Use of nerve elongator to repair short-distance peripheral nerve defects: a prospective randomized study
Lu Bai, Tian-bing Wang, Xin Wang, Wei-wen Zhang, Ji-hai Xu, Xiao-ming Cai, Dan-ya Zhou, Li-bing Cai, Jia-dong Pan, Min-tao Tian, Hong Chen,, Dian-ying Zhang, Zhong-guo Fu, Pei-xun Zhang,, Bao-guo Jiang,
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Hand Surgery, Ningbo No.6 Hospital, Ningbo, Zhejiang Province, China
Use of nerve elongator to repair short-distance peripheral nerve defects: a prospective randomized study
Lu Bai1, Tian-bing Wang1, Xin Wang2, Wei-wen Zhang2, Ji-hai Xu2, Xiao-ming Cai2, Dan-ya Zhou2, Li-bing Cai2, Jia-dong Pan2, Min-tao Tian2, Hong Chen2,*, Dian-ying Zhang1, Zhong-guo Fu1, Pei-xun Zhang1,*, Bao-guo Jiang1,*
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Hand Surgery, Ningbo No.6 Hospital, Ningbo, Zhejiang Province, China
Repair techniques for short-distance peripheral nerve defects, including adjacent joint fexion to reduce the distance between the nerve stump defects, “nerve splint” suturing, and nerve sle eve connection, have some disadvantages. Therefore, we designed a repair technique involving intraoperative tension-free application of a nerve elongator and obtained good outcomes in the repair of short-distance peripheral nerve defects in a previous animal study. The present study compared the clinical outcomes between the use of this nerve elongator and performance of the conventional method in the repair of short-distance transection injuries in human elbows. The 3-, 6-, and 12-month postoperative follow-up results demonstrated that early neurological function recovery was better in the nerve elongation group than in the conventional group, but no significant difference in long-term neurological function recovery was detected between the two gro ups. In the nerve elongation group, the nerves were sutured without tension, and the duration of postoperative immobilization of the elbow was decreased. Elbow function rehabilitation was signifcantly better in the nerve elongation group than in the control group. Moreover, there were no security risks. The results of this study confrm that the use of this nerve elongator for repair of short-distance peripheral nerve defects is safe and effective.
nerve regeneration; peripheral nerve deficiency; nerve elongator; British Medical Research Council scale; neurological function; prognosis; NSFC grants; neural regeneration
Funding:This study was supported by grants from the National Natural Science Foundation of China, No. 31171150, 31271284, 81171146, 30971526, 30801169; the National Program on Key Basic Research Project of China (973 Program), No. 2014CB542206; Program for New Star in Science and Technology of Beijing of China, No. A-2008-10; Program for New Century Excellent Talents in University of Ministry of Education of China, No. BMU20110270; and the National Natural Science Foundation of China for Distinguished Youth, No. 31100860.
Bai L, Wang TB, Wang X, Zhang WW, Xu JH, Cai XM, Zhou DY, Cai LB, Pan JD, Tian MT, Chen H, Zhang DY, Fu ZG, Zhang PX, Jiang BG (2015) Use of nerve elongator to repair short-distance peripheral nerve defects: a prospective randomized study. Neural Regen Res 10(1):79-83.
Introduction
Peripheral nerve regeneration is a slow process, and its clinical outcome is often poor. No breakthroughs have been made in the repair of transection injuries of peripheral nerves in the past 10 years. Clinical outcomes worsen if nerve injuries are combined with broken nerve ends (Barbaro, 2011). Clinical research on peripheral nerve defects has importance for further studies on nerve regeneration and restoration.
