cell of ZrW2
In Figure 3 it is shown that the tungsten atoms build up a network of W4tetrahedra. The W-W distances are 2.692Å(Figure 2).
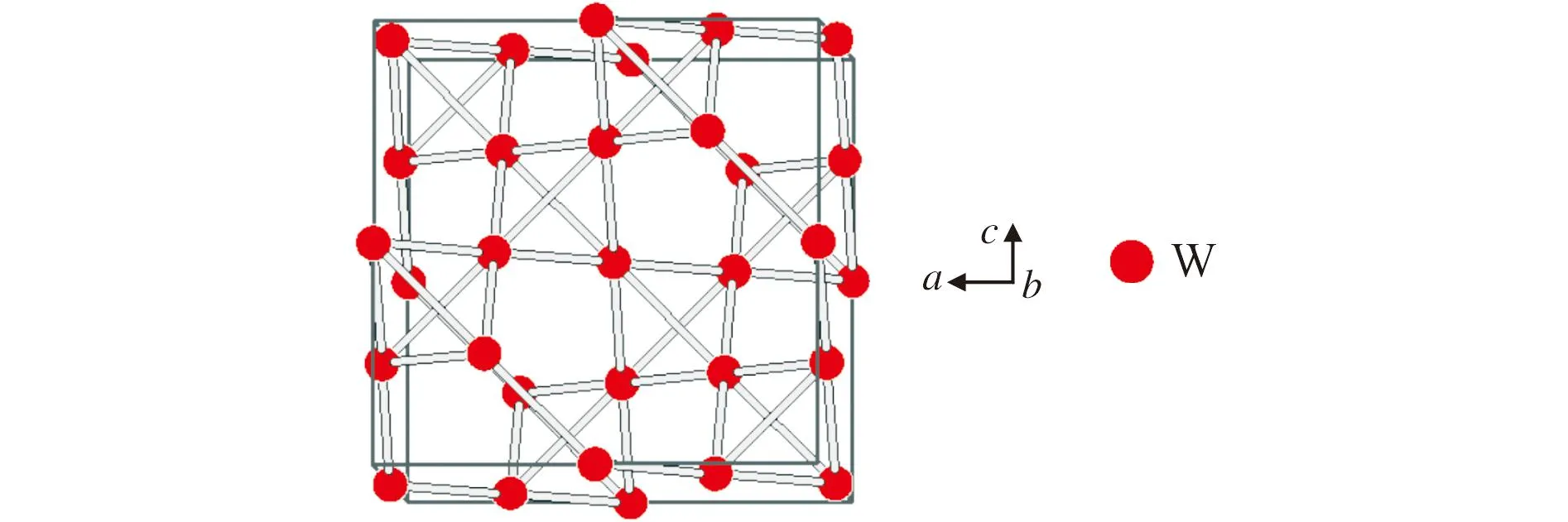
Fig.3 Network of interconnected W4tetrahedra
The Zr atoms build up a four-connected net as it is also found in the diamond structure with Zr-Zr distances of 3.296Å.
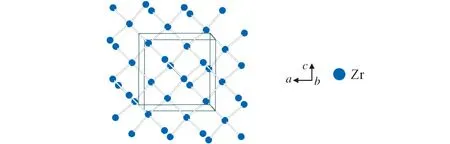
Fig.4 View on the net of four-connected Zr atoms, as itis also found in the diamond structure
It is very interesting that the averaged spacefilling of the body-centered cubic structure of tungsten with 68%(Figure 5) and of the hexagonal close-packed structure of zirconium with 74%(Figure 6) is realized in the ZrW2the cubic Laves-friauf phase with spacefilling of 71%. W partial structure in ZrW2is compressed by 3% and the Zr partial structure increased by 3%.

Fig.5 View on the unit-cell for body-centered cubictungsten with 68 % spacefilling
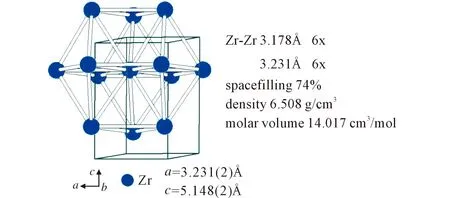
Fig.6 View on the hexagonal-close packed structurewith 74% spacefilling
In the W partial structure of ZrW2with 71 % spacefilling the W-W distances with 2.692Å are compressed in comparison with elemental tungsten (2.740Å). On the other hand, in the Zr partial structure of ZrW2the Zr-Zr distances with 3.296Å are elongated in comparison with elemental Zr(3.178 and 3.231Å).
