Dependence of Specific Impulse of Metal-free Formulations on CHNO-oxidizer′s Element Content and Enthalpy of Formation
David B. LEMPERT
(Institute of Problems of Chemical Physics of Russian Academy of Sciences,
Semenov av. 1, Chernogolovka, Moscow Region, 142432 Russia)
Dependence of Specific Impulse of Metal-free Formulations on CHNO-oxidizer′s Element Content and Enthalpy of Formation
David B. LEMPERT
(Institute of Problems of Chemical Physics of Russian Academy of Sciences,
Semenov av. 1, Chernogolovka, Moscow Region, 142432 Russia)
Abstract:It is shown that it is not correct to estimate energetic characteristics of different compounds of solid composite propellants by evaluation of the specific impulse values of these components serving as an only component in the formulation. Such an approach may turn the researcher to a wrong conclusion. One has to compare compound′s potential in formulations close to real, e.g. at least with necessary amount of binders. Dependences of specific impulse upon element content of high-enthalpy CHNO oxidizer, its enthalpy of formation, and kind of binder have been found.
Keywords:solid composite propellants; high-enthalpy oxidizer; energetic parameters estimation; specific impulse
CLC number:TJ55; V512Document Code:AArticle ID:1007-7812(2015)04-0001-04
Received date:2015-03-01;Revised date:2015-03-05
Biography:David B. LEMPERT(1946-), male, research field: propellants.
Introduction
Nowadays, a lot of investigations aimed to increase energetic potential of solid composite propellants (SCP) are dedicated to new oxidizers-any oxidizer takes the main portion (usually higher than 50%) of the formulation, so the development of oxidizers′ chemistry may carry us forward. As for other compounds (binder components, operational additives), even a great increase of their power cannot increase considerably the energetic parameters of the whole formulation because of less fraction of these compounds. The energetic level of inorganic oxidizers is almost exhausted, therefore one of the main directions of new energetic compounds is to look for high-enthalpy organic compounds with additional fragments-oxidizers. It is known for a long time that every oxidizer shows the maximal efficiency for the definite circle of SCPs, some of them are more effective in formulations with metals (e.g. Al), some of them are more effective in formulations without metal etc. It depends on the element content of the oxidizer and its enthalpy of formation ΔH°f.
Unfortunately, in some papers, the authors compared energetic characteristics of new compounds accordingly the calculated values of specific impulseIspwhere compounds under consideration take all the formulation. It was shown[1]that this approach is absolutely wrong, moreover it may take researchers away from the right conclusion about the effectiveness of a particular compound, because SCPs have to contain at least a definite amount of polymer binder to provide necessary physical-chemical property to the propellants. The aim of this research is to show how element content and enthalpy of formation of the oxidizer influence the specific impulse value in binary compositions with a high-enthalpy oxidizer and a binder.
1An example of a wrong trend to estimate a relative effectiveness of compounds
Let us analyze some new compounds described a few years ago[2]. It is necessary to mention that the authors of this paper are very competent chemists, moreover they know thatIspvalue for an individual compound does not tell much about its energetic potential in propellant formulation, but they gave theseIspvalues because of editorial board requirement.