Traumatic nerve transection injuries are often combined with crushing of the nerve stoma and defects associated with the broken nerve ends. In surgical management of such injuries, a 1- to 3-cm defect is always left after debridement of the broken nerve ends. Such defects can be resolved by the following three methods: (1) flexion/extension of an adjacent joint to decrease the distance between the broken ends (Wolfe and Hotchkiss, 2010), (2) use of a modified splint technique to reduce tension on the nerve (Hall and Buncke, 1981; Jabaley, 1991), and (3) nerve tubulization, in which a chitin tube is used to fll the gap and create a selective growth environment (Konofaos and Ver Halen, 2013). However, these three methods have some defciencies. Joint fexion/extension may fll the gap and decrease anastomotic tension, but fxation with a plaster stone for 6 weeks is required, which may cause joint stiffness (Müller et al., 2013). Direct suturing may increase tension at the anastomosis site and lead to transient nerve ischemia, electrophysiological changes, or new nerve injury; furthermore, postoperative immobilization of the joint may lead to joint stiffness and muscle atrophy (Abe et al., 2004; Alluin et al., 2009; Barbaro, 2011). Nerve tubulization is a good choice for selective regeneration, but is not widely used in the clinical setting.
We created an intraoperative elongation technique to overcome the disadvantages of the direct suture technique. A nerve elongator that was used in an experimental rabbit study exhibited beneficial effects (Baoguo et al., 2004). We assumed that this elongator would also have certain benefcial effects for human nerve defects.
In the present study, we presumed that the elongator would allow for regional tension-free nerve suturing and elevate clinical effects. We performed a randomized controlled trial to answer the following questions: Can broken end defects be intraoperatively extended 2 to 3 cm by the elongator? If the broken ends of the nerve trunk are sutured under little tension, the duration of splint immobilization can be shortened; therefore, does use of the elongator accelerate the recovery of adjacent joint function?
Subjects and Methods
Subjects
From August to November 2011, 18 patients with peripheral nerve injury of the upper extremity were treated with nerve debridement and microsurgical repair. Five patients were excluded: four had moved and could not be contacted, and one was involved in a traffc accident. Thus, this study included 13 patients (11 male, 2 female) with an average age of 32.6 ± 9.5 years (range, 19-47 years). The median delay between trauma and surgery was 3.6 hours (range, 2-6 hours). All participants provided written informed consent. The study protocol was approved by the Ethics Committee of People’s Hospital of Peking University in China and was performed in accordance with the Helsinki Declaration. The approval was authorized in 2008 16# File of Clinical Trial Approval. The 13 patients’ general characteristics are shown inTable 1.
The patients were randomly divided into a nerve elongation group and a control group. In the nerve elongation group, lengthening surgery was performed at the distal end of the injured nerve using the above-mentioned nerve elongator. The nerve was sutured without tension. Patients in the control group were treated with the classic technique: fexion of the elbow or stretching of the nerve to match the nerve endings.
Microsurgical procedures
All procedures were performed by one group of experienced surgeons. All patients were treated with a standard antithrombotic prophylaxis regimen. Mecobalamin (WeiCai China Pharmaceutical Co., Ltd., Suzhou, Jiangsu Province, China) was used as a nerve nutrient in all patients (0.5 mg orally, three times a day for 6 weeks). Debridement was routinely performed. Concurrent injuries such as vascular injury or tendon rupture were repaired frst. Before nerve repair, the distance between the two nerve ends was measured. In the nerve elongation group, the broken ends were exposed for debridement. The distal end of the injured nerve was fxed to the nerve elongator by a soft rubber band. Saline was slowly injected into the elongator to cause the distal nerve fbers to elongate at an approximately constant speed. After debridement, 8 to 10 cm of the distal end of the involved nerve was exposed. Elongation of the gap by 1 to 2 cm required 20 minutes. The nerve elongator and surgical procedures are shown inFigures 1and2. Prolene (8-0; Ethicon Johnson & Johnson, Somerville, NJ, USA) was used as the suture material.
Postoperative splint immobilization was performed to create stable fxation of the repaired tendon. In the nerve elongation group, splint fxation was conducted for 4 weeks. In the control group, 6 weeks of immobilization was needed to prevent elbow extension and protect the nerve anastomosis and repaired tendon without tension. Rehabilitation training was routinely performed in all patients. After splint removal, joint exercises were performed gradually. At 6 weeks after surgery, full active range-of-fexion/extension exercises were required. At 9 to 12 weeks, progressive core stability exercises and muscle strength training were performed.
