Large animal models of human cauda equina injury and repair: evaluation of a novel goat model
Wen-tao Chen, Pei-xun Zhang,, Feng Xue,, Xiao-feng Yin, Cao-yuan Qi, Jun Ma, Bo Chen, You-lai Yu, Jiu-xu Deng, Bao-guo Jiang,
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Radiology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
Large animal models of human cauda equina injury and repair: evaluation of a novel goat model
Wen-tao Chen1, Pei-xun Zhang1,*, Feng Xue1,*, Xiao-feng Yin1, Cao-yuan Qi2, Jun Ma2, Bo Chen1, You-lai Yu1, Jiu-xu Deng1, Bao-guo Jiang1,*
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Radiology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
Previous animal studies of cauda equina injury have primarily used rat models, which display signifcant differences from humans. Furthermore, most studies have focused on electrophysiological examination. To better mimic the outcome after surgical repair of cauda equina injury, a novel animal model was established in the goat. Electrophysiological, histological and magnetic resonance imaging methods were used to evaluate the morphological and functional outcome after cauda equina injury and end-to-end suture. Our results demonstrate successful establishment of the goat experimental model of cauda equina injury. This novel model can provide detailed information on the nerve regenerative process following surgical repair of cauda equina injury.
nerve regeneration; spinal cord injury; goat; animal model; radiography; magnetic resonance imaging; diffusion tensor imaging; fiber bundle; diagnosis; injury; physiology; neuroimaging; NSFC grants; neural regeneration
Funding:This study was supported by grants from the National Program on Key Basic Research Project of China (973 Program), No. 2014CB542200; Program for Innovative Research Team in University of Ministry of Education of China, No. IRT1201; the National Natural Science Foundation of China, No. 31271284, 31171150, 81171146, 30971526, 31040043, 31371210, 81372044, 31471144; Program for New Century Excellent Talents in University of Ministry of Education of China, No. BMU20110270; and the Natural Science Foundation of Beijing of China, No. 7142164.
Chen WT, Zhang PX, Xue F, Yin XF, Qi CY, Ma J, Chen B, Yu YL, Deng JX, Jiang BG (2015) Large animal models of human cauda equina injury and repair: evaluation of a novel goat model. Neural Regen Res 10(1):60-64.
Introduction
Attempts at repairing the injured cauda equina have had limited success (LeBlanc et al., 1969; Gertzbein et al., 1988). Nonetheless, there are a number of successful cases of cauda equina repair in both experimental studies and in the clinical setting (Cheng et al., 2004). Previous studies have shown that the transected nerve roots can be surgically repaired and axonal regeneration achieved (Conzen and Sollmann, 1985). However, it is diffcult to repair the fragile nerve roots without attachment to an intact epineurium (LeBlanc et al., 1969; Konno et al., 1995). Little is known about cauda equina regeneration following transection. Most animal models of cauda equina injury use rats, and have a number of shortcomings (Blaskiewicz et al., 2009; Mackenzie et al., 2012). The cauda equina is much smaller in rats than in humans, making surgery and repair difficult. A novel experimental animal model mimicking cauda equina injury, allowing for surgical repair and monitoring of surgical outcome, was developed in our laboratory. There are three major aims of our study: (1) to establish a novel animal model that mimics intrathecal cauda equina injury and that allows for surgical repair of the spinal cord; (2) to identify the electrophysiological properties of muscles innervated by the cauda equina; and (3) to develop effective methods to evaluate cauda equina morphological and functional recovery using histology and MRI.
Materials and Methods
Animals
Three 6-month-old male goats, weighing 20, 24 and 27 kg (Lvyuanweiye Farm, Shunyi District, Beijing, China; license No. 2013-60), were used in this pilot study. The study protocol was carried out in strict accordance with the recommendations of the Institutional Animal Committee of Experimental Animals, Peking University People’s Hospital (Beijing, China). Anesthesia was performed using intramuscular injection of Sumianxin II combined with ketamine (1:1, v/v), 0.2 mL/kg. Goats were raised as a closed herd and kept under a strict quarantine protocol.
