Recent Developments and Challenges in FlameRetardation of Textiles and Fibres
A Richard Horrocks ,Liu Wei
(1.Institute for Research and Innovation,University of Bolton,Bolton,BL1 5LL,UK)
(2. Sichuan Fire Research Institute of Ministry of Public Security, Chengdu 610036,China)

Recent Developments and Challenges in FlameRetardation of Textiles and Fibres
A Richard Horrocks1,Liu Wei2
(1.Institute for Research and Innovation,University of Bolton,Bolton,BL1 5LL,UK)
(2. Sichuan Fire Research Institute of Ministry of Public Security, Chengdu 610036,China)
Abstract:Almost 50 years ago, the 1950-1960 period witnessed the development of the chemistry underlying most of today’s successful and durable flame retardant treatments for fibres and textiles. In today’s more critical markets in terms of environmental sustainability, chemical toxicological acceptability, performance and cost, many of these are now being questioned. “Are there potential replacements for established, durable formaldehyde-based flame retardants such as those based on tetrakis (hydroxylmethyl) phosphonium salt and alkyl-substituted, N-methylol phosphonopropionamide chemistries for cellulosic textiles?” is an often-asked question. “Can we produce char-forming polyester flame retardants?” and “Can we really produce effective halogen-free replacements for coatings and back-coated textiles?” are others. These questions are addressed initially within the context of a historical review of research undertaken in the second half of the twentieth century which is the basis of most currently available, commercialised flame retardant fibres and textiles. Secondly, research reported during the first decade of the twenty first century and which primarily addresses the current issues of environmental sustainability and the search for alternative flame retardant solutions, the need to increase char-forming character in synthetic fibres and the current interest in nanotechnology are critically discussed. The possible roles of micro- and nano-surface treatments of fibre surfaces and their development using techniques such as plasma technology and layer-by-layer and sol gel chemistries are also reviewed.
Key words:flame retardant; halogen; phosphorus; textile; fibre; environment; nanotechnology; plasma CLC number:TB 383;TQ328.3Document code: AArticle ID:1674-3962(2015)09-0659-16
1Introduction
Within recent years there have been several comprehensive reviews that not only have critically reviewed the research period between about 1950-1980 during which time most of the presently used commercial flame retardants for fibres and textiles were developed[1], but also have considered developments since that time[2-5]. During the period up to about 1970-1980, the established durable and flame retardant treatments for natural fibres as well as those additives and comonomers introduced into both regenerated (e.g. viscose) and synthetic (notably polyester, polypropylene and the modacrylics) fibres during manufacture were synthesised and developed into commercially-acceptable products. In fact the majority of currently available flame retardants for textiles and fibres reviewed very recently by Weil and Levchik[4]derive from chemical developments prior to 1980.
2“Golden period” of flame retardant research during the 1950-1980 and its developments during the 1980-late 1990 period
With the recognition that durable flame retardant treatments, initially developed during the Second World War for service personnel had usefulness in a peace-time environment in which personal safety was becoming more important than hitherto, flame retardant research have been fruitful during the 1950-1980. The commercial development of flame retarded fibres and textiles was subsequently and continues to be driven by legislation and regulation. For example, the patents for a number of durable organophosphorus-based for cotton stemmed from the 1950 period and gave rise to the current flame retardants based on cross-linked tetrakis(hydroxymethyl) phosphonium salt adducts (often based on so-called THPX chemistry) and N-alkyl-substituted phosphonopropionamide derivatives[1,4].Furthermore, flame retardant versions of the conventional synthetic fibres and heat resistant fibres often based on aromatic-structured polymeric chains, have been produced, such as the aramids, exemplified by the poly(meta-aramid) fibre Nomex®(Du Pont)[6]and the poly(para-aramid) fibre Kevlar®(Du Pont), as well as the poly(benzimidazole) or PBI fibre and many other polyheterocyclic fibre-forming polymers[7]. The more commercially successful aromatic-structured fibres have been comprehensively reviewed elsewhere[8-9]. This same period saw a particularly significant research effort into the development of phosphorus-containing species as possible candidates for commercial flame retardants. And it was during this time that researchers like Ed Weil at Stauffer Chemicals developed and patented the chemistry from which derived a whole portfolio of phosphorus-containing and phosphorus-halogen-containing “Fyrol” commercial products[10-12], the products Fyrol 6, 51 and 76 each of which was developed for textile applications. However, this “golden age” was unfettered by the constraints of subsequent health and safety requirements and now the realisation that many of these species may be indeed quite toxic. Due to their potentially toxicity, many flame retardants have disappeared from the portfolio of available commercial flame retardants since 1977[13-15]. The principal flame retardants researched and developed during this period and which still retain commercial significance for textiles as corroborated by a recent more commercially-focussed review of textile flame retardants have been reviewed[5, 16].
Competing with flame retardant treatments has been the fibre industry’s attempts to develop inherently flame retardant varieties since the 1950 period. Polyester and polypropylene fibres present particular flame retardancy challenges because neither fibre has an inherent char-forming property and flame retardancy is introduced either by ensuring that molten drips self-extinguish as in the case of Trevira CS®(Trevira GmbH) or by use of bromine chemistry to introduce gas phase retardancy. The low melt reactivity and process temperatures of polypropylene enable continued use of synergised bromine additives although the recent introduction of hindered amine species has rendered the need for antimony III oxide unnecessary[17].
The next twenty years or so from 1980 to 2000, witnessed very little new research into novel chemical species and in the main progress, was made in refining earlier chemistry and the addressing the realisation that some flame retardants were demonstrating certain environmental problems, coupled with bio-accumulative and bioactive effects. However, the UK furnishing regulations of 1980 first amended in 1983 for cigarette resistance and then later in 1988 for both cigarette and simulated match resistance[18]promoted the development of flame retardant back-coatings primarily in furnishing fabrics[19]. This increasing need for barrier fabrics in the furnishing and other sectors also signalled an increased need for char-promoting flame retardant textiles and the challenges posed by this and the increasing interest in application of intumescents to textiles was reviewed by ourselves in 1996[20].
3Attempts to replace established flame retardants with cheaper and environmentally more sustainable alternatives
Interest here has focussed mainly on finding formaldehyde-free flame retardants for cellulosics as well as bromine-free back-coatings for furnishing and barrier fabric fabrics[5].
3.1Formaldehyde-free flame retardants for cellulosics
The main targets for replacement are the two major and commercially dominant generic types of durable flame retardants for cotton and cotton-rich blends, namely those based on tetrakis(hydroxymethyl) phosphonium salt (THPX) condensates and those based on N-methylol dimethylphosphonopropionamide (N-MDMPA) derivatives. The former is typified by the Proban®(Rhodia) product which is based on tetrakis(hydroxymethyl) phosphonium-urea condensate which, after padding on to cloth is cross-linked by ammonia gas and followed by peroxide oxidation to stabilise the resulting polymeric matrix which is interdispersed throughout the interfibrillar fine structure of the cotton fibres present[1]. However, there is commercial evidence that some formaldehyde may be released during its use although this is dependent upon the effectiveness of the ammonia/oxidative curing process, which if rigorously undertaken reduces HCHO emissions to <20 ppm. N-MDMPA derivatives which are typified by Huntsman product Pyrovatex CP®and which is available in various modifications such as the dimethylol derivative is claimed to improve durability and/or reduce formaldehyde release during application[2, 4]. Since the condensation reactions involved are equilibria in which formaldehyde is a product, this will always be present during both application and regenerated during service life. Unlike the Proban®product which uses a patented ammonia cure process and requires specialised plant, the Pyrovatex product and its many alternative versions[1-2, 4]may be applied by a conventional pad-dry-cure process.
In order to be able to replace either of these products and their derivatives, it is appropriate to summarise their respective strengths and weaknesses as in Table 1, which explains why both have been the dominant durable flame finishes for cotton and blends for the last 50 years.
Within recent years a considerable literature has appeared in attempts to develop formaldehyde-free flame retardant replacements. While this review is not intended to be a comprehensive review[4], some of the more salient alternatives will be briefly discussed. Abdel-Mohdy et al. have published the use of aminomethyl phosphonic acid diamide, and derivatives[21], and triethylamino phosphine oxides[22]as phosphorus- and nitrogen-containing synergistic flame retardants for cotton but unfortunately their respective methylolation using formaldehyde is an essential feature for their subsequent reactivity with anhydroglucopyranose-OH groups. In a not dissimilar vein, ICL have re-introduced their former Fyrol 51 product[4, 23]as Fyroltex HP[24]which with a phosphate-phosphonate oligomeric structure has the potential for being a durable flame retardant for cellulosic textiles as initially reported by Wu and Yang in 2003[25]. Their further research has shown[26-28]that if it is to achieve acceptable levels of multiple laundering durability, its application requires the presence of methylolated resin species like dimethylol dihydroxyethylene urea or methylated formaldehyde-urea, indicating that the problem of formaldehyde release will still remain.

Table 1 Comparison of tetrakis(hydroxymethyl) phosphonium salt condensate- and N-methylol N,N’-dimethylpropionamide derivative-based flame retardants for cotton[1-2]
However, the quest for a truly formaldehyde-free, durable, and effective flame retardant for cellulosics in particular remains a challenge sufficient to attract interest. More recently Yang et al.[29]have applied a novel halogen- and formaldehyde-free flame retardant (named Neo-FR) to cellulosic fabrics and show that Neo-FR system is a durable flame retardant for cellulosic fabrics. Significantly the mechanical properties of the samples treated with Neo-FR system are much less affected than those treated by a commercial durable flame retardant agent (Pyrovatex CP®New).The synthesis of Neo-FR and its reaction with the cross -linking agent are shown as below:
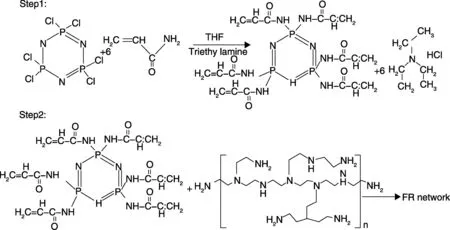
Work by Yang and colleagues has combined 1,2,3,4-butane tetracarboxylic acid or BTCA as the cellulose bridging species with phosphorylated species such as the hydroxyalkyl organophosphorus oligomer, Fyroltex, discussed above to enhance both flame retardancy, and durability[24, 30]. While BTCA forms a bridge between the oligomer and cellulose molecules, and durability is somewhat improved, the ease of ion exchange between free carboxylic acid group hydrogen ions with calcium ions during washing in hard water is accompanied by a loss in flame retardancy as a consequence of calcium salt formation[27]. Addition of triethanolamine (TEA) reduces the calcium ion pick-up as a consequence of free carboxylic acid group esterification and using a Fyroltex/BCTA/TEA combination applied to a 35%/65% cotton/Nomex blend, acceptable levels of durability were achieved with vertical strip test (ASTM D6413-99) passes after 30 home launderings[30]. A more recent publication extends this work to show that the mixed Fyroltex/BCTA system may be applied to silk to yield a 15 hand-wash level of durability[31]. Yang’s research team has recently developed this idea further and reported that treatment of cotton fleece with maleic acid and sodium hypophosphite enables Class 1 passes to 16 CFR 1610 (US Federal Standard for the flammability of Clothing Textiles) to be achieved when exposed to the 45° ASTM D1230-94A apparel test after 20 home launderings[32]. Subsequent work has extended this to include succinic, malic and tartaric acids to yield similar flame retardant performance[33-34]. A phosphorus-containing maleic acid oligomers shorted as PMAO is considered to be a mixture of species, which is applied to cotton fleece fabrics again in the presence of sodium hypophosphite with no significant changes in fabric properties[35]. Their most recently reported work re-examines the possible role of Fyroltex and BCTA in 100% cotton fleece and when TEA is present also achieves Class I after multiple home launderings[36].