Follow-up
The patients underwent physical examinations on an outpatient basis at 3, 6, and 12 months postoperatively. Nerve recovery was assessed using the British Medical Research Council scale (Seddon, 1975). Using this scale, muscle function was assigned a grade of M0 (no muscle contracture) to M5 (totally recovered). Sensation was assigned a grade of S0 (no sensation) to S4 (normal sensation). Elbow function was evaluated using the Disabilities of the Arm, Shoulder, and Hand score and Mayoelbow score (Hudak et al., 1996).
Complications and safety control
Vital signs of all patients were monitored during hospitalization. All adverse events possibly associated with nerve elongation were recorded and evaluated. The physiological conditions of all patients were compared between the two groups (Table 2).
Statistical analysis
Statistical analysis was performed with SPSS 18.0 software (SPSS, Chicago, IL, USA). All measurement data are presented as the mean ± SD. Data were compared with the unpaired t-test, and categorical data were compared with the chisquare test and Fisher’s exact test. A significant difference was defned as that with a P < 0.05.
Results
Nerve elongation improved neurological function recoveryAfter 3 months of follow-up, the British Medical Research Council function grades were better in the nerve elongation group than in the control group (χ2= 7.252, P = 0.027). No statistically significant difference was found in the British Medical Research Council function grades between the two groups as time progressed (6 months: χ2= 2.270, P = 0.321; 12 months: χ2= 3.343, P = 0.342;Figure 3A). No signifcant difference was observed in the British Medical Research Council sensation grades between the two groups (P > 0.05;Figure 3B).