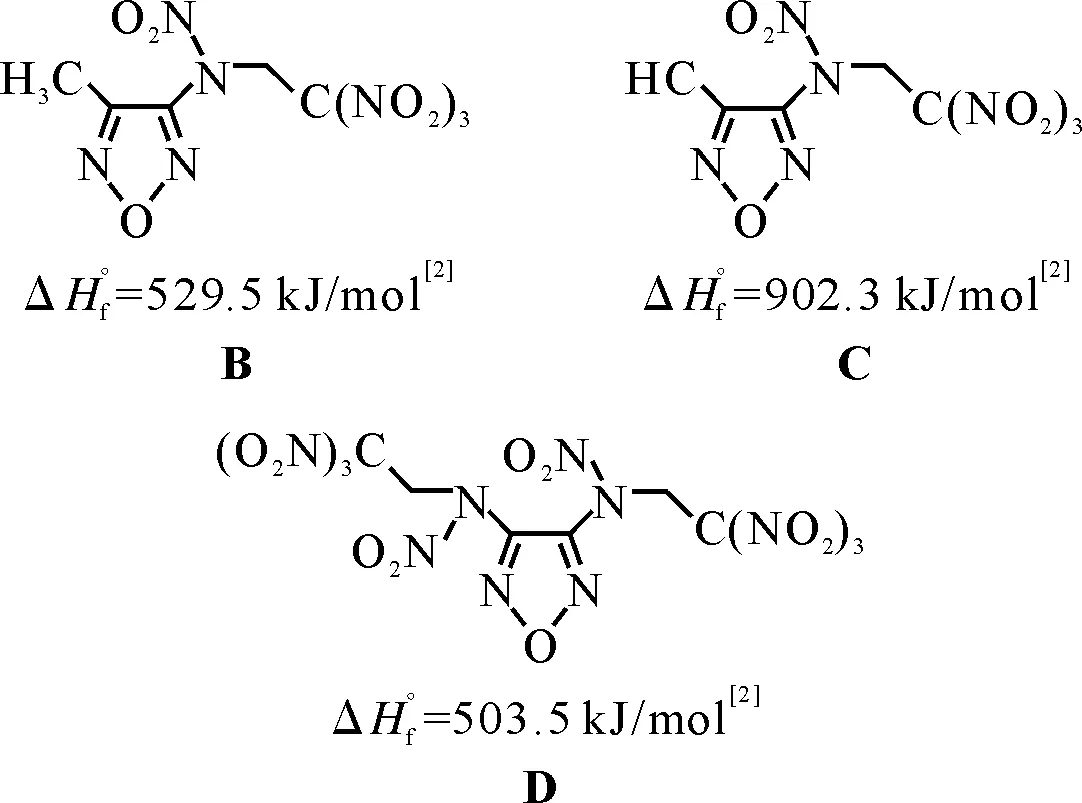
Actually, theIspvalues were calculated correctly in[2], and according to these data one could deduce that the energetic efficiency falls in the row B>C>D. But as any propellant needs a polymer binder (at least 18%-19% volume fraction), let us considerIspvalues of binary compositions oxidizer (either B, or C, or D, or HMX, or ADN) with a binder (either standard hydrocarbon binder SHCB, or so called active binder AB). The element contents, ΔH°f values, and densities of these two binders are as following[3]:
SHCB- C73.17H120.9;ΔH°f=-393kJ/kg,ρ=0.91g/cm3;
AB-C18.96H34.64N19.16O29.32;ΔH°f=-757kJ/kg,ρ=
1.49g/cm3.
The specific impulseIsp(at pressure at combustion camera and nozzle section 4.0 and 0.1MPa accordingly) were calculated using the standard code TERRA for thermo-chemical equilibrium calculations[4]. Fig.1 illustrates the dependence ofIspon the binders volume content in binary formulations oxidizer+binder. To the right from the vertical line (no binder) is SHCB, to the left-AB. Oxidizer D withIspvalue lowerIspof C and D in absence of binder turned out the best oxidixer among all three compounds (B, C, D) in presence of binder. The first reason of this “paradox” is the fact that D has the oxygen access coefficientα(α=O/(2C+0.5H)) higher than 1, thus, the addition of SHCB as well as AB up to defined values increasesIsp. On the other hand compounds B and C have a deficiency of oxygen, so an addition of a binder decreasesIspbecause it decreasesαmore. The most impressive example is the dependence ofIspon the binder content for binary mixtures ADN+binder. If one decides to create a propellant with ADN only, it will be a propellant with too lowIsp-a high oxygen potential of ADN is not realized in this case.