Quite different from the above approaches is the recently introduced Firestop product Noflan, a phosphorus-, and nitrogen-containing molecule[37]. While this is obviously a formaldehyde-free molecule, it may react only with cellulosic substrates via the phosphoramidate-NH2group. It is most likely that for this to be effective in cellulosic-based textiles, it may be applied either in a resin binder or cross-linked using a methylolated resin.
An interesting and novel approach has been published by Chang et al.[38]from the USDA Southern Regional Research Centre in New Orleans where much of the pioneering research into durable flame retardant finishes for cotton was undertaken during the 1950-1970 period[1]. This group has synthesised two new monomers (2-methyl-oxiranylmethyl)-phosphonic acid dimethyl ester and [2-(dimethoxy-phosphorylmethyl)-oxyranylmethyl]-phosphonic acid dimethyl ester which together with dicyandiamide and citric acid impart flame resistance to woven 100% cotton and 80/20 cotton/polyester fleece fabrics. The resulting mono- and bis-(dimethoxy-hydroxymethyl phosphonyl) cyanurate derivatives may be padded on to fabrics and while the former can give rise to LOI values up to 25.5 vol% at about 21 wt% add-on, higher LOI values above 28 vol% were obtained when the latter was applied at add-ons below 20 wt%. Fabrics passed the standard 45° and vertical strip tests ASTM D1230-94 and D6413-99 before laundering. Durability is not, however, very good with only about 5 wash cycles being achievable whilst maintaining acceptable levels of flame retardancy in spite of the claimed cellulose reactivity of cyanurate derivatives. Further research by this group was recently described at FRPM09[39]. Finally, one other very recent paper attempts to eliminate the need for a formaldehyde-based cross-linking resin from the N-DMDPA system by converting the latter to the N-1-chloroisopropyl alcohol derivative which may then directly bond to viscose cellulose[40]. This creates the flame retardant, cellulose-reactive species:
(CH3·O)2·P(O)-CH2·CH2·CO·NH·CH2CH(OH)·CH2·Cl + HO-Cell → (CH3·O)2·P(O)-CH2·CH2·CO·NH·CH2CH(OH)·CH2·O·Cell
Recent interest has also been shown in the potential for combining phosphorus, nitrogen and silicon on to cellulose substrates to create the potential for carbonaceous and silicaceous char-forming characteristics. Lecoeur et al.[41-42]have combined monoguanidine diphosphate (MGDP) and 3-aminopropyltriethoxysilane applied in the presence of phosphoric acid as catalyst if water-soak durability (20 min in hard water at room temperature) is to be achieved. Treated cottons behave typically of those containing char-promoting flame retardants in that flame retardancy is improved (M1 rating to NF P 92-503), cone calorimetric ally determined peak heat release rate (PHRR) reduces and residual char increases. Other recent research by Easson et al.[43]has focussed mainly on the potential use of cyanuric chloride derivatives in flame retardant applications to cotton fabric. The results from standardized thermogravimetric, limiting oxygen index, and vertical flame methods indicated the acceptable levels of flame retardancy were achieved by the introduction of two cyanuric chloride derivatives. Other recent work by these USDA researchers and reviewed by Horrocks[44]involves reacting functional flame retardant species with the C6 primary hydroxyl group in a manner similar to reactive dye chemistry. In this work, Nguyen and coworkers[45]reported the synthesis and reaction with cotton of tetramethyl (6-chloro-1,3,5-triazine-2,4,diyl)bis(oxy)bis(methylene) diphosphonate, which was padded on to cotton in 50% aqueous isopropanol, dried at 100 ℃ for 5 min and cured at 140 ℃ for 5 min at add-ons from 5 to 19 wt%. Treated fabrics were white and only the highest add-ons (17 and 19 wt%) were self-extinguishing with LOI>35 vol%.
Also very recently, some proteins containing phosphorus, nitrogen and sulphur from animal or microbial sources have been investigated as novel green flame retardants for cotton fabrics. Alongi[46]has used caseins or hydrophobins as the novel green flame retardants, which could enhance the formation of char and decrease the total burning rate during combustion. Deoxyribonucleic acid (DNA) has also been proven to be an efficient renewable, natural flame suppressant and retardant for cotton fabric, due to its intrinsic intumescent features[47]. Alongi et al.[48]also showed that DNA decomposes to produce an intumescent effect that undergoes a transformation into a multicellular, foamed and thermally insulating material at a relatively low temperature (160~200 ℃).
3.2Non-halogen-containing back-coatings
Within the UK’s furnishing textile back-coatings market, the standard formulations based on antimony III oxide and brominated hydrocarbons, notably decabromodiphenyl ether (decaDBE), and hexabromocyclododecane (HBCD) have until recently dominate the market until environmental concerns have driven their gradual replacement (see ref.[5] for a current discussion on commercial alternatives). The challenge of replacing these systems by phosphorus-containing species only has been investigated by ourselves[49-50]and reviewed by Weil and Levchik[4]. Our research has shown that while replacement by a number of phosphorus-nitrogen formulations including intumescent formulations and cyclic organophospate species is possible, their effectiveness is limited by durability following the 40 ℃ water soak required in the 1988 UK Furniture, and Furnishing (Fire) (Safety) Regulations[18]prior to testing to BS 5852: Part 1:1979 for match, and cigarette ignition resistance. Thus notwithstanding these issues, the outcomes of our previous research[49- 50]have led to three strategies that may be proposed to achieve these requirements:
i.The sensitisation of decomposition or flame retarding efficiency of phosphorus-based systems We have demonstrated that the inclusion of small amounts of certain transition metal salts, notably those of zinc II and manganese II can reduce the onset of decomposition of ammonium polyphosphate (APP) from 304 to 283 ℃ in the case of 2 wt% manganese II sulphate addition[50]. When applied in a back-coating formulation with APP, the presence of metal ions increases LOI values slightly from 25.1 for APP-only coated cotton to 26.6 vol% in the presence of 2% manganese acetate. However, all coated fabrics still failed the simulated small flame ignition version of BS 5852[49]. And it should be pointed out the problem of durability to water soaking would still remain.
ii.The reduction in solubility of successful but soluble systems Work by Bourbigot and coworkers[51]has shown that microencapsulation of otherwise soluble flame retardants like ammonium phosphate with polyurethane shells can improve the durability of coatings containing them. However, the preparation of these microencapsulated agents is not an easy process and different techniques are being developed in order to improve yields[52-53].
iii.The introduction of a volatile and possible vapour-phase active, phosphorus-based flame retardant component In accordance with previous research, our recent works[54-55]initially considered four potentially volatile phosphorus flame retardants selected from their reported boiling or decomposition data. TGA studies of monomeric cyclic organo-phosphate (Antiblaze CU, Rhodia), tributyl phosphate (TBP), triphenyl phosphate (TPP) and triphenylphosphine oxide (TPPO) suggested that TBP (b.pt.=289 ℃ with decomposition) would be most suitable for furnishing fabrics because its initial decomposition temperature (150 ℃), well below the melting temperature of polypropylene (~165 ℃) and the ignition temperature of cotton (~350 ℃).
Further evidence of the volatile phosphorus activity was gained by determining the retention of phosphorus in charred residues from back-coated samples containing APP, melamine phosphate (MP), Amgard CU, and the oligomeric phosphate-phosphonate Fyrol 51 where the two last are liquids and are potentially vapour-phase active flame retardants[54-55]. Phosphorus loss was lowest for fabrics containing the char-promoting APP and MP and highest for Amgard CU and Fyrol 51 which also exhibited the highest coated fabric LOI values. These results suggested that an ideal back-coating might comprise a non-volatile, char-former like APP or MP in combination with volatile phosphorus- or even nitrogen-containing species. Thus since melamine (Mel) is an insoluble and yet volatile solid which sublimes above 400 ℃ or so, it was introduced and shown to further raise LOI values of all samples above 27 vol% before water soaking. Unfortunately, similarly high performance was not observed after coated fabrics had been subjected to a water soak at 40 ℃ as shown in Table 2. This result points the way towards achieving passes after water soaking and after 20 s ignition time if the water insolubility of the char-former present can be increased.