Nerve elongation improved elbow function recovery
At 3, 6, and 12 months of follow-up, elbow function wasbetter in the nerve elongation group than in the control group (Figure 4).
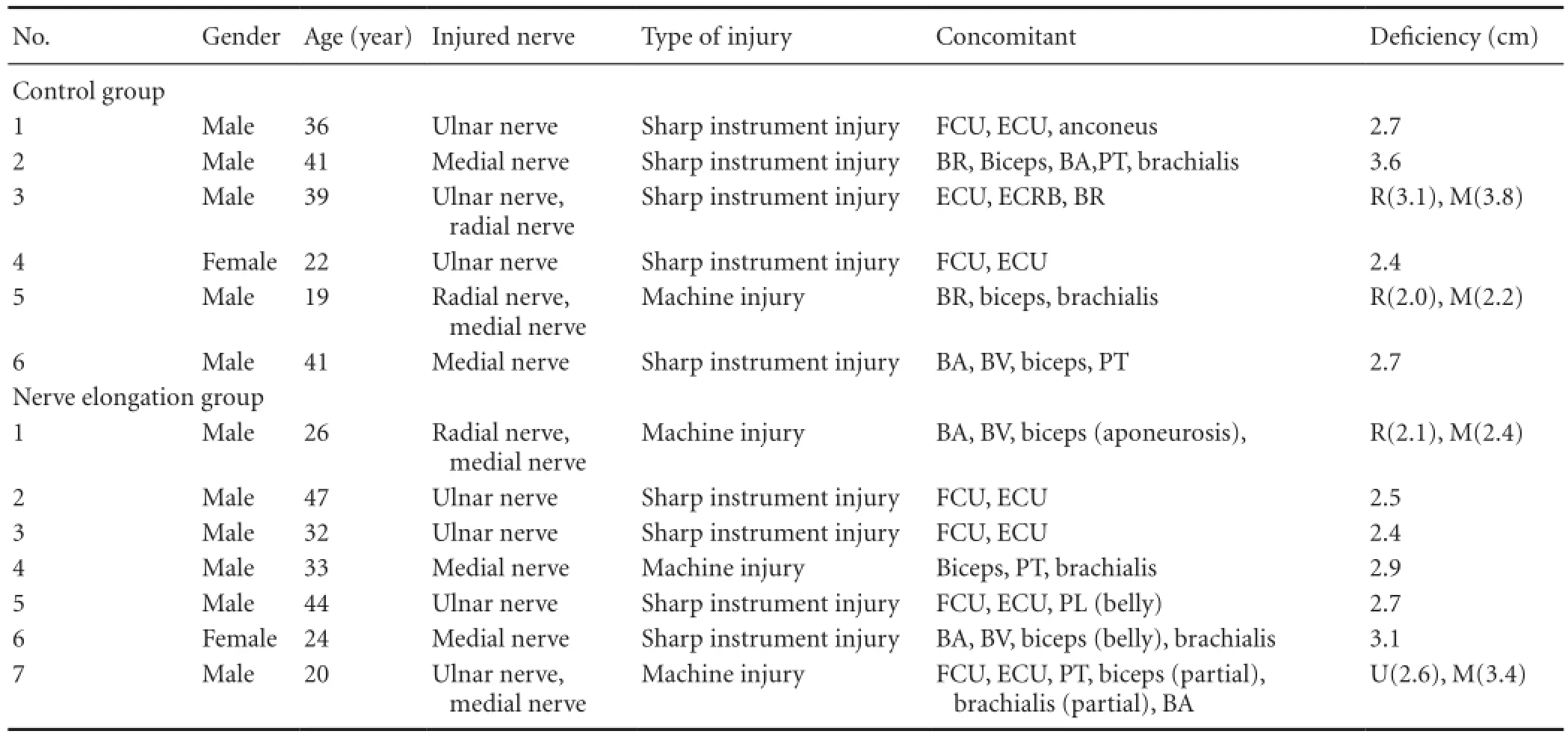
Table 1 Patients’ clinical characteristics
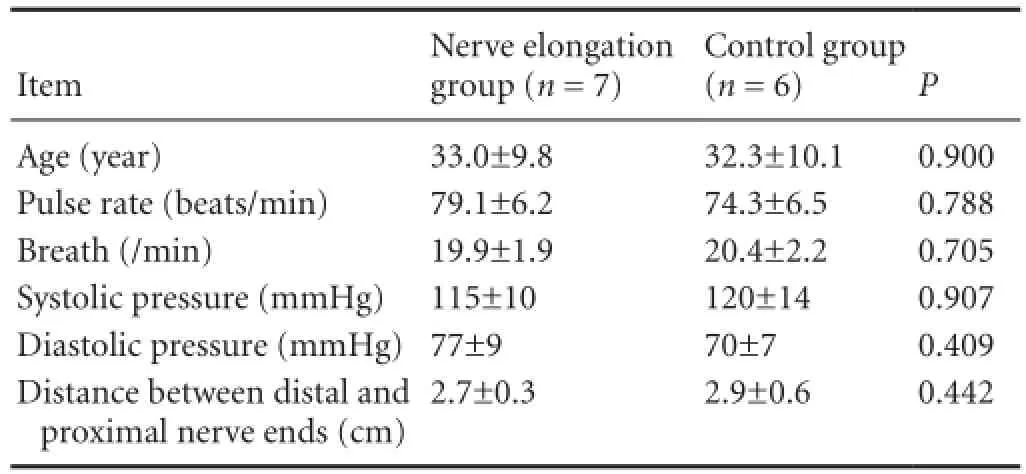
Table 2 Physiological conditions of all patients
Discussion
In recent studies, selective nerve regeneration (Ichihara et al., 2008; Jiang et al., 2010), protection of targeted muscles, and improvement of the microenvironment of the injured site (delayed release of nerve growth factor, blood supply to injured ends, and tension-free suturing) were the main methods used to obtain better peripheral nerve injury repair outcomes (Clark et al., 1992; Barbaro, 2011). In the clinical setting, injured peripheral nerves in periarticular regions often had to be sutured under tension. Based on our experience, if the length of the nerve defect is more than four times the diameter of the nerve, substantial tension would be present at the anastomotic stoma. A conventional technique involves flexion of the joint and wide dissection to reduce tension. However, this does not eliminate the tension at the nerve ends. Conversely, wide dissection may harm the blood supply to the epineurium, and joint immobilization may increase the risk of joint stiffness and tendon adhesion.