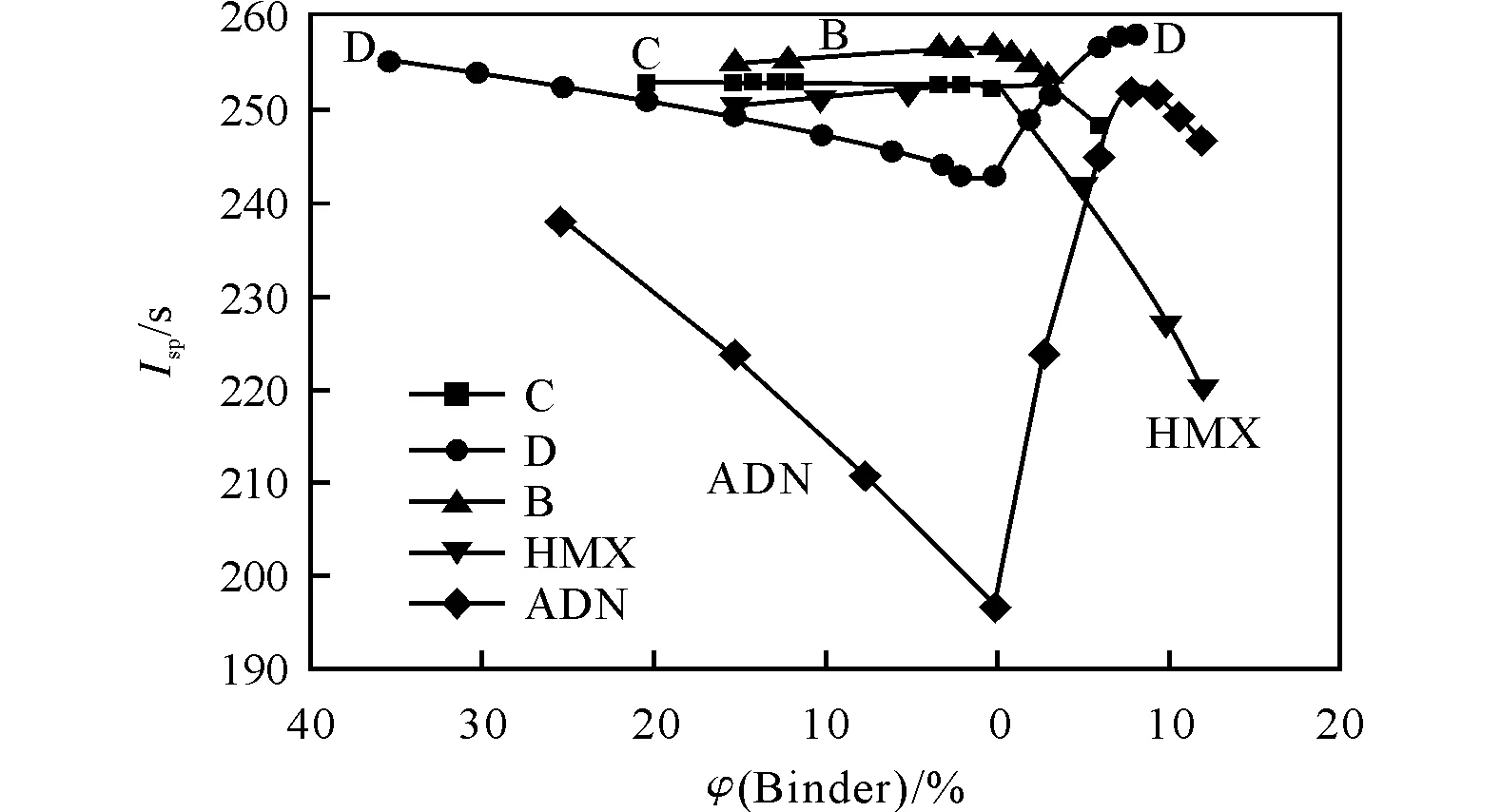
Fig.1 Ispas function of oxidizer nature and binder percentage(To the right from zero point on the X-axis there are data for SHCB,to the left -data for AB)
2Analysis of the relative efficiency of high-enthalpy CHNO-compounds
Thus, the analysis described above confirms the statement that it is not allowed to estimate the energetic abilities of oxidizers for SCPs basing only on theIspvalue of this compound taken alone. Formulations with no metal have maximalIspvalues at zero binder content only for compounds havingαvalues close to that of HMX (0.65-0.75). But this affirmation is correct only for formulations with ΔH°f close to the ΔH°f of HMX too.
On the other hand the value of ΔH°f of the oxidizer not only influences theIsp, but also influences the optimal propellant formulation, e.g. at fantastic ΔH°f values (15000kJ/kg or so) polynitrogenes (Nx) achieve colossalIsp[5]even with no need of any other compounds, with no oxygen atoms in the molecules.
As nowadys most investigations in new oxidizers for SCPs are aimed to the search of high-enthalpy compounds that do not need aluminun addition, an analysis of formulations with no metal have been carried out basing on binders (SHCB or AB) with a wide row of model hypothetic oxidizers with ΔH°f from +300kJ/kg up to+5400kJ/kg and different element contents (Table 1). Element content was selected by the manner to construct some couples of oxidizers with the sameαvalues, but different H/C ratio. It was found out howαand H/C values influence the oxidizer effectiveness.