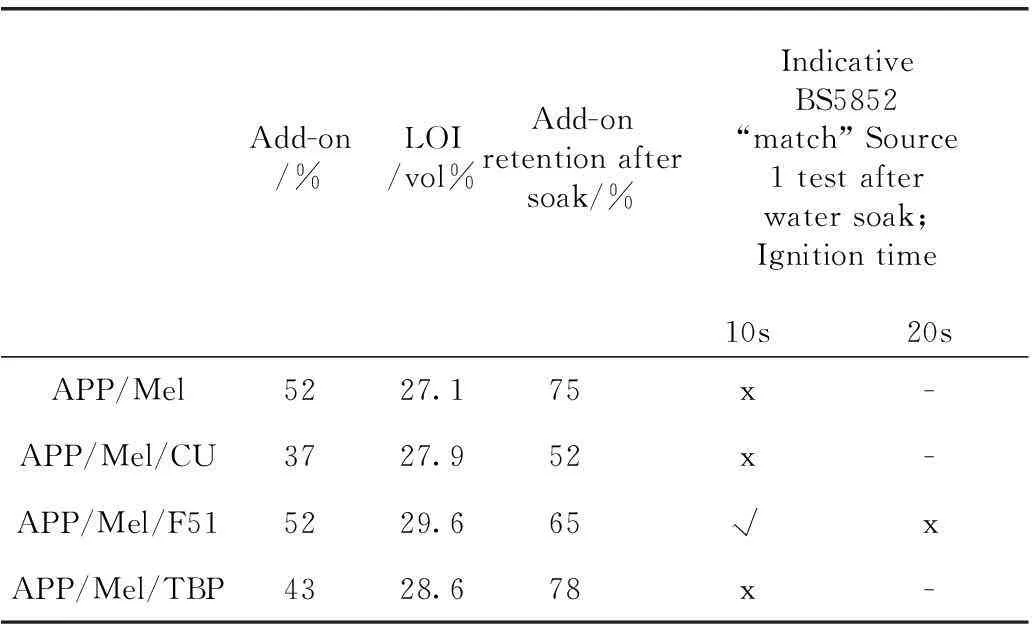
Note: “√” denotes a pass, “x” denotes a fail and “-“ denotes no test undertaken
4Attempts to improve melt-dripping of melt fusible fibres like polyester and polyamide during combustion
Apart from the use of back-coatings which may be applied to any textile comprising any fibre type of blend, most flame retardants applied to fusible fibre-forming polymers act by increasing melt dripping with the added requirement that flaming drips are absent or quickly self-extinguishing. PET and other aliphatic fibre-forming polyesters tend to volatilise when heated above their melting points and show little cross-linking and hence char-forming characteristics. PP is in a similar position except that molten drips are difficult to extinguish without the presence of a gas phase flame retardant present.
Ideally, there is a need for flame retardants that reduce melt dripping and encourage char formation thereby allowing pure synthetic fibre-containing fabrics to behave as barriers as do flame retarded cellulosics, wool and high performance, aromatic-structured fibres. However, any agent that reduces melt dripping will increase the polymer and textile flammability unless it has an additional flame retarding character. Wang’s recent review[56]discusses this need to reduce melt dripping and the challenges that it poses. He and Bourbigot[3]have reviewed recent Chinese research that demonstrates a number of recent novel routes for polyester fibres and fabrics. For instance Chen et al.[57]have applied intumescent polymeric units such as poly (2-hydroxy propylene spirocyclic pentaerythritol bisphosphonate)

topically on to PET fabrics and show that at levels approaching 10 wt% not only are melt dripping and afterflame suppressed but also char formation is evident and fabrics pass vertical strip testing and general thermal stability is increased. Wang[56]also reviews his own group’s contemporary work in which novel copolyesters comprising phosphaphenanthrene-10-oxide derivative comonomers and organomontmorillonite clays reduced melt dripping[58]. Although this work was not based on fibre and fabric substrates, earlier Russian work introduced the same polymer as an additive under the name Ukanol®(available from Schill + Seilacher Aktiengesellschaft) to demonstrate its effectiveness in fibres yielding an LOI of 27.7 vol%[59].
Most recently, Wang and his coworkers have pioneered a novel flame-retardant method with no conventional flame retardant but via smart thermal cross-linking reactions at high temperatures[60], and solved the contradiction between the non-flammability and non-dripping of polyesters. They reported the copolymerization of terephthalic acid and ethylene glycol together with a pendent phenylethynyl-based monomer named 4-(phenylethynyl) di(ethylene glycol) phthalate (PEPE), which exhibited a cross-linkable nature at a defined temperature. TG-DSC simultaneous thermal analysis, FTIR, dissolution tests and rheological investigations proved the thermal cross-linking behaviour of the copolyester, which increases the viscosity at high temperature, leading to compact char residue formation and flame-retardant and anti-dripping effects in the absence of any flame-retardant element (bromine, chlorine, phosphorus, or nitrogen, etc.)[60]. This flame-retardant-free, flame retardant idea will also open a new field in “green” flame retardation of polymeric materials. Besides, some other high temperature cross-linkable compounds can be used as the comonomers to offer a good balance between flame retardance and anti-dripping properties of copolyester. Jing et al.[61]have reported that the novel polyester (PEAT) containing azobenzene units was synthesized via esterification and copolycondensation of terephthalic acid, ethylene glycol and azobenzene-4,4’-dicarboxylic acid (Fig.1). The introduction of the high temperature cross-linkable azobenzene groups into the main-chain of PEAT as a new method can solve the conflict between the flame retardance and anti-dripping of poly(ethylene terephthalate). The cone calorimetric analysis and LOI tests confirmed the self-extinguishing properties and inhibition of melt-dripping.

Fig.1 The chemical structure of PEAT
Interestingly, a recent paper[62]investigates the mechanism by which this additive functions and finds that it acts mainly by stabilising the thermal processes in the condensed phase although char formation is not enhanced. Comparison with the Phosgard®additive 2-carboxyethyl(phenyl phosphinic) acid, which is not dissimilar to the comonomer in Trevira CS®, except that it has a pendant aromatic group, while showing no char promotion, does show evidence of gas phase activity. While this and other cited work demonstrate that the melt-dripping issue can be addressed in PET, it must be emphasised that any flame retardant product that can replace the currently established Trevira CS-type of product must be producible in large quantities at a price that justifies its costs.
5Challenges and opportunities for nanotechnology
Recent applications of nanotechnology as a means of improving the flame retardancy and fire performance of fibres and textiles has been reviewed recently by Bourbigot[3,63]and Horrocks with regard to potential applications[64]. They demonstrate that the main issues that influence whether or not nanotechnology can be exploited may be considered to include[64]:
•Compatibility of nanoparticles with polymers during and after processing
•Effects on rheology of adding nanoparticles to polymeric extrusion and coating fluids
•Dispersion of nanoparticles during processing
•Levels of flame retardancy or improved fire performance achieved
While reported fire performance based on cone calorimetric data of bulk polymers[65-67]typically shows that the presence of nanoclays reduces peak heat release rates, they more often than not reduce time-to-ignition and extend total burning periods while affecting little the total heat release of the polymeric substrate. However, they also encourage increased char formation even in some polymers that are not char-formers[66-67].
5.1Nanocomposite fibres
Bourbigot et al.[68-69]reported the first fire performance studies of nanocomposite polyamide 6 filaments and these were converted into fabric having an area density of 1 020 g/m2and thickness 2.5 mm. When exposed to 35 kW/m2heat flux in a cone calorimeter, PHRR was reduced by 33%, ignition resistance was significantly reduced and total heat release was little, if any, affected. It was clear that the fibres were not flame retardant in the more accepted sense in that ignition resistance was not increased by inclusion of nanoclays alone. A further problem with fibres and fabrics with respect to bulk polymers is their high specific surface areas and their thermally thin character. This is significant since Kashiwagi et al.[70]suggested that the effectiveness of nanoclays in reducing PHRR values and related fire performance may be a function of sample or composite thickness. Thinner samples appear to show lower PHRR reductions because of competition between the formation of a surface carbonaceous-silica shield and the volatilisation to fuel of surrounding polymer. This can be considered as the difference between so-called thick and thin thermal behaviour[71]so that in “thin” textile fabrics the “shield-forming” mechanism observed for bulk polymer nanocomposites may be too slow for effective improvement in fire performance. Thus it may be suggested that nanoclay presence alone in fibres, films and textiles will only be significant at lower heat fluxes. More recent work by Bourbigot et al.[72]has extended their polyamide research to include nanoclays into melt-spun poly(lactic acid) (PLA) filaments.
However, as observed for bulk polymers, combination of nanoparticles with conventional flame retardants such as APP may promote overall additive and even synergistic activity[73-74]. Our work considered both additive and/or synergistic effects of adding selected phosphorus-containing flame retardants into PA6, and PA66 polymer films (~80 μm thick) in the presence of commercial and experimental nanoclays[75-76]. The effectiveness of adding nanoclay is shown by the ability to reduce by 25-33 wt% the concentration of APP necessary to create a defined level of flame retardancy. There have been other attempts to observe the effect of introducing nanoclays in the presence of flame retardants in other fibre-forming polymers, such as PP[77]and polyester[57].
Bourbigot et al.[78]also introduced poly(vinylsilsesquioxane) (POSS) nanoparticles at 10 wt% loadings in polypropylene from which multifilament yarns and knitted fabrics were produced. Unlike the same group’s results for PA6 nanocomposite fabrics[68-69], no reduction in PHRR values occurred relative to the pure fibre-containing samples. However, the time-to-ignition under a heat flux of 35 kW/m2increased from 21 s for the latter to 76 s for the POSS-PP fabrics and TGA results indicate that the presence of POSS stabilizes the PP to the initial stages of thermal oxidative degradation. However, other work described the introduction of 1wt% multiwalled carbon nanotubes into polypropylene filaments and fabrics[79]and showed both an increase in the thermal stability arising from their presence and a 50% reduction in PHRR values when examined by cone calorimetry at 35 kW/m2heat flux. As seen for the earlier PA6 fabrics, so here the presence of nanoparticles reduced the time-to-ignition considerably from 60 s to 30 s.
Our work considered the effects of nanoclays alone[80]as well as in the presence of more conventional flame retardants[81]. Table 3 presents tensile and flammability data for polypropylene fibres and fabrics containing Cloisite 20A clay and a maleate-grafted polypropylene at various concentrations[80]. All polymer samples were twice compounded to maximise dispersion prior to fibre extrusion and sample 5 differed from sample 4 in its having been produced as a more concentrated masterbatch before being let down during the extrusion stage. While it is clear that the presence of nanoclay alone promotes an expected improvement in fibre tenacity and modulus, there is also a decrease in peak heat release rate determined by cone calorimetry at a heat flux of 35 kW/m2. Addition of the compatibilising maleate-grafted PP reduces the tensile properties as expected and, apart from sample 3, suggests that it causes further reduction in PHRR values.