Figure 1 Illustration of tissue expander (nerve elongator) used for nerve elongation.
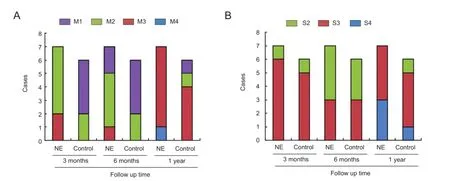
Figure 3 Effects of nerve elongation on neurological function.
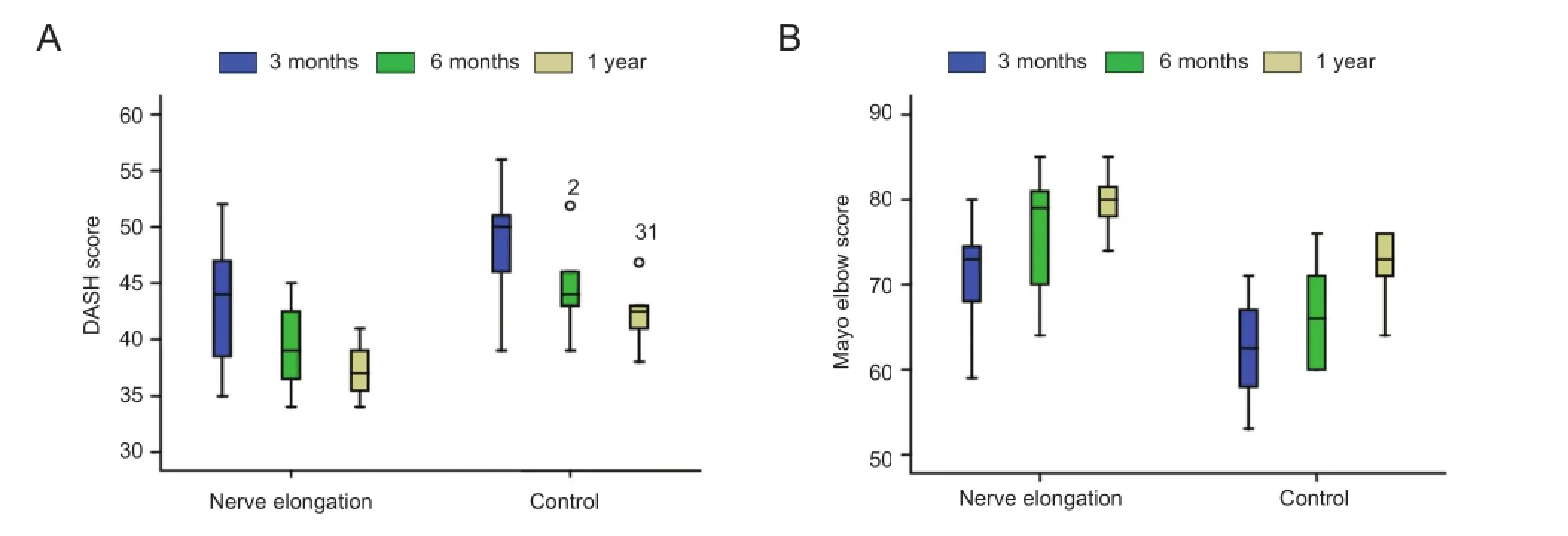
Figure 4 Effects of nerve elongation on elbow function.

Figure 2 Surgical application of nerve elongator.