Although above we blamed the trend to study relative effectiveness of compounds comparingIspvalues of these compounds being alone in formulation, it is useful to analyze first of all how enthalpy of formation,αand H/C values of the oxidizer under consideration influence theIspvalue at zero binder content. It is useful only for understanding the basic regularities amongIsp, enthalpy of formation,αand H/C values, but not for practical advices concerning relative effectiveness of this compound among other ones.

Table 1 Hypothetic oxidizers under consideration. ΔH°f
2.1Ispas function of enthalpy of formation,αand H/C values of compounds under consideration (Table 1) being the only compound of propellant
The dependence ofIspupon ΔH°f is principally known-Ispincreases almost linearly, but at combustion temperature increase (that is at ΔH°f increase) the derivative valuedIsp/d ΔH°f falls a bit permanently (Fig.2). The analysis of received data shows that at constant ΔH°f values the dependenceIsp=f(α, H/C) may be represented with an empiric formula for the given ΔH°f value
Isp=q+b·α+c·(H/C)(Ⅰ)
Coefficientsq,b, and c are represented in Table 2.

Table 2 Coefficients of the equation (Ⅰ)
Naturally, the coefficientsq,b, andcmay be applied only for compounds withαand H/C values are inside the diapason indicated in Table 1 (0.57<α<0.8 and 1<(H/C)<3).
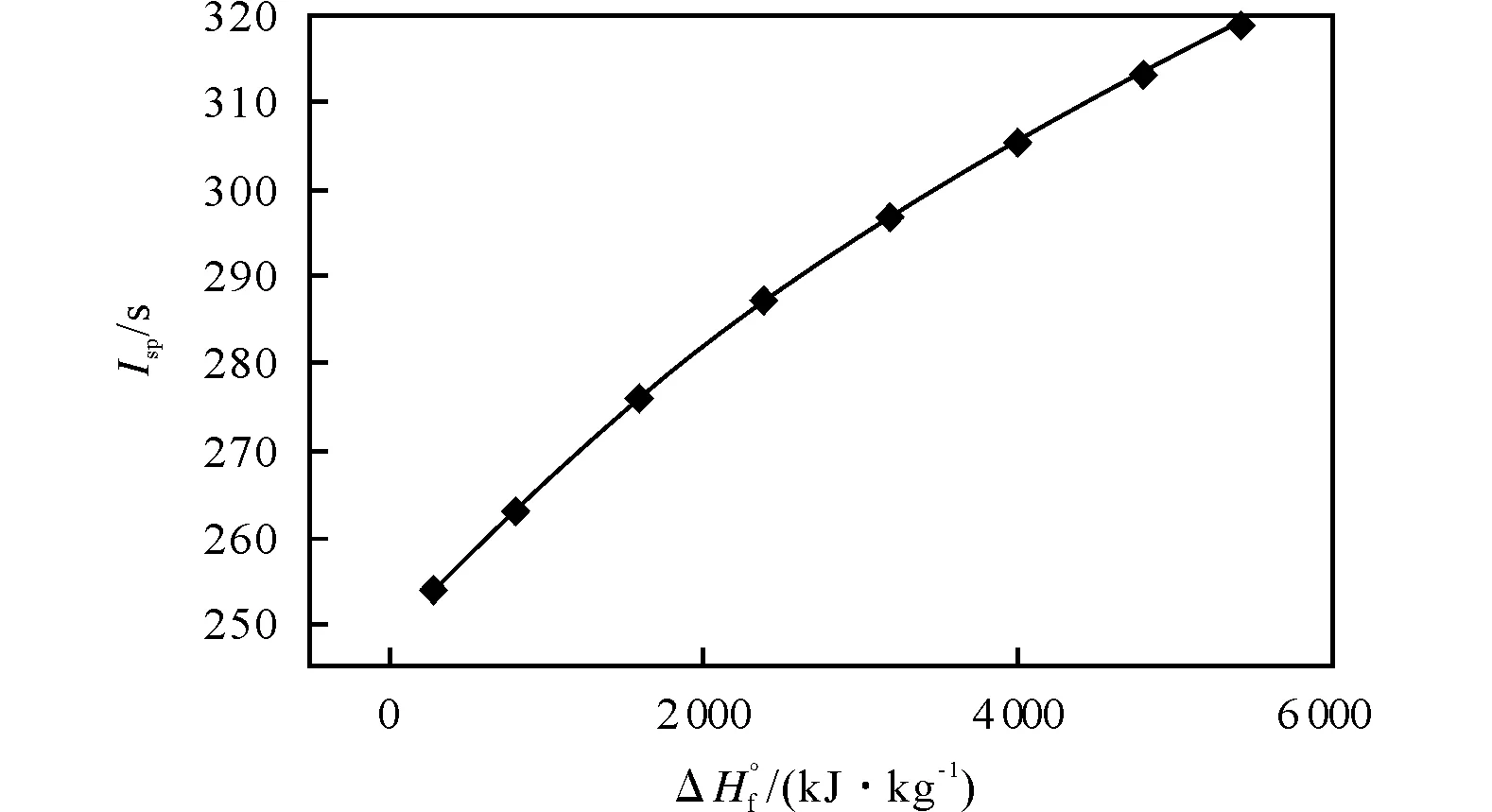
Fig.2 Ispof the compound C1H2N2O2takingalone as function of its ΔH°f value
There is a rather interesting result (Table 2)-as ΔH°f quickly increases the coefficientbdecreases considerably. It means that if the ΔH°f increases theαvalue would fall for providing the maximalIsp. This phenomene may be explained simply-at higher ΔH°fvalues the energetic material needs the