Table 3 PP fibre compositions and tensile properties and fabric PHRR values at 35 kW/m2 heat flux[81]
Note: * only one sample tested; ** sample 5 is produced as a concentrated masterbatch before being let down during extrusion to yield the stated additive concentrations
Subsequent work[81], investigated the effect of introducing selected flame retardants APP, melamine phosphate (MP), pentaerythritol phosphate (PEP), tris (tribromopentyl) phosphate (TTBPP) and tris(tribromophenyl)cyanurate (TTBPC)and the hindered amine stabiliser NOR 116 (Ciba)[17]. These were compounded with selected clays (Cloisite 20A and 30B, Bentone 107 (a bentonite clay; Elementis), and a montomorillonite modified with vinyltriphenyl phosphonium bromide) and compatibilisers ((Polybond) Pb and polypropylene grafted with diethyl-p-vinylbenzyl phosphonate (DEP)). Extrusion into filaments proved to be challenging because of problems with optimising clay and flame retardant dispersion and this was especially the case when APP was present because of its very poor dispersion and relatively large particle size (25~30 μm). The result shows that while the melting temperature of the formulations are unaffected by their contents, melt flow indices generally are seen to increase indicating a general reduction in melt viscosity possibly driven by thermal degradation associated with the extrusion process. Char yields at 800 °C in air are close to the nominal inorganic residues expected. LOI values are also unaffected by either the presence of clays and/or flame retardant at the low concentrations (5 wt% except for NOR 116 at 1 wt%)[17]. However, the burning behaviours of knitted fabrics having the formulations were recorded as time to burn for successive 60 mm distances. While 100% polypropylene fabrics burnt their entire length quite rapidly, those containing 3 wt% clay alone, showed slightly longer time to reach the 60 mm mark and hence had slower burning rates during the first 60 mm of sample. For the Bentone 107 clay-containing formulations, fabric burning rates were in the decreasing order:PP>PP-B107>PP-Pb-B107-APP>PP-Pb-B107>PP-NOR-Pb-B107-PEP>PP-NOR-Pb-B107-TTBPP which demonstrates the obvious effect of small amounts of added flame retardant. Conversely, the order for Cloisite 20A-containing formulations was:PP>PP-Pb-20A-APP>PP-Pb-20A which suggested that APP has a deleterious effect. However, samples also burned differently and the flames flickered quite significantly, probably due to the poor dispersion. Because APP concentrations above 20% would normally be required to render PP flame retarded[17]then the observation that in the presence of nanoclay only 5% can cause marked effects in PP fabrics is encouraging.
Other work in our laboratories[82], has shown that fibre-grade poly(acrylonitrile) copolymer when polymerised in the presence of a functionalised nanoclay, may absorb ammonium polyphosphate during filament extrusion and yield fibres having LOI > 40 vol%. In these fibres, a clear synergy between nanoclay and flame retardant is observed and filament properties are little changed from those acceptable for normal textile applications. Unfortunately, APP is not durable to water soaking or washing and so introduction of a cross-linkable or insoluble flame retardant would be required to achieve required levels of launderability.
Alongi et al.[83-85]have demonstrated similar improvements in fire performance of poly (lactic) acid and poly(ethylene terephthalate) (PET) fibres respectively in the presence of nanoparticles. However, only when both clay and flame retardant were present in PET fibres did derived fabrics show both reduced PHRR values and increased LOI values (LOI = 33 vol% for a PET/sepiolite/zinc phosphinate formulation)[85].
5.2Nanotechnology applied to fibre and textile surface treatments, coatings and back-coatings
Examples of the potential for use of nanoparticulate fillers to enhance the fire performance of polymer coatings have largely been restricted to coatings for textile substrates including back-coatings. Bourbigot et al.[69,78,86]have shown that addition of nanoclays and poly(silsosesquioxanes) when present in polyurethane coatings on cotton and polyester fabrics can reduce the peak heat release rates compared to respective uncoated fabrics. However, the presence of these nanoparticles alone again reduced the time-to-ignition and prolonged the time of burning-exactly the opposite of what is required for flame retarded coated textiles.
As mentioned above, Horrocks et al.[48,54,55]have shown that if a back-coating is to be effective it must have a transferable flame retardant activity from the coating on the reverse face of the textile when ignited from the front face in tests such as BS 5852: Part 1 1979 and 1990 and EN1021: Parts 1 and 2. The use of purely char-promoting flame retardants within the coating does not allow this transferable flame retardant activity to occur unless the retardant species becomes mobile and can diffuse through the fabric to the front face. Furthermore, the addition of nanoclay to a back-coating polymeric film has been shown to have no beneficial effect when alone[54]. It was also noted that when fumed (nano) silica was added with ammonium polyphosphate to the back-coating formulation, not only was there an adverse effect noted with respect to formulation rheology, but also the flame retardant character as determined by LOI was reduced with increasing silica content.
Clearly the potential applications of nanocomposites within the coating area, especially with respect to coated textiles, must be questioned based on the present data available and especially in light of the effectiveness of nanocomposites being inversely related to thickness as also discussed above[70].
6Adding value to currently flame retardant systems: smarter flame retardancy
The above review has outlined that replacing currently accepted flame retardant treatments for textiles and chemical systems for synthetic fibres is indeed a challenge given that these are well-proven and have been able to demonstrate acceptable general and ecotoxicological properties. However, there is the good possibility that any given flame retardant substrate maybe have additional fire resisting value added to it by subsequent aftertreatment using surface modification as a most probable means. If such aftertreatments are to have minimal effects on the underlying textile characteristics then we are probably considering surface modifications and coatings at the microlevel at worst and nanolevel at best. This also introduces the possibility of so-called smart coatings that may be in principle applied to any number of different flame retardant fibre and textile substrates[87].
A major issue when considering surface flame retardant treatments for fibres and textiles compared with other treatments such as water or soil repellency is the high concentration required. Hence if the level of flame retardancy to be conferred is high in that the underlying substrate is highly flammable like cotton for instance, then the level of flame retardant formulation applied may be within the region of 20-100 wt% with respect to the underlying fabric and the surface treatment will be of tens and possibly hundreds of microns thick. Finally, not only must such surface coatings render the underlying fibres flame retardant but also the resin matrix in which they are embedded, unless it is inherently flame retardant like poly(vinyl chloride), for example.
However, there are the possibilities of gaining some degree of heat and fire protection using coatings or films applied at the nanolevel if they are not seen to be simple replacements for conventional flame retardant coatings. In normal flame retardant textiles and coated fabrics which may be classed as thermally thin materials[71]unless they are quite thick (>3~5 mm), the ability to form a thick, surface insulating char is limited and the underlying fibres soon reach temperatures approaching that of the igniting source (>500 ℃) when they degrade and may ignite. However, if we are able to convert a thermally thin textile into one showing so-called thermally thick behaviour, its overall fire protective character will increase and many conventional surface treatments and coatings, especially those comprising intumescent additives, attempt to do this. It is highly unlikely that nanocoatings could promote a similar effect unless they could offer a heat shield property of unusual efficiency.
In the area of heat protective textiles[88], use is made of the deposition of reflective metal films on to fabric surfaces to reduce the effects of heat radiation from a fire source and it is in this area that nanofilm and nanocoating deposition may have opportunities.
6.1Plasma technology and its potential
Plasma technology offers a means of achieving the means of developing novel nanocoatings having the desired thermal shielding effects. Shi has demonstrated that low pressure, radio frequency discharge plasma treatment of a number of polymer surfaces including poly(ethylene terephthalate) in the presence of gaseous (CF4/CH4) leads to flame retardation[89]. Later studies in which ethylene-vinyl acetate copolymers were plasma-exposed for time up to 15 min followed by immersion into acrylamide, gave very high yields of surface grafted poly(acrylamide) and LOI values approaching 24 vol% at 47 wt % grafting levels[90]. The subsequent studies of low pressure argon plasma graft polymerization by Tsafack et al.[91-92]have reported the successful grafting of phosphorus-containing acrylate monomers to polyacrylonitrile (PAN) fabrics (290~300 g/m2). In the presence of a grafting agent, ethylene glycol diacrylate (EGDA), graft yields were optimised (as high as 28 wt%) resulting in limiting oxygen index values as high as 26.5 vol%, although after accelerated laundering this reduced to 21 vol%. This and Shi’s techniques would not be expected to provide nanofilms since this type of grafting may be perhaps best considered as a variation of established polymer surface and textile-grafting procedures[93]and the high yields (28 wt% in the case of grafted dimethyl(acryloyloxymethyl)phosphonate, DMAMP) would explain both the level of flame retardancy and poor launderability achieved. When extended to cotton (120 and 210 g/m2), low pressure argon plasma graft polymerization of these same acrylate monomers[94], again yielded grafted fabrics having elevated LOI values as high as 26.0 vol% in the case of DMAMP. However, even higher and more acceptable levels of flame retardancy were achieved only if synergistic nitrogen was also present in grafts, which they demonstrated following the grafting of the phosphoramidate monomers. The improved durability in the case of the cross-linking agent (EGDA) with a high concentration can be achieved due to the greater reactivity of the plasma-activated cellulose chains compared with those generated on PAN fibre surfaces. While no grafted film thicknesses have been reported, they are probably within the micron range and not the nanometre range.
The possibility that plasma deposition of silicon-based films might improve the flame retardancy of underlying polymer surfaces has been reported by Jama et al.[95]. Here normal and nanocomposite polyamide 6 films were activated by a low pressure (4.2 mbar) cold nitrogen plasma and then transferred to a reactor containing 1,1,3,3-tetramethyldisiloxane (TMDS) vapour in an oxygen carrier gas for 20 min. This remote plasma-assisted polymerization is similar to that used by Tsafack et al. above except that the monomer is in the vapour phase prior to polymeric deposition. Thermogravimetry showed that increasing the oxygen flow rate considerably increased the thermal stability in air of deposited coatings as the increasingly oxygenated polysiloxane coating transforms to a silica-based structure at about 800 ℃. This gives the opportunity for a thermal barrier effect coupled with a moderate increase in flame retardancy of a coated polyamide 6 film and a surprising increase in the flame resistance of the nanocomposite polyamide 6 films, with LOI values exceeding 45 vol% for the latter. Clearly this rise in LOI is impressive given the low levels of surface polymer present. Char residues mirror respective LOI trends with the former rising from zero with no plasma deposition, through to 53 and 75 residual weight percent (from TGA in air) as the LOI respectively rises from about 23, through to about 47 vol%.
Analysis shows that those from the coated nanocomposite films are largely silica-based while those for coated normal polyamide 6 films are essentially polysiloxane-like. The presence of the nanoclay at 2 wt% appears to have synergised the formation of silica from the plasma-generated coating. The thermal barrier efficiency of the coated nanocomposite films is demonstrated by cone calorimetric analysis under an incident heat flux of 35 kW/m2where PHRR values of plasma-coated nanocomposite films are reduced in intensity by 25% compared to the uncoated films. A subsequent paper[96]demonstrated that the film thicknesses obtained were about 48 μm in thickness whereas those from the larger low pressure plasma source and reactor reduced to only 1.5 μm thickness and coated nanocomposite polyamide 6 films continued to yield LOI values as high as 48 vol%.