Modeling procedure
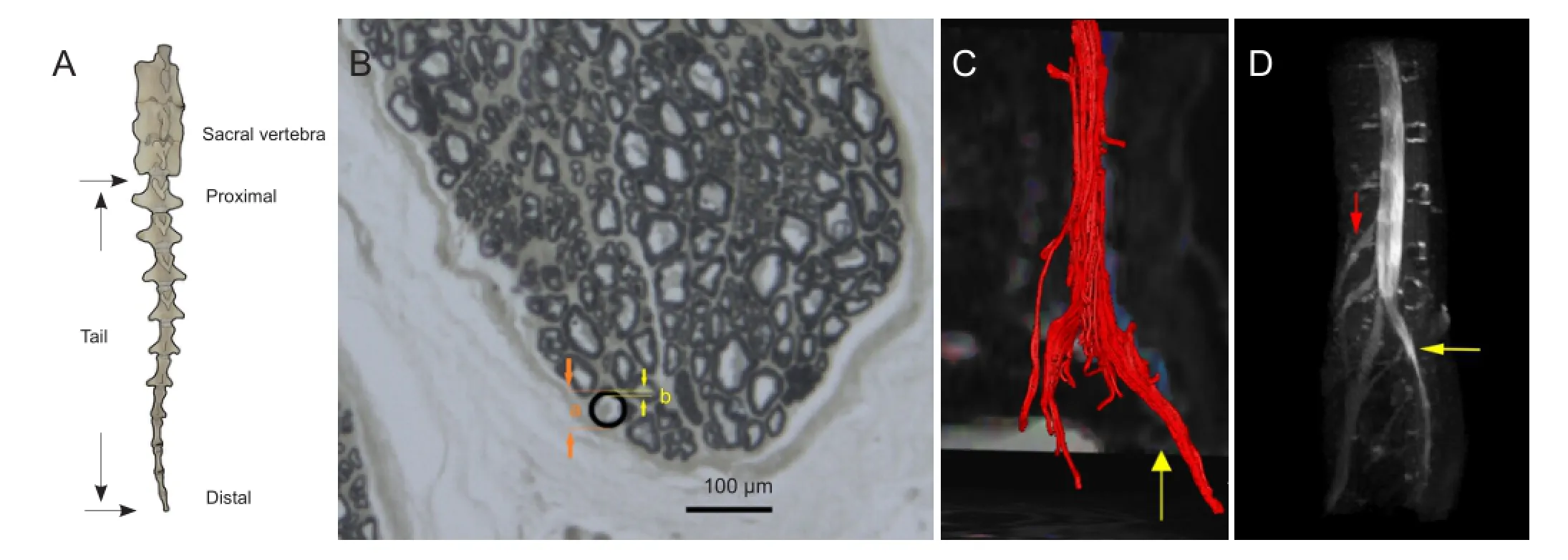
Figure 2 The gross skeletal anatomy of the goat tail, morphology of the goat cauda equina and magnetic resonance reconstruction images of the normal goat cauda equina.