heat of C or H oxidation less and less, it becomes more profitably to gasify all elements (even carbon) on account of the inner energy (ΔH°f) of the compound. At ΔH°f=~1650kJ/kg the coefficientbfalls already to zero. Obtained quantitative regularities explain why at the ΔH°f values increase the table of ranks for oxidizers with differentαand H/C values changes, why at ΔH°f=300kJ/kg the compounds with higherαare the best while at ΔH°f=4800kJ/kg the best compounds are those with lowα, but higher H/C values-the coefficient “b” falls with the ΔH°f value increase, at ΔH°f=2400kJ/kg or so “b” is already negative, which means that at such high ΔH°f values the system does not need any more an additional heat because of carbon or hydrogen oxidation.
The coefficientqdepends on ΔH°f accordingly empiric formulaq=199.4+0.0407· ΔH°f-2.926·10-6·(ΔH°f)2.
The physical sense of the second member (0.0407·ΔH°f) of the equation is evident, the third member (-2.926·10-6·(ΔH°f)2) means the following: the higher isTc, the higher is the dissociation level of gaseous O2, H2, N2, so a definite part of extra-energy is consumed for such reactions as H2=2 H, but not for the thrust increase. That is why the view of the dependenceq=f(ΔH°f) is close to the curve of Fig.2.
2.2Ispas function of enthalpy of formation,αand H/C values of compounds under consideration in the mixture with small amount of binder
When we are considering binary compositions (oxidizer from Table 1 with a small amount of binder at fixed ΔH°f) we can see (Fig.3) that at ΔH°f=+300kJ/kg an addition of any binder (SHCB or AB) decreasesIsp(though at AB addition this fall ofIspis too small), while at ΔH°f=+2400kJ/kg the SHCB addition up to definite content increasesIspin the mixtures with oxidizers withα>~0.72. At ΔH°f=+4800kJ/kg the addition of SHCB to each oxidizer incresesIsp, and the higher isαthe higher is theIspgrowth. If we use AB we can see that applying all considered oxidizers at all ΔH°f values an addition of AB decreasesIsp. The higher is ΔH°f, the more considrable is theIspfalls.