More recent work in our own laboratories has led to a patented process[97-98]in which using atmospheric plasma we have demonstrated that the flash fire resistance of a both pure cotton, flame retarded cotton and poly(meta-aramid) fabrics may be improved by surface treatment in the presence of a clay and a silicon-containing monomer such as hexamethylene disiloxane (HMDSO). At the relatively low heat flux of 35 kW/m2, cone calorimetry showed that time-to-ignite (TTI) and time-to-peak (TTP) values of cotton increased for argon plasma-treatment of 15 min because of the effect the clay present. Similar treatments to a Proban®-treated cotton sample showed that while TTI values are unaffected, TTP values are again increased following clay deposition.
Table 4 shows the changes in cone calorimetric behaviour of a 200 gsm woven meta-aramid (Nomex®) fabric subjected to an argon plasma alone and in the presence of clay and/or HMDSO. The fabric alone failed to ignite when exposed at the more typical heat flux of 50 kW/m2but did ignite when exposed at 60 kW/m2. Flash fire testing is usually associated with heat fluxes of 80 kW/m2[99]or more and this level was not achievable by our equipment. The results showed that even after argon plasma treatment alone, slight increases in both TTI and TTP are observed with a similarly slight reduction in PHRR. Clearly the already high heat flux ignition resistance of the meta-aramid fabric was being significantly improved following plasma treatment. Increasing the incident heat flux to 70 kW/m2caused untreated and treated fabrics to ignite but the presence of the plasma treatment reduced PHRR values both before and after a simulated laundering.
Table 4The cone calorimetric behaviour of poly(meta-aramid)-containing fabrics exposed to 60 kW/m2heat flux after subjecting them to various atmospheric plasma treatments[97-98]

SampleandtreatmentMasschange/%Time-to-ignition,TTI/sTime-to-peakheatrelease,TTP/sPeakheatreleaserate,PHRR/kW·m-2Meta-aramidalone-131683Argonplasmaonly-2.8162073Argonplasmawithsilicon-containingmonomer(HMDSO)-0.6NI*--Argonplasmawithnanoclay1.6NI*--Argonplasmawithsilicon-containingmonomerandnanoclay3.5NI*--
Note: NI indicates that the sample did not ignite.
Other recent work from Tata et al.[100]shows that polyester fabrics could be etched to give a surface pre-treatment by cold oxygen plasma and further finished with hydrotalcite, nanometric titania and silica aqueous suspensions. It reveals that the immersion time is fundamental for the best fire performances. Furthermore, combustion data indicated that only hydrotalcite-containing treatments promote consistent increases in time-to-ignite (TTI), and hence, yield acceptable flame retardancy levels. The subsequent study[101]in their laboratory uses plasma surface activation combined with nano-montmorillonite adsorption to affect the thermal stability of fabrics in air. Cone calorimetry reveals the best sample has a remarkable improvement in terms of TTI (up to 104%) and a slight reduction in the PHRR (ca. 10%) compared to neat PET fabric. Meanwhile, thermal analysis showed a temperature delay of the mass loss process for the treated samples due to the presence of nanoparticles. Other research from Totolin[102]involves grafting/crosslinking sodium silicate layers on to viscose and cotton flannel substrates by using atmospheric pressure plasma to prepare an environmentally friendly, flame-retardant cellulosic material. The combustion time of the treated cellulose samples are significantly increased because of the introduction of silicate and silica layer acting as a thermal insulator, allowing the coated fabrics to decompose at lower rates compared with untreated ones. The presence of the silicate on the surface of the fabrics even after ultrasound washes could be confirmed by XPS and SEM. The possible plasma-induced crosslinking mechanism has also been proposed as shown in Fig.2.

Fig.2 Suggested plasma-induced crosslinking mechanism[102]
Kamlangkla et al.[103]report that diethyl 2-(acryloyloxyethyl) phosphate (DEAEP) and diethyl 2-(acryloyloxyethyl) phosphoramidate (DEAEPN) could be grafted on the surface of silk fabrics to enhance its flame retardancy by means of a two steps process, i.e. argon-induced graft polymerization of phosphorus containing monomers followed by a SF6plasma treatment. The procedure of the two-step treatment is illustrated in Fig.3. This flame retardant silk fabric showed only a slight variation in the tensile strength and the colour fastness compared with untreated silk. In addition, acceptable flame retardant behaviour remained after 50 cycles of laundering (using the McSherry method).
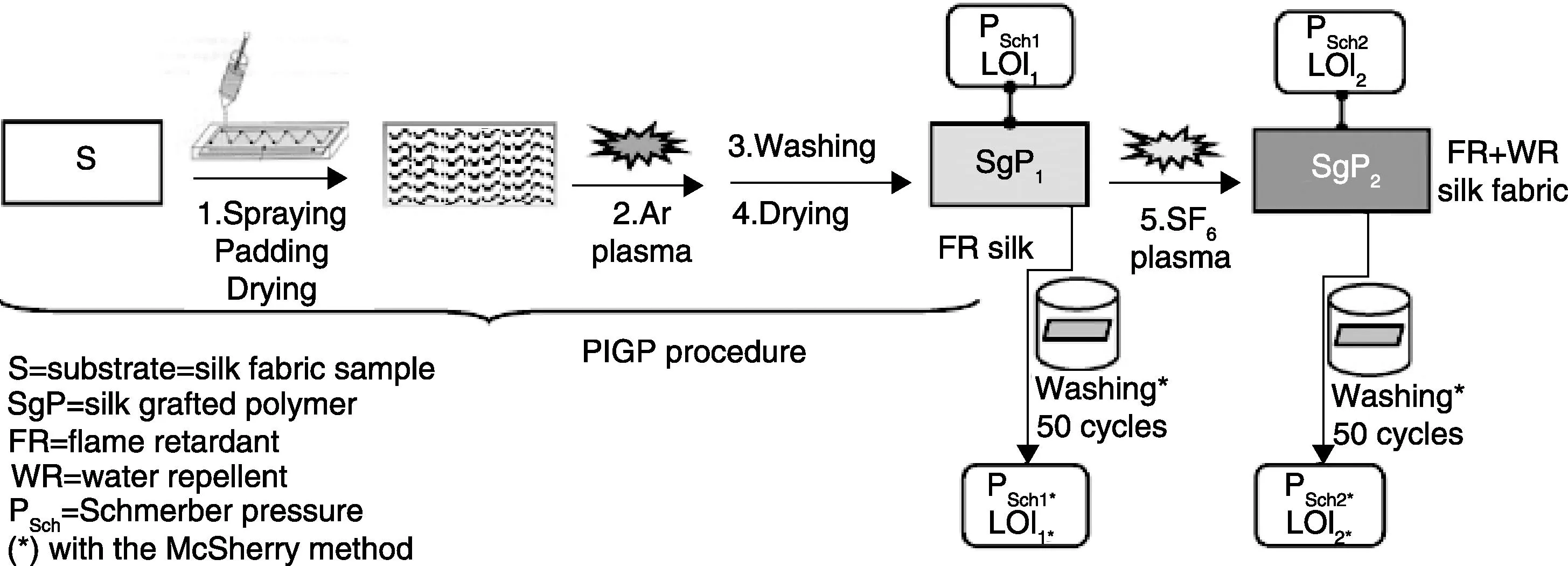
Fig.3 Procedure of the two-step treatment. Step 1: PIGP process of FR monomers onto silk fabrics. Step 2: SF6 plasma on the FR fabrics[104]
In conclusion, it should be noted that plasma technological modification of fibre and textile surface has a history spanning about 40 years and although it has gained commercial significance within industrial sectors such as microelectronics and more recently in improving paint/coating adhesion to plastics for automotive and other applications, its adoption by the textile industry has been slow[104-106]. One of the main reasons for this is that the majority of successful plasma applications occurred using low pressure plasma and it is only recently that atmospheric pressure plasma technologies have been developed which are considered to be more appropriate to continuous processing of textile fabrics[105].
6.2Other technologies
In addition to plasma technology, many other technologies, recently reviewed by Alongi et al.[107]used for surface treatment of fibres and textiles include layer-by-layer (LbL) assembly, gamma and UV-induced grafting/polymerization all of which have been used to improve the flame retardancy of fabrics. The LbL assembly has emerged as a promising way for the formation of multilayer thin films (typically no thicker than 1 μm) on to the surface of textiles to obtain the special function, due to its low cost, easy process and controllable thickness[108-109].
Li et al.[110-112]and Cariosos et al.[109]have extended its application range further and reported that multilayer films consisting of silica nanoparticles have been fabricated by LbL assembly in an effort to improve the flame retardancy of PET fabric. In this study, PET fabric was alternately immersed into positive alumina coated silica (Ludox CL) and two different kinds of negatively charged colloidal silica (Ludox TM40 and Ludox SM30 ) to generate conformal colloidal layers on each fibre surface. Results from cone calorimetry and the vertical flame tests have shown that the fabric coated by CL/ SM with 5BL has an increased time to ignition of 99 s and a reduced PHRR of 20% with no melt dripping due to the formation of an effective inorganic barrier.
Since then these same workers have undertaken further research which has shown[112]that the first ever intumescent nanocoating can be achieved using LbL assembly of poly(sodium phosphate) (PSP),which acts as the acid source and is negatively charged in water, and poly(allylamine) (PAAm), which is used as the blowing agent and is positively charged in water. The bubbles on the fibre surface can be observed from SEM images after burning, demonstrating the intumescent behavior of these nanocoatings. The publication shows that the PHRR and total heat release of fabric show a 43% and 51% reduction compared to the control fabric, with only 1.7 wt% coating added (with 5BL). All coated fabric samples have a lower degradation temperature and a higher char yield.
More recent work by Laufer and colleagues has combined LbL assembly technology with renewable polyelectrolytes such as cationic chitosan (CH) and anionic phytic acid (PA) to form an environmentally friendly intumescent multilayer nanocoating[113]. Film thickness can be tailored by varying the pH of aqueous depositing solutions. The result shows that coatings made with pH 4 solutions (the thinnest films with a PA content of 66 wt%) resulted in the better flame retardancy due to a greater phosphorus content (in the form of PA).