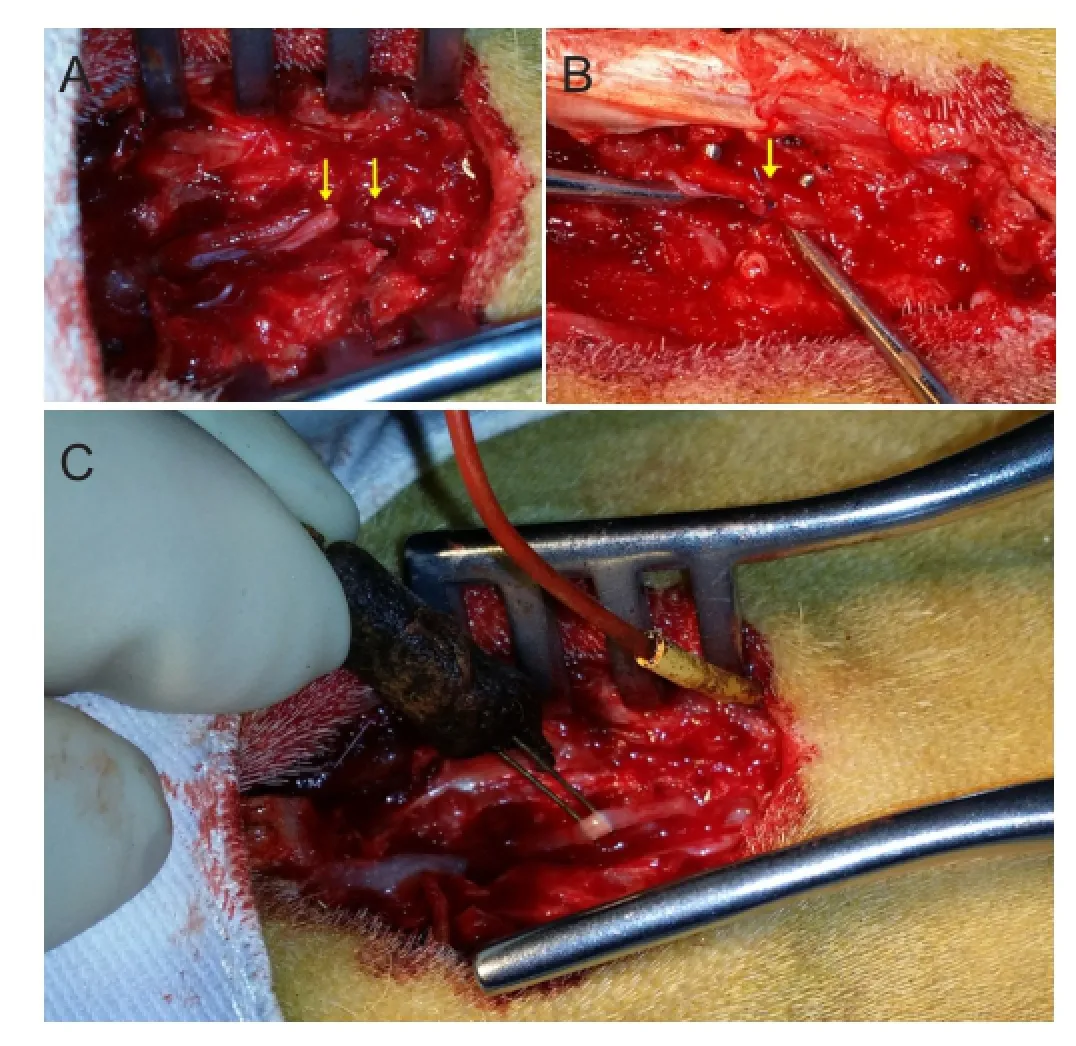
Figure 1 The surgical procedure for repairing cauda equina injury and placement of electrodes for electrophysiological examination of cauda equina nerves in goats.
The skin over the lumbosacral junction was shaved. The goats were fxed in the prone position for surgery. After routine disinfection and draping, a 5.0-6.0-cm longitudinal skin incision was made straight along the spinous processes originating from the posterior superior iliac spine. The subcutaneous tissue and fascia were incised to expose the lamina. The tissues were retracted laterally, and the posterior lamina was removed using a rongeur and micro-curette. Then, a window in the vertebral lamina was formed via the posterior approach under direct visualization of the spinal cord. The dura was then cut, and the cauda equina was exposed and isolated. After the electrophysiological examination was done, all cauda equina nerves were transected and repaired with end-to-end suture (Figure 1A, B). The dura was then closed with a 6-0 suture. The procedure was performed by the same senior doctor.