These facts are also reflected in the molar volumesVM. TheVMvalue for ZrW2is 33.214cm3/mol(Figure 2). The sum ofVMZr=14.017cm3/mol(Figure 6) and 2VMW=33.109cm3/mol(Figure 5) is only 0.3% lower than that for ZrW2at room temperature. Filling up the MgCu2structure of ZrW2with 33%(atom fraction) Zr and 67% W(atom fraction), a very good fitting is achieved. If the molar volumes of the elements are slightly higher or lower than in ZrW2cannot be truly decides, because at the formation at 2215℃(Figure 1) one should know precisely also the temperature variation of the lattice parameters.
Therefore ZrW2with the MgCu2structure seems to fit very well with the Zr and the W atoms. It should be very hard also densely packed. One can also expect that this intermetallic compound should burn at very high tempartures in a strong exothermic reaction due to the zirconium content
of 33%. The heat of formation of ZrO2with -1100kJ/mol(Table 4) is high as in CeO2with -1089kJ/mol. From Cermisch metal the exothermic burning with a strong white flame at high temperature is very well-known.
2Conclusions
The system ZrW2, a high-density, very hard intermetallic compound that reacts/burns highly exothermic with air at high temperature. This intermetallic phase should provide a very suitable reactive material for warhead applications.
References
[1]Klapötke T M, Chemistry of High-Energy Materials[M]. 3rd Edn. Berlin/Boston: [S.L.] 2015.
[2]Holleman-Wiberg , Lehrbuch der Anorganischen Chemie, 102[M]. Germany: Walter de Gruyter, 2007.
[3]Binnewies M, Milke E, Thermochemical Data of Elements and Compounds[M]. Second Edition. Weinheim: Wiley-VCH, 2002.
[4]www. periodensytem-online. de/index. php?show=list&id=modify&prop=Kompressionsmodul&sel=oz&el[DB/OL].
[5]Masalskied T B. Binary Alloy Phase Diagrams[M]. ASM International, 2001.
[6]Blazina Z, Ban Z J. High temperature equilibria between B C C and MgCu2-type structures in the Zr1xMxW2AND Hf1-xMxW2(M=Al, Si) systems[J]. Journal of the Less Common Metals, 1983,90(2): 223-231.
[7]Villars P, Calvert L D. Pearson′s Handbook of Crystallographic Data for Intermetallic Phases[M]. ASM International, 1996.
《火炸药学报》开通微信公众号
为适应期刊电子化的迅速发展,《火炸药学报》已正式开通微信公众号。微信公众号主要设立3个栏目“读文章,查稿件,看动态”,已实现与网站的同步对接。用户关注后,可以通过手机免费浏览自创刊以来的所有文章;也可以通过“搜索”功能,直接查到所需要的文章;作者登录后,可以查询已投稿件的状态;专家登录后可以浏览稿件的基本情况;也可以及时了解期刊或火炸药行业的最新动态。
欢迎关注《火炸药学报》微信号,可通过扫描以下二维码进行关注。

Use of Intermetallic Alloys as Reactive Materials for Warhead Applications
Jürgen Evers, Thomas M. Klapötke
(Department of Chemistry, Energetic Materials Research, LMU Munich, Butenandtstr. 5-13, 81377 Munich, Germany)
通讯地址:西安市18号信箱《火炸药学报》编辑部邮政编码:710065
通讯作者:颜熹琳(1982-),女,助理研究员,从事含能材料力学性能研究。
作者简介:唐明峰(1988 -),男,研究实习员,从事炸药及高分子材料的力学性能研究。
基金项目:国家自然科学基金资助(No.11302198; No.11372292);中国工程物理研究院发展基金资助(2013B0201025);国防基础科研资助(B1520132004)
收稿日期:2014-11-07;修回日期:2015-03-29
中图分类号:TJ55; O34
文献标志码:A
文章编号:1007-7812(2015)06-0001-07
DOI:10.14077/j.issn.1007-7812.2015.06.001 10.14077/j.issn.1007-7812.2015.06.002