The LbL method when combined with other technologies has also been used to improve the flame retardance of cotton fabrics. Wang et al.[114]have applied an intumescent coating composed of a nitrogen-modified silane hybrid and phytic acid deposited on to cotton fabric through the combination of sol-gel process and layer-by-layer techniques in order to reduce flammability of the fabric. The cationic solution for the cotton fabric impregnation was synthesized by the sol-gel technique. The chemical structure of nitrogen-modified silane hydrid (SIN) and its related finishing procedure are illustrated in Fig.4. In a vertical flame test, fabrics coated with 15 bilayers (BLs) of SiN-PA extinguished the flame immediately upon removing the ignition source, while untreated cotton was completely burnt out. The results show that 15BL-coated cotton resulted in a 31% and 38% reduction in peak heat release rate and total heat release, respectively, relative to those of the uncoated control.
Alongi et al.[115]have used alumina-coated silica and silica as the positive and the negative counterpart to deposit on the cotton fabric by a horizontal spray method. The result shows that a 40% increase of TTI and a 20 to 30% reduction of PHRR and TSR were observed in the cone calorimeter.

Fig.4 Schematic illustration of synthesis of nitrogen-modified silane hybrid (a) and LbL assembly of SiN and PA on cotton fabrics (b)[114]
Grafting and polymerization induced by UV and gamma radiation have been used to impart flame retardancy to textiles because of their economy and effectiveness and with little alteration of the bulk properties. A study from Zhang’s research group has worked on introducing surface photografting technology into the flame retardant finishing of fabric since 2007 and has shown that photografting with maleic anhydride (MAn) followed by a reaction with triethanolamine as a two-step surface modification method can improve the flame retardancy of PA6,6 fabric[116]. Their further research has reported that PA6,6 fabric photografted with acrylamide (AM) shows no melt dripping and a shorter char length during the vertical burning test with LOI values exceeding 26 vol%[117]. Subsequent work has extended this to PET fabric to yield similar performance[118].
Recent interest has also been shown in the potential use of gamma radiation to induce polymerization and grafting of a novel phosphorus-, nitrogen-, and sulphur-containing monomer, diethyl(acryloyloxy) ethylthiophosphoramidate on to cotton fabric to obtain levels of flame retardancy[119]. It can be concluded, therefore, that the major advantage of these methods is that in principle they may be applied to any textile substrate retrospectively and so offer great opportunity for enhancing the heat and fire resistance of a range of textile substrates.
参考文献References
[1]Horrocks A R. Flame Retardant Finishing of Textiles [J].ReviewofProgressTextileColoration, 1986,16: 62-101.
[2] Horrocks A R. Flame Retardant Finishes and Finishing [M]// Heywood D.TextileFinishing. Bradford: Society of Dyers and Colourists, 2003: 214-250.
[3]Bourbigot S. Flame Retardancy of Textiles-New Approaches [M]// Horrocks A R, Price D.AdvancesinFireRetardantMaterials. Cambridge, Woodhead Publishing, 2008: 9-40.
[4]Weil E D, Levchik S V. Flame Retardants in Commercial Use or Development for Textiles [J〗.JournalofFireScience, 2008, 26 (3): 243-281.
[5] Horrocks A R. Overview of Traditional Flame-Retardant Solutions [M]// Alongi J, Horrocks A R, Carosio F,etal.UpdateonFlameRetardantTextiles:StateoftheArt,EnvironmentalIssuesandInnovativeSolutions. Shawbury: Smithers Rapra, 2013: 123-178.
[6]Proceedings of Conference on Materials for Improved Fire Safety [C]. Washington D C: NASA Spec Publ, 1971: 155.
[7]Hughes A J, McIntyre J E, Clayton G,etal. The Production of Man-Made Fibres [J].TextileProgress, 1976, 8(1): 97-177.
[8] Horrocks A R, Eichhorn H, Schwaenke H,etal. Thermally Resistant Fibres [M]// Hearle J W S.HighPerformanceFibres. Cambridge: Woodhead Publishing, 2001: 281-321.
[9]Bourbigot S, Flambard X. Heat Resistance and Flammability of High Performance Fibre: A Review [J].FireandMaterials, 2002, 26(4/5): 155-168.
[10] Weil E,etal. U.S. Pat. No.: 3,746,572 [P]. 1970-7-17.
[11] Weil E.ProcessforFlameproofingTextiles: United States, 3695925[P]. 1972-10.
[12]Weil E.CopolycondensedVinylphosphonates: United States, 3855359[P]. 1974-12-17.
[13] J G Leitner.FlameRetardant-SmolderResistantTextileBackcoating: United States, 4404313[P]. 1983-9-13.
[14]Blum A, Ames B N.TextileIndustries,1980, 144(8): 48.
[15] Tesoro G C.JournalofFireRetardantChemistry, 1979, 6(4): 239.
[16]Hicklin R, Padda R, Lenotte G. Trends in Textile Flame Retardants- A Market Review [M]// Hull T R, Kandola B K.FireRetardancyofPolymers,NewStrategiesandMechanisms. London: RSC Publishing, 2009: 255-265.
[17] Zhang S, Horrocks A R. A Review of Flame Retardant Polypropylene Fibres [J].ProgressinPolymerScience, 2003, 28: 1 517-1 538.
[18]The Upholstered Furniture (Safety) (Amendment) Regulations, 1983, No. 519, Consumer Protection. London: Her Majesty’s Stationery Office; Consumer Protection Act, 1987(a): The Furniture and Furnishing (Fire) (Safety) Regulations, 1988 (amended 1989 and 1993), SI 1324. London: Her Majesty’s Stationery Office.
[19]Dombrowski R. Flame Retardants for Textile Coatings [J].JournalofCoatedFabrics, 1996, 25: 224-238.
[20]Horrocks A R. Developments in Flame Retardants for Heat and Fire Resistant Textiles-the Role of Char Formation and Intumescence [J].PolymerDegradationandStability, 1996, 54: 143-154.
[21]Abdel-Mohdy F A, Nawar G A M.JournalofTextileAssociation, 1999, 9-10: 121-128.
[22]Abdel-Mohdy F A, Belyakova M K, Gaballa M M.Colourage, 2002, 49(11): 27-34.
[23]Weil E D.Proceedingsof2ndConferenceonAdvancesinFlameRetardantPolymers[C]. Norwalk, CT: Business Communications Inc., 1991: 15-32.
[24]Stowell J K, Weil E D, Coble W L,etal.Formaldehyde-FreeFlameRetardantTreatmentforCellulose-ContainingMaterials: United States, 6,365,070 [P]. 2002-4-2.
[25]Wu W, Yang C Q.Proceedingsof14thConferenceonAdvancesinFlameRetardantPolymers[C]. Norwalk, CT: Business Communications Inc., 2003.
[26]Yang C Q, Wu W. Combination of a Hydroxyl-Functional Organo-phosphorus Oligomer and a Multifunctional Carboxylic Acid as a Flame Retardant Finishing System for Cotton: Part I. The Chemical Reactions [J].FireandMaterials, 2003, 27: 223-238.
[27]Yang C Q, Wu W. Combination of a Hydroxyl-Functional Organophosphorus Oligomer and a Multifunctional Carboxylic Acid as a Flame Retardant Finishing System for Cotton: Part II. Formation of Calcium Salt during Laundering [J].FireandMaterials, 2003, 27: 239-251.
[28]Wu W, Yang C Q. Comparison of DMDHEU and Melamine Formaldehyde as the Binding System for a Hydroxy-Functional Organophosphorus Flame Retarding Agent on Cotton [J].JournalofFireScience, 2004, 22:125-143.
[29]Yang Z Y, Wang X W. A Durable Flame Retardant for Cellulosic Fabrics [J].PolymerDegradationandStability, 2012, 97: 2 467-2 472.
[30]Yang H, Yang C Q. Nonformaldehyde Flame Retardant Finishing of Nomex/Cotton Blend Fabric Using a Hydroxyl-Functional Organophosphorus Oligomer [J].JournalofFireScience, 2007, 25: 425-446.
[31]Guan J, Yang C Q, Chen G. Formaldehyde-Free Flame Retardant Finishing of Silk Using a Hydroxyl-Functional Organophosphorus Oligomer [J].PolymerDegradationandStability, 2009, 94: 450-455.
[32]Wu X, Yang C Q. Flame Retardant Finishing of Cotton Fleece Fabric: Part III-The Combination of Maleic Acid and Sodium Hypophosphite [J].JournalofFireScience, 2008, 26: 351-368.
[33] Yang C Q, Wu X. Flame Retardant Finishing of Cotton Fleece Fabric. Part IV-Bifunctional Carboxylic Acids [J].JournalofFireScience, 2009, 27: 431-444.
[34]Wu X L, Yang C Q. Flame Retardant Finishing of Cotton Fleece: Part VII. Polycarboxylic Acids with Different Numbers of Functional Group [J].Cellulose, 2010, 17: 859-870.
[35]Cheng X, Yang C Q. Flame Retardant Finishing of Cotton Fleece Fabric: Part V. Phosphorus-Containing Maleic Acid Oligomers [J].FireandMaterials, 2009, 33: 365-375.
[36]Cheng X, Yang C Q. Flame Retardant Finishing of Cotton Fleece Fabric: Part VI. The Combination of a Hydroxyl-Functional Organophosphorus Oligomer and 1,2,3,4-Butanetetracarboxylic Acid [J].JournalofFireScience, 2009, 27: 583-600.
[37] Galbraikh L S, Zubkova N S, Butylkina N G,etal.MethodfortheFlame-RetardantProcessingofTextileMaterials.AssignedtoIsleFirestopChemicals: United States, 6,541,068[P]. 2003-4-1.
[38] Chang S C, Sachinvala N D, Sawhney A P,etal. Epoxy Phosphonate Crosslinkers for Providing Flame Resistance to Cotton Textiles [J].PolymerforAdvancedTechnologies, 2007, 18 (8): 611-619.
[39]Chang S C, Condon B, Edwards V J,etal. Study of Phosphorus and Nitrogen Containing Economic Flame Retardant Materials and Their Textile Application [C]//ProceedingsofFlameRetardantPolymersandMaterials(FRPM09). 2009.
[40]Hu J T, Yau Y N, Liu X S,etal. The Application of a Novel Flame Retardant on Viscose Fibre [J].FireandMaterials, 2009, 33: 145-156.