Anastomotic tension may adversely affect nerve regeneration. Our hypothesis is that if tension of the anastomotic stoma could be reduced during the operation, the blood supply and regeneration environment of the nerve trunk may be preserved. This may lead to better clinical outcomes. Furthermore, if we can provide a tension-free anastomosis, the elbow joint would not need to be fxed for a long period of time, and exercise can be performed in the early postoperative period. According to previous nerve elongation studies involving acute nerve stretching, acute elongation up to 6% of the length of the nerve may lead to a 70% decline in the potential conduction velocity, and stretching of more than 12% may lead to irreversible damage (Clark et al., 1992; Abe et al., 1996; Driscoll et al., 2002). Additionally, tension may affect the blood flow of the nerve fibers (Clark et al., 1992). If a peripheral nerve is acutely stretched by 10% of the length of the nerve, the node of Ranvier may widen and the bands of Fontana would disappear; at acute stretching of more than 20%, the node of Ranvier would fracture (Baoguo et al., 2004; Wang et al., 2010). These phenomena suggest the feasibility of intraoperative elongation of the nerve trunk. Manders et al. (1987) frst reported that defects can be flled by nerve elongation with a tissue expander. Their results showed no difference between nerve elongation and nerve transplantation. Gradual elongation of medial nerves and suturing without tension in rabbits resulted in outcomesequally as good as those achieved with nerve transplantation (Baoguo et al., 2004). A better result was obtained using a tissue expander than the conventional technique to elongate nerves (Jou et al., 2000). If the gap between the broken ends of a nerve can be sutured in one stage, the result would be better than that achieved with nerve transplantation (Baoguo et al., 2004; Matsuzaki et al., 2004; Pfster et al., 2011). Thus, one-stage gradual nerve elongation and “tension-free” anastomosis appear to have the potential to cure peripheral nerve injury.
Some previous studies on nerve elongation produced discouraging results. Nogueira et al. (2003) showed that 9.3% of patients who underwent lower limb lengthening (1 mm per day in four sessions) developed nerve lesions. Simpson et al. (2013) reported signifcant electrophysiological changes during limb lengthening. A nerve stretch test in rabbits (Ikeda et al., 2000) found that lengthening at 0.8 mm per day may not harm the nerves. The above evidence seems to discourage the performance of intraoperative elongation of injured nerves. In the present study, only the distal end of the affected nerve was lengthened. This is because when the nerve trunk begins to regenerate, it does so proximal to distal. Therefore, the distal end was not involved until the regeneration arrived at the anastomotic stoma. For this reason, we elongated the distal end to reduce the tension and protect the proximal end from stretching. The nerve elongation group showed better early motion recovery. Intraoperative nerve stretching could not be avoided, and its clinical manifestation was the transient loss of neural function. Elongation of the distal end of the nerve may decrease stretch injury to the proximal nerve fbers. No signifcant differences were observed in the sensation grade between the two groups. Motor nerve fbers may be more sensitive to elongation than sensory nerve fbers. Another advantage of nerve elongation is that better elbow and upper extremity function recovery is obtained than with the conventional technique. A shorter immobilization duration and earlier exercise may contribute to this better functional recovery.
Absolute randomization among the patients was diffcult owing to the nature of their condition (peripheral nerve injury). A limitation of this study is that we intentionally focused on cases of periarticular elbow injury to decrease the differences among the cases.
Author contributions:LB, HC, PXZ, and BGJ designed the study and wrote the paper. LB, TBW, XW, WWZ, JHX, XMC, DYZ, LBC, JDP, MTT, DYZ, and ZGF performed the study and analyzed the data. All authors approved the final version of the paper.
Conficts of interest:None declared.
Abe I, Ochiai N, Ichimura H, Tsujino A, Sun J, Hara Y (2004) Internodes can nearly double in length with gradual elongation of the adult rat sciatic nerve. J Orthopaed Res 22:571-577.
Abe Y, Doi K, Katoh Y, Yamamoto H, Kataoka H, Kawai S (1996) The limit of low speed peripheral nerve elongation; neurological and circulatory aspects. J Neurol Sci 140:61-66.
Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P (2009) Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30:363-373.
Baoguo J, Shibata M, Matsuzaki H, Takahashi HE (2004) Proximal nerve elongation vs. nerve grafting in repairing segmental nerve defects in rabbits. Microsurgery 24:213-217.
Barbaro NM (2011) Peripheral nerve injury. J Neurosurg 114:1516-1519.
Clark WL, Trumble TE, Swiontkowski MF, Tencer AF (1992) Nerve tension and blood fow in a rat model of immediate and delayed repairs. J Hand Surg Am 17:677-687.
Driscoll PJ, Glasby MA, Lawson GM (2002) An in vivo study of peripheral nerves in continuity: biomechanical and physiological responses to elongation. J Orthopaed Res 20:370-375.
Hall EJ, Buncke HJ (1981) Microsurgical techniques to reconstruct irreparable nerve loss. Orthop Clin North Am 12:381-401.
Hudak PL, Amadio PC, Bombardier C (1996) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 29:602-608.
Ichihara S, Inada Y, Nakamura T (2008) Artifcial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury 39 Suppl 4:29-39.
Ikeda K, Tomita K, Tanaka S (2000) Experimental study of peripheral nerve injury during gradual limb elongation. Hand Surg 5:41-47.
Jabaley ME (1991) Modified technique of nerve repair: epineurial splint. In: Operative Nerve Repair and Reconstruction (Gelberman RH, ed). Philadelphia: JB Lippincott.
Jiang B, Zhang P, Jiang B (2010) Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol 38:1-4.
Jou IM, Lai KA, Shen CL, Yamano Y (2000) Changes in conduction, blood fow, histology, and neurological status following acute nervestretch injury induced by femoral lengthening. J Orthop Res 18:149-155.
Konofaos P, Ver Halen JP (2013) Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg 29:149-164.
Müller AM, Sadoghi P, Lucas R, Audige L, Delaney R, Klein M, Valderrabano V, Vavken P (2013) Effectiveness of bracing in the treatment of nonosseous restriction of elbow mobility: a systematic review and meta-analysis of 13 studies. J Shoulder Elbow Surg 22:1146-1152.
Manders EK, Saggers GC, Diaz-Alonso P, Finn L, Sipio JC, Glumac T, Au VK, Wong RK, Mottaleb M (1987) Elongation of peripheral nerve and viscera containing smooth muscle. Clin Plast Surg 14:551-562.
Matsuzaki H, Shibata M, Jiang B, Takahashi HE (2004) Distal nerve elongation vs. nerve grafting in repairing segmental nerve defects in rabbits. Microsurgery 24:207-212.
Nogueira MP, Paley D, Bhave A, Herbert A, Nocente C, Herzenberg JE (2003) Nerve lesions associated with limb-lengthening. J Bone Joint Surg Am 85-A:1502-1510.
Pfster BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK (2011) Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng 39:81-124.
Seddon HJ (1975) Results of Repair of the Nerves. Edinburgh: Churchill Livingstone.
Simpson AHRW, Halliday J, Hamilton DF, Smith M, Mills K (2013) Limb lengthening and peripheral nerve function-factors associated with deterioration of conduction. Acta Orthop 84:579-584.
Wang W, Zhang P, Yan J, Han N, Kou Y, Zhang H, Jiang B (2010) Histological analysis of single peripheral nerve fber in acute nerve elongation process. Artif Cells Blood Substit Immobil Biotechnol 38:165-168.
Wolfe SW, Hotchkiss RN (2010) Green’s Operative Hand Surgery. Philadelphia: Churchill Livingstone.
Copyedited by Morben A, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Bao-guo Jiang, M.D., jiangbaoguo@vip.sina.com. Pei-xun Zhang, M.D., zhangpeixun@126.com. Hong Chen, chenhong_6612@163.com.
10.4103/1673-5374.150710
http://www.nrronline.org/
Accepted: 2014-12-11
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

