Metachronous metastasis of renal cell carcinoma to bilateral testis
Sameer Nain, Suman Kharkwal, Onkar Kaur
1Department of Surgery, ESI PGIMSR Hospital, Basaidarapur, New Delhi 110015, India.
2Department of Pathology, ESI PGIMSR Hospital, Basaidarapur, New Delhi 110015, India.
Metachronous metastasis of renal cell carcinoma to bilateral testis
Sameer Nain1, Suman Kharkwal1, Onkar Kaur2
1Department of Surgery, ESI PGIMSR Hospital, Basaidarapur, New Delhi 110015, India.
2Department of Pathology, ESI PGIMSR Hospital, Basaidarapur, New Delhi 110015, India.
Unusual site metastasis as a presenting complaint of renal cell carcinoma (RCC) has been reported previously in the literature. RCC is a tumor with notoriously unpredictable behavior. The authors report an unusual case of metachronous bilateral testicular metastasis in a patient who operated for RCC. The case highlights the unique behavior of RCC with an unusual site of metastasis. A 72-year-old patient presented with bilateral scrotal swelling of 1-month duration. There was a history of left radical nephrectomy for RCC 4 years prior. He underwent a bilateral high inguinal orchidectomy and diagnosis of chromophobe RCC was made on histopathological examination.
Metachronous metastasis, renal cell carcinoma, testis
Ⅰntroduction
Renal cell carcinoma (RCC) is a relatively rare adult solid tumor accounting for 3.0% of malignancies worldwide. It is an unpredictable entity due to its atypical metastatic prof le at presentation. Thirty percent of these tumors may be accompanied by synchronous metastatic disease at diagnosis.[1]The organs most affected by metastatic spread are: lung, bone, liver, brain, and lymph nodes.[2]However, other structures can also be affected by RCC metastases: eyes, mouth, neck and thyroid, heart, breast, rectum abdominal muscle, intra-scrotal structures, and vagina.[3]Although metastatic foci are present in about 30.0% of RCCs at the time of primary diagnosis (synchronous), metastatic disease can develop as part of the latency of the tumor, with delayed development of metastases, especially if the tumor is well-differentiated.
Case Report
A 72-year-old smoker presented to surgical outpatient department (OPD) with a complaint of a progressively increasing bilateral scrotal swelling of 1-month duration. There was a history of left radical nephrectomy for RCC 4 years prior. His general physical examination was unremarkable. Local examination showed bilateral hard testicular masses, 12 cm × 5 cm on the right side and10 cm × 6 cm on the left side, extending to epididymis, with absent testicular sensation. Examination of the abdomen did not reveal any abnormality. Blood samples for serum lactate dehydrogenase, β human chorionic gonadotropin, and serum alpha fetal protein were sent, which were found to be within normal limits. Metastatic workup was done: contrast-enhanced computed tomography of whole abdomen and pelvis was within normal limits. Ultrasound of testis showed bilateral homogenous enlargement of testis size 10 cm × 5 cm × 3 cm on right side and 10 cm × 5 cm × 3 cm on left side extending to epididymis, with focal areas of necrosis suggestive of testicular malignancy. After all routine hematological and biochemical investigations, he was consented and undertook a bilateral high inguinal orchidectomy. Post-operative period was uneventful.
Biopsy f nding
On gross examination: right testicular mass of 13 cm × 8 cm × 6 cm with cut surface showing a solid variegated appearance. Left testicular mass of 10 cm × 8 cm × 6 cm with cut surface showing a solid variegated appearance. Microscopy revealed malignant tumor cells arranged in large islands and nests in the interstitium. The tumor cells were large with clear cytoplasm in a fair number of cells, and eosinophilic cytoplasm in other cells with moderate nuclear pleomorphism along with some mitotic f gures [Figure 1a]. The seminiferous tubules and epididymis were pushed to one side [Figure 1b], but all the margins were free and testicular vein was not involved on either side.
Immunohistochemistry was done epithelial membrane antigen positive and cytokeratin pan, S100, CD10 were negative. A diagnosis of chromophobe variety of RCC was made.