[41] Lecoeur E, Vroman I, Bourbigot S,etal. Flame Retardant Formulations for Cotton [J].PolymerDegradationandStability, 2001, 74: 487-492.
[42]Lecoeur E, Vroman I, Bourbigot S,etal. Optimization of Monoguanidine Dihydrogen Phosphate and Amino Propylethoxysilane Based Flame Retardant Formulations for Cotton [J].PolymerDegradationandStability, 2006, 91: 1 909-1 914.
[43]Easson M, Condon B, Megumi Y T. Cyanuric Chloride Derivatives for Cotton Textile Treatment-Synthesis, Analysis, and Flammability Testing [J].AATCCReview, 2011, 11: 60-66.
[44]Horrocks A R. Flame Retardant and Environmental Issues, in Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions [M]. Shawbury, 2013: 207-238.
[45]Nguyen T-M D, Chang S C, Condon B,etal. Synthesis and Characterization of a Novel Phosphorus-Nitrogen-Containing Flame Retardant and Its Application for Textile [J].PolymersforAdvancedTechnologies, 2012, 23: 1 034-1 044.
[46]Alongi J, Carletto R A, Bosco F,etal. Caseins and Hydrophobins as Novel Green Flame Retardants for Cotton Fabrics [J].PolymerDegradationandStability, 2014, 99:111-117.
[47] Alongi J, Di Blasio A, Milnes J,etal. Thermal Degradation of DNA, an All-in-One Natural Intumescent Flame Retardant [J].PolymerDegradationandStability, 2015, 113(3): 110-118.
[48]Alongi J, Carletto R A, Di Blasio A,etal. Intrinsic Intumescent-Like Flame Retardant Properties of DNA-Treated Cotton Fabrics [J].CarbohydratePolymers, 2013, 96:296-304.
[49]Wang M Y, Horrocks A R, Horrocks S,etal. Flame Retardant Textile Back-Coatings. Part 1: Antimony-Halogen System Interactions and the Effect of Replacement by Phosphorus-Containing Agents [J].JournalofFireSciences, 2000, 18: 265-294.
[50]Horrocks A R, Wang M Y, Hall M E,etal. Flame Retardant Textile Back-Coatings. Part 2: Effectiveness of Phosphorus-Containing Retardants in Textile Back-Coating Formulations [J].PolymerInternational, 2000, 49: 1 079-1 091.
[51]Giraud S, Bourbigot S, Rochery M,etal. Flame Retarded Polyurea with Microencapsulated Ammonium Phosphate for Textile Coating [J].PolymerDegradationandStability, 2005, 88 (1):106-113.
[52]Saihi D, Vroman I, Giraud S,etal. Microencapsulation of Ammonium Phosphate with a Polyurethane Shell Part I: Coacervation Technique [J].ReactiveandFunctionalPolymer, 2005, 64 (3): 127-138.
[53] Saihi D, Vroman I, Giraud S,etal. Microencapsulation of Ammonium Phosphate with a Polyurethane Shell. Part II. Interfacial Polymerization Technique [J].ReactiveandFunctionalPolymers, 2006, 66 (10): 1 118-1 125.
[54]Horrocks A R, Davies P, Alderson A,etal. The Challenge of Replacing Halogen Flame Retardants in Textile Applications: Phosphorus Mobility in Back-Coating Formulations [C]//Proceedingsof10thEuropeanMeetingofFireRetardantPolymers. Berlin: 2005: 141-158.
[55]Horrocks A R, Davies P, Alderson A,etal. The Potential for Volatile Phosphorus-Containing Flame Retardants in Textile Back-Coatings [J].JournalofFireSciences, 2007, 25 (6): 523-540.
[56] Wang Y Z. Halogen-Free Flame Retardants[M]// Horrocks A R, Price D.AdvancesinFlameRetardantMaterials. Cambridge: Woodhead Publishing, 2008: 67-94.
[57] Chen D Q, Wang Y Z, Hu X P,etal. Flame-Retardant and Anti-Dripping Effects of a Novel Char-Forming Flame Retardant for the Treatment of Poly(ethylene terephthalate) Fabrics [J].PolymerDegradationandStability, 2005, 8: 349-356.
[58]Wang D Y, Wang Y Z, Wang J S,etal. Thermal Oxidative Degradation Behaviours of Flame-Retardant Copolyesters Containing Phosphorous Linked Pendent Group/Montmorillonite Nanocomposites [J].PolymerDegradationandStability, 2005, 87: 171-176.
[59]Aizenshtein E M, Anan'eva L A, Okuneva O P,etal. Polyester Fibre with Low Combustibility [J].FibreChemistry, 2002, 34(3): 172-176.
[60]Zhao H B, Chen L, Yang J C,etal. A Novel Flame-Retardant-Free Copolyester: Cross-Linking Towards Self Extinguishing and Non-Dripping [J].JournalofMaterialsChemistry, 2012, 22(37): 19 849-19 857.
[61]Jing X K, Wang X S, Guo D M,etal. The High-Temperature Self-Crosslinking Contribution of Azobenzene Groups to the Flame Retardance and Anti-dripping of Copolyesters [J].JournalofMaterialsChemistryA, 2013, 1: 9 264-9 272.
[62] Lecomte H A, Liggatt J J. Commercial Fire-Retarded PET Formulations-Relationship between Thermal Degradation Behaviour and Fire-Retardant Action [J].PolymerDegradationandStability, 2008, 93: 498-506.
[63]Bourbigot S, Duquesne S, Bellayer S,etal. Novel Developments in Flame Retardancy of Textiles[C]//AdvancesintheFlameRetardancyofPolymericMaterials-CurrentPerspectivesPresentedatFRPM’05. Norderstedt: 2007: 159-180.
[64]Horrocks A R. Nanocomposites II: Potential Applications for Nanocomposite-Based Flame Retardant Systems [M]// Horrocks A R, Price D.AdvancesinFlameRetardantMaterials. Cambridge: Woodhead Publishing, 2008: 124-158.
[65]Gilman J W, Awad W H, Davis R,etal. Improved Thermal Stability of Crown Ether and Imidazolium Treatments for Flame Retardant Polymer-Layered Silicate Nanocomposites [M]. London: Interscience Publications Ltd., 2002:139-146.
[66]Gilman J W, Kashiwagi T. Polymer-Layered Silicate Nanocomposites with Conventional Flame Retardants[M]// Pinnavia T J, Beall G W.Polymer-ClayNanocomposites. Chichester and New York: Wiley, 2000:193-206.
[67]Gilman J W. Flammability and Thermal Stability Studies of Polymer Layered-Silicate (Clay) Nanocomposites [J].AppliedClayScience, 1997, 15: 31-49.
[68]Bourbigot S, Devaux E, Rochery M,etal. Nanocomposite Textiles: New Routes for Flame Retardancy [C]//Proceedingsofthe47thInternationalSAMPESymposium. 2002, 47:1 108-1 118.
[69]Bourbigot S, Devaux E, Flambard X. Flammability of Polyamide-6/Clay Hybrid Nanocomposite Textiles [J].PolymerDegradationandStability, 2002, 75: 397-402.
[70] Kashiwagi T, Shields J R, Harris Jr R H,etal. Flame Retardant Mechanism of a Polymer Clay Nanocomposite [C]//Proceedingsofthe14thConferenceon“AdvancesinFlameRetardantPolymers”. Stamford: 2003.
[71] Drysdale D.AnIntroductiontoFireDynamics, 2ndEdition[M]. Chichester: John Wiley and Sons: 1999: 212-222.
[72]Solarski S, Mahjoubi F, Ferreira M,etal. Polylactide/Clay Nanocomposite Textile: Thermal, Mechanical, Shrinkage and Fire Properties [J].JournalofMaterialsScience, 2007, 42(13): 5 105-5 117.
[73] Monticelli O, Musina Z, Frache A,etal. Influence of Compatibilizer Degradation on Formation and Properties of PA6/Organoclay Nanocomposites [J].PolymerDegradationandStability, 2007, 92: 370-378.
[74]Morgan A B, Wilkie C A.FlameRetardantPolymerNanocomposites[M]. Hoboken, New Jersey: Wiley-Interscience, 2007.
[75] Padbury S A, Horrocks A R, Kandola B K. The Effect of Phosphorus-Containing Flame Retardants and Nanoclay on the Burning Behaviour of Polyamides 6 and 6.6 [C]//Proceedingsofthe14thConferenceon“AdvancesinFlameRetardantPolymers”. Stamford: 2003.
[76]Horrocks A R, Kandola B K, Padbury S A. The Effect of Functional Nanoclays in Enhancing the Fire Performance of Fibre-Forming Polymers [J].JournaloftheTextileInstitute, 2005, 94: 46-66.
[77]Zhang S, Horrocks A R, Hull T R,etal. Flammability, Degradation and Structural Characterization of Fibre-Forming Polypropylene Containing Nanoclay-Flame Retardant Combinations [J].PolymerDegradationandStability, 2006, 91: 719-725.
[78]Bourbigot S, Le Bras M, Flambard X,etal. Polyhedral Oligomeric Silsesquioxanes: Applications to Flame Retardant Textiles [M]// Le Bras M, Wilkie C A, Bourbigot S,etal.FireRetardancyofPolymers:NewApplicationsofMineralFillers. London: Royal Society of Chemistry, 2005: 189-201.
[79]Bellayer S, Bourbigot S, Flambard X,etal.Proceedingsofthe4thAUTEXConference[C]. Roubaix: 2004.
[80]Horrocks A R, Kandola B K, Smart G,etal. Polypropylene Fibres Containing Dispersed Clays Having Improved Fire Performance. Part I: Effect of Nanoclays on Processing Parameters and Fibre Properties [J].JournalofAppliedPolymerScience, 2007, 106: 1 707-1 717.
[81]Smart G, Kandola B K, Horrocks A R,etal. Polypropylene Fibres Containing Dispersed Clays Having Improved Fire Performance Part II: Characterisation of Fibres and Fabrics from Nanocomposite PP Blends [J].PolymerforAdvancedTechnologies, 2008, 19: 658-670.
[82] Horrocks A R, Hicks J, Davies P,etal. Flame Retardant Copolymeric Polyacryonitrile Fibres Containing Dispersed Phyllosilicate Clays [C]//Proceedingsof11thEuropeanConferenceonFireRetardantPolymers. Cambridge, UK :The Royal Society of Chemistry, 2009: 307-330.
[83]Alongi J, Frache A. Poly(ethylene terephthalate)-Carbon Nanofiber Nanocomposite for Fiber Spinning; Properties and Combustion Behaviour [J].E-polymer, 2010, 70: 1-10.