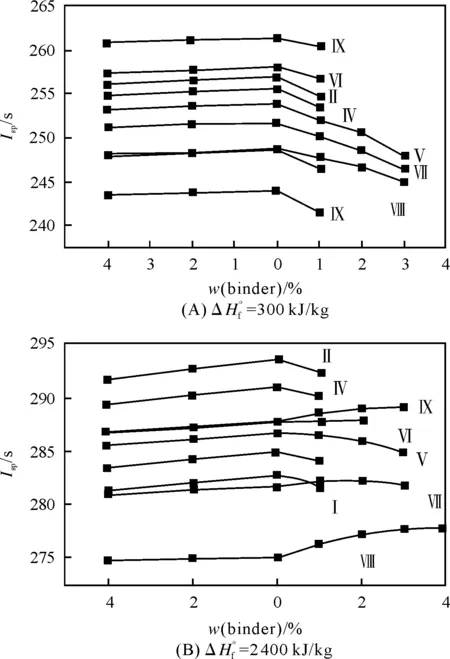

Fig.3 Ispas function of oxidizer nature and binder percentage.To the right from zero point on the X-axis there aredata for SHCB, to the left-data for AB
2.3Ispas function of enthalpy of formation,αand H/C values of compounds under consideration in the mixture with 18%-19% (volume fraction) of binder
Real SCP has to contain a binder with concentration not lower than 18%-19% for providing required physical-chemical parameters of cured composition and required rheological parameters of the initial propellant mixture. How an introduction of binder in required volume percentage (not a few percents, that we analyzed above in chapter 2.2) influencesIsp? Table 3 and Fig.4 represent the obtained data. At SHCB density equal 0.91g/cm3, AB density 1.49g/cm3, and oxidizer density~1.85g/cm3the mass fraction has to be ~10.4 % for formulations with SHCB and ~16 % for formulations with AB to provide binder volume percentage with 18%-19%. Fig.4 shows that if the oxidizer′s enthalpy of formation is not very high (e.g. 300kJ/kg) theIspis considerably higher in formulations with AB in case of all oxidizers (sure, if the requirement “binder content is not lower than 19 vol/%” is assured). If ΔH°f=2400kJ/kg or so AB remains still better than SHCB, but theIspadvantage (compared with the formulation with SHCB) is quite smaller.


Fig.4 Ispvalues of formulations oxidizer with no binder(central column in each of nine column sets), and with~19% ofbinder (the left columns-AB, the right columns-SHCB).Oxidizers symbols (Ⅰ-Ⅸ) accordingly Table 1
Only if oxidizer′s ΔH°f becomes so high as 4800kJ/kg or so the most of oxidizers show higherIspvalues in mixture with SHCB, only oxidizers with comparatively lowαvalues haveIspa bit higher in mixture with AB, because as it was mentioned above (2.1) at oxidizer′s ΔH°f increase the propellant needs oxygen less and less.
3Conclusions
(1)Even in preliminary estimations there is no sense to evaluate energetic abilities of any components by comparingIspvalues of components being alone in the formulation.
(2)Effectiveness of compounds, especially high-enthalpy oxidizers of solid composite propellants, has to be estimated in mixture with binders with different element content.
Depending on element content of the oxidizer and its enthalpy of formation one has to select binder with an appropriate element content to obtain the maximal specific impulse.
(3)The higher the enthalpy of formation of the main compound, the less should be its oxygen content.
References:
[1]Lempert D B. Preliminary Estimation of the effectiveness of new and predicted energetic compounds as oxidizers for solid composite propellants[C]∥ In Proc 17th International Seminar “New Trends in Research of Energetic Materials”. 2014: 281-287.
[2]Sheremetev A, Alexandrova N, Palysaeva N, et al. Ionic Liquids as Unique Solvent in one-pot synthesis of 4-(N,2,2,2-tetranitroethylamino)-3-R-Furazans[J]. Chem Eur J, 2013, 19: 12446-12457.
[3]Lempert D B, Nechiporenko C N, Manelis G B. Energetic capabilities of high-density composite solid propellants[J]. Combustion, Explosion, and Shock Waves, 2011, 47(1): 45-54.
[4]Trusov B G. Program system TERRA for simulation phase and thermal chemical equilibrium.[C]∥ In Proc of the XIV Intern Symp on Chemical Thermodynamics St-Petersburg, 2002: 483-484.
[5]Lempert D B, Nechiporenko G N, Soglasnova S I. Energetic potential of compositions based on high-enthalpy polynitrogen compounds[J]. Combustion, Explosion, and Shock Waves, 2009, 45(2): 160-168.
DOI:10.14077/j.issn.1007-7812.2015.04.001
- 火炸药学报的其它文章
- 高能钝感炸药MAD-X1合成简讯
- 《微纳米含能材料》新书介绍