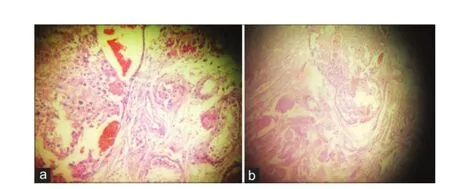
Figure 1:(a) Histopathology shows malignant tumor cells arranged in large islands and nests in the interstitium; (b) histopathology shows the seminiferous tubules and epididymis were pushed to one side
Post-operative follow-up
Patient was followed up every 3 months in surgical OPD. Clinical examination routine hematological investigations and abdominal ultrasound were undertaken at each visit. He was asymptomatic till 2 years post-operatively but is now lost to follow-up.
Discussion
An unpredictable clinical behavior is often characteristic of RCC. The occurrence of metastatic localizations in unusual sites is widely described. The interval between primary diagnosis and the occurrence of distant metastasis can vary from synchronous to very long.[4]RCC metastasizing to testes is rare. Amongst the urinary tract malignancies, prostate is the most common primary site for testicular secondaries constituting 35% of all testicular malignancies.[5]The left testis is more involved than right, and it is believed that metastasis from RCC to left testis occurs via left testicular vein.[6]The pathogenesis of right testicular involvement is less clear and is probably of hematogenous nature by involvement of inferior vena cava by invasion.[7]Tran et al.[8]published a rare case of metastasis of RCC to ipsilateral spermatic cord in 2013. With regard to metastatic testicular involvement, the incidence of secondary testicular tumors ranges from 0.3% to 3.6%.[9]In this case, the patient had bilateral testicular metastasis following RCC similar to simultaneous bilateral testicular metastases from renal clear cell carcinoma.
1. Martel CL, Lara PN. Renal cell carcinoma: current status and future directions. Crit Rev Oncol Hematol 2003;45:177-90.
2. Stankard CE, Karl RC. The treatment of isolated pancreatic metastases from renal cell carcinoma: a surgical review. Am J Gastroenterol 1992;87:1658-60.
3. Pereira BJ, Brandão A, Borges R, Leão R, Grenha V, Coelho H, Peralta P, Sobral F. Spermatic cord metastasis as initial presentation of renal cell carcinoma. Acta Urol Março 2011;1:49-51.
4. Göğüş C, Kiliç O, Tulunay O, Tulunay O, Bedük Y. Solitary metastasis of renal cell carcinoma to the parotid gland 10 years after radical nephrectomy. Int J Urol 2004;11:894-6.
5. Nabi G, Gania MA, Sharma MC. Solitary delayed contralateral testicular metastasis from renal cell carcinoma. Indian J Pathol Microbiol 2001;44:487-8.
6. Steiner G, Heimbach D, Pakos E, Müller S. Simultaneous contralateral testicular metastasis from a renal clear cell carcinoma. Scand J Urol Nephrol 1999;33:136-7.
7. Dieckmann KP, Düe W, Loy V. Intrascrotal metastasis of renal cell carcinoma. Case reports and review of the literature. Eur Urol 1988;15:297-301.
8. Tran M, Daly P, Tran T, Heathcote P. Renal cell carcinoma metastases to the spermatic cord: review of the literature and case presentation. J Clin Urol 2013;6:427-31.
9. Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep 2011;5:429.
How to cite this article:Nain S, Kharkwal S, Kaur O. Metachronous metastasis of renal cell carcinoma to bilateral testis. J Cancer Metastasis Treat 2015;1:39-40.
Received:11-01-2015;Accepted:09-03-2015.
Source of Support:Nil,Conf ict of Interest:None declared.
Dr. Sameer Nain, Department of Surgery, ESI PGIMSR Hospital, Basaidarapur, New Delhi 110015, India. E-mail: sameer.nain@gmail.com
10.4103/2394-4722.153913
 Journal of Cancer Metastasis and Treatment2015年1期
Journal of Cancer Metastasis and Treatment2015年1期
- Journal of Cancer Metastasis and Treatment的其它文章
- Patient-derived xenograft models for oncology drug discovery
- Cancer preventing spices
- Complete response with fotemustine and bevacizumab after early progression following radiotherapy and temozolomide treatment in patient with glioblastoma multiforme
- Malignant ascites with omental metastasis: a rare event in prostate cancer
- Early stage squamous cell carcinoma of the tonsil presenting with multiple organ metastases including skin and brain after successful local treatment
- Incidence of bone metastasis in squamous cell carcinoma of the buccal mucosa