[84]Alongi J, Frache A, Giofferdi E. Fire-Retardant Poly(ethylene terephthalate) by Expandable Combination with Graphite and Layered Clays for Plastics and Textiles [J].FireandMaterials, 2011, 35: 383-398.
[85]Alongi J. Investigation on Flame Retardancy of Poly(ethylene terephthalate) for Plastics and Textiles by Combination of an Organic-Modified Sepiolite and Zn Phosphinate [J].FibersandPolymers, 2011, 12: 166-173.
[86]Bourbigot S, Devaux E, Rochery M. Polyurethane/Clay and Polyurethane/POSS Nanocomposites as Flame Retarded Coating for Polyester and Cotton Fabrics [J].FireandMaterials, 2002, 26: 149-154.
[87]Horrocks A R. Flame Retardant/Resistant Textile Coatings and Laminates[M]// Horrocks A R, Price D.AdvancesinFireRetardantMaterials. Cambridge: Woodhead Publishing, 2008:159-187.
[88]Horrocks A R. Thermal (Heat and Fire) Protection [M]// Scott R.TextilesforProtection. Cambridge: Woodhead Publishing, 2005: 398-440.
[89]Shi L S. Investigation of Surface Modification and Reaction Kinetics of PET in CF4-CH4 Plasmas [J].JournalofPolymerEngineering, 1999, 19: 445-551.
[90]Shi L S. An Approach to the Flame Retardation and Smoke Suppression of Ethylene-Vinyl Acetate Copolymer by Plasma Grafting of Acrylamide [J].Reactive&FunctionalPolymers, 2000, 45: 85-93.
[91]Tsafack M J, Hochart F, Levalois-Grützmacher J. Polymerization and Surface Modification by Low Pressure Plasma Technique [J].EuropeanPhysicalJournal-AppliedPhysics, 2004, 26: 215-219.
[92]Tsafack M J, Levalois-Grützmacher J. Plasma-Induced Graft-Polymerization of Flame Retardant Monomers onto PAN Fabrics [J].SurfaceandCoatingsTechnology, 2006, 200: 3 503-3 510.
[93]Bhattacharya A, Misra B N. Grafting: A Versatile Means to Modify Polymers. Techniques, Factors and Applications [J].ProgressinPolymerScience, 2004, 9:767-814.
[94] Tsafack M J, Levalois-Grützmacher J. Flame Retardancy of Cotton Textiles by Plasma-Induced Graft-Polymerization (PIGP) [J].SurfaceandCoatingsTechnology, 2006, 201 (6): 2 599-2 610.
[95] Jama C, Quédeé A, Goudmand P,etal. Fire Retardancy and Thermal Stability of Materials Coated by Organosilicon Thin Films Using a Cold Remote Plasma Process [J].ACSSymposiumSeries, 2001, 797: 200-213.
[96]Queédeé A, Mutel B, Supiot P,etal. Plasma-Assisted Process for Fire Properties Improvement of Polyamide and Clay Nanocomposite-Reinforced Polyamide: A Scale-Up Study. Fire Retardancy of Polymers [M]// Le Bras M, Wilkie C A, Bourbigot S,etal.NewApplicationsofMineralFillers. London: Royal Society of Chemistry, 2005: 276-290.
[97] Horrocks A R, Kandola B K, Nazaré S,etal.FlashFireResistantFabric: UK, 0900069.6 [P]. 2009-1-5.
[98] Horrocks A R, Nazaré S, Masood R,etal. Surface Modification of Fabrics for Improved Flash-Fire Resistance Using Atmospheric Pressure Plasma in the Presence of a Functionalised Clay and Polysiloxane [J].PolymerforAdvancedTechnologies, 2011, 22(1): 22-29.
[99]NFPA 2112 Standard on Flame Resistant Garments for Protection of Industrial Personnel against Flash Fire [S].National Fire Protection Association, USA, 2007.
[100] Tata J, Alongi J, Frache A. Optimization of the Procedure to Burn Textile Fabrics by Cone Calorimeter: Part II. Results on Nanoparticle-Finished Polyester [J].FireandMaterials, 2012, 36(7): 527-537.
[101] Carosio F, Alongi J, Frache A. Influence of Surface Activation by Plasma and Nanoparticle Adsorption on the Morphology, Thermal Stability and Combustion Behavior of PET Fabrics [J].EuropeanPolymerJournal, 2011, 47(5): 893-902.
[102]Totolin V, Sarmadi M, Manolache S O,etal. Environmentally Friendly Flame-Retardant Materials Produced by Atmospheric Pressure Plasma Modifications [J].JournalofAppliedPolymerScience, 2012, 124: 116-122.
[103]Kamlangkla K, Hodak S K, Levaois-Grützmacher J. Multifunctional Silk Fabrics by Means of the Plasma Induced Graft Polymerization (PIGP) Process [J].SurfaceandCoatingsTechnology, 2011, 205(13-14):3 755-3 762.
[104]Shishoo R.PlasmaTechnologiesforTextiles[M]. Cambridge: Woodhead Publishing, 2007.
[105] Herbert T. Atmospheric-Pressure Cold Plasma Processing Technology [M]// Shishoo R.PlasmaTechnologiesforTextiles. Cambridge: Woodhead Publishing, 2007: 79-128.
[106] Stegmaier T, Dinklemann, Arnim V,etal. Corona and Dielectric Barrier Discharge Plasma Treatment of Textiles for Technical Applications [M]// Shishoo R.PlasmaTechnologiesforTextiles. Cambridge: Woodhead Publishing, 2007: 129-157.
[107] Alongi J, Carioso F, Malucelli G.Smart(nano)Coatings.UpdateonFlameRetardantTextiles:StateoftheArt,EnvironmentalIssuesandInnovativeSolutions[M]. Smithers Rapra.
[108] Wang Q, Hauser P J. Developing a Novel UV Protection Process for Cotton Based on Layer-by-Layer Self-Assembly [J].CarbohydratePolymers, 2010, 81(2): 491-496.
[109]Carosio F, Laufer G, Alongi J. Layer-by-Layer Assembly of Silica-Based Flame Retardant Thin Film on PET Fabric [J].PolymerDegradationandStability, 2011, 96(5): 745-750.
[110] Li Y C, Schulz J, Grunlan J C. Polyelectrolyte/Nanosilicate Thin Film Assemblies: Influence of pH on Growth, Mechanical Behavior, and Flammability[J].AppliedMaterials&Interfaces, 2009, 1(10): 2 338-2 3347.
[111]Li Y C, Schulz J, Mannen S,etal. Flame Retardant Behavior of Polyelectrolyte-Clay Thin Film Assemblies on Cotton Fabric [J].ACSNano, 2010, 4(6): 3 325-3 337.
[112]Li Y C, Mannen S, Grunlan J C. Intumescent All-Polymer Multilayer Nanocoating Capable of Extinguishing Flame on Fabric [J].AdvancedMaterials, 2011, 23(34): 3 926-3 931.
[113] Laufer G, Kirkland C, Grunlan J C. Intumescent Multilayer Nanocoating, Made with Renewable Polyelectrolytes, for Flame-Retardant Cotton [J].Biomacromolecules, 2012, 13(9): 2 843-2 848.
[114]Wang X, Romero M Q, Zhang X Q,etal. Intumescent Multilayer Hybrid Coating for Flame Retardant Cotton Fabrics Based on Layer-by-Layer Assembly and Sol-Gel Process [J].RSCAdvances, 2015, 5:10 647-10 655.
[115]Alongi J, Carosio F, Frache A,etal. Layer by Layer Coatings Assembled through Dipping, Vertical or Horizontal Spray for Cotton Flame Retardancy [J].CarbohydratePolymers, 2013, 92: 114-119.
[116]Liu W, Zhang S. Surface Photografting: New Application for Flame Retardant Finishing of Polyamide6.6 (PA6.6) Fabric [J].JournalofAppliedPolymerScience, 2011, 119(1): 66-72.
[117] Liu W, Zhang S. Thermal Behavior and Fire Performance of Nylon-6,6 Fabric Modified with Acrylami6de by Photografting [J].PolymerDegradationandStability, 2010, 95 (9): 1 842-1 848.
[118] Yu L H, Zhang S, Liu W,etal. Improving the Flame Retardancy of PET Fabric by Photo-Induced Grafting [J].PolymerDegradationandStability, 2010, 95(9):1 934-1 942.
[119]Verma S K, Kaur I. Gamma-Induced Polymerization and Grafting of a Novel Phosphorous-, Nitrogen-, and Sulfur-Containing Monomer on Cotton Fabric to Impart Flame Retardancy [J].JournalofAppliedPolymerScience, 2012, 125(2): 1 506-1 512.
(编辑惠琼)
Received date:2015-05-08
Corresponding author:A Richard Horrocks,Professor,Email:arh1@bolton.ac.uk
纤维与纺织品的阻燃新进展与挑战
A Richard Horrocks1,Liu Wei2
(1.Institute for Research and Innovation,University of Bolton,Bolton,BL1 5LL,UK)(2. 公安部四川消防研究所,四川 成都 610036)
摘要:20世纪50~60年代,各种织物、纤维的耐久阻燃整理技术飞速发展,这些产品和工艺现已被大量应用于织物和纤维的阻燃耐久整理。但随着市场对环境、化学品毒性、成本及性能等方面的要求愈加严苛,其中多数产品的使用受到了质疑。“可否找到使用中释放甲醛类阻燃剂的替代品用于纤维素织物的耐久阻燃”已成为常被提及的问题。而关于“能否生产出成炭性能良好的聚酯阻燃剂”和“能否制备出高效无卤阻燃剂用于织物涂层和背涂”也备受关注。相关研究回顾表明,人们从20世纪下半叶就在寻求这些问题的解决方法,而这些工作也为目前最新的商业化阻燃纤维和纺织品发展奠定了基础;其次,本世纪前十年的研究主要侧重于解决环境可持续发展问题、寻找可替代的阻燃配方、满足合成纤维提高成炭的需求以及纳米技术的应用。另外,文章还对纤维的微表面改性和纳米表面改性的作用以及整理过程中等离子体、层层自组装、溶胶-凝胶等技术在织物阻燃整理中的应用进行了综述。
关键词:阻燃; 卤素; 磷元素;织物;纤维;环境;纳米技术;等离子体
DOI:10.7502/j.issn.1674-3962.2015.09.04