Electrophysiological examination
After exposure and isolation of the cauda equina, multichannel stimulus-evoked electromyography was used to monitor action potentials of tail muscles innervated by the cauda equina. A spaced (approximately 1-mm) pair of wire hooks were placed gently onto the cauda equina for bipolar stimulation. To avoid contact with cerebrospinal fluid, the hooks were elevated slightly (Figure 1C). Four pairs of parallel needle electrodes were inserted symmetrically along the tail and connected to preamplifers for recording evoked electromyograhies. Every single cauda equina nerve was stimulated. Single-pulse electrical stimulation (0.09 mA in intensity, 0.1 ms in duration) (MedlecSynergy; Oxford Instrument Inc., Oxford, UK) was applied to the cauda equina. The initial intensity was 0.06 mA, and then adjusted to 0.09 mA to evoke the electromyography response from the tail muscles. When the test was completed, the nerve was transected. Stimulation was then applied to the proximal stump of the transected nerve, and the electromyography was recorded. Action potentials evoked by the electrical stimulation of the nerve were recorded. The amplitude and duration of action potentials preoperatively and postoperatively were compared.
Histological examination

Figure 3 Electromyography recordings from the four recording electrodes (0.09 mA electrical stimulation) before and after transection of the cauda equina.

Table 1 The fractional anisotropy values and apparent diffusion coefficients for different slices by magnetic resonance diffusion tensor imaging
Several tissue samples were obtained from the cauda equina and cut into 2-mm pieces. The samples were fixed in 4% formaldehyde in 0.1 mM phosphate buffer for 24 hours at 4°C and then rinsed in water for 12 hours. After this step, the samples were stained in 1% osmium tetroxide for 12 hours, rinsed in water for 6 hours, immersed in neutral buffered formalin twice for 10 seconds, dehydrated through a graded ethanol series (50%, 70%, 90%, 95%, absolute), immersed in xylene twice for 40 seconds, and embedded in paraffn for sectioning. The blocks were cut into 2-µm-thick cross-sections.
The images were observed under a DFC 300FX color digital camera (Leica, Heidelberg, Germany). The total number and diameter of myelinated axons were measured (Wang et al., 2013). The thickness of the myelin was subsequently evaluated. The number and diameter of the proximal and distal ends of the transected cauda equina will be determined and compared at different postoperative time points in a future study.
MRI procedure
All images were acquired with a 3.0T MR scanner (Siemens Healthcare, Erlangen, Germany). The animals were placed on the surface coil in the supine position. The protocol consisted of spin-echo (repetition time = 4.0 seconds, echo time = 98 ms, feld of view = 350 cm × 350 cm, slice thickness = 4.0 mm), half-Fourier acquisition single-shot turbo spin-echo, SPACE-STIR, MEDIC, and diffusion tensor imaging (DTI; repetition time = 4.0 seconds, echo time = 98 ms, field of view = 280 cm × 280 cm, slice thickness = 4.0 mm) sequences. The parameters for the DTI sequence were as follows: spatial resolution, 2.0 mm × 2.0 mm × 4.0 mm; coverage area, L2-coccygeal vertebra; b-value, 600; scanning time, 14 minutes 59 seconds. A volume coil and surface coil (Siemens Healthcare, Erlangen, Germany) were used in the scanning. The inside diameter of the volume coil was 49 cm, and was used for radiofrequency transmission. The diameter of the surface coil was 52 cm, and was used for receiving radiofrequency signals. Image reconstruction for tractography was performed using a workstation (Neuro 3D, Siemens, Germany) by selecting regions of interest. The region of interest was between the middle part of L4and the inferior part of S1. Fractional anisotropy (0.2) and angle threshold (30 degrees) were used for tractography. The complete cauda equina couldbe visualized and compared in reconstructed tractography images.
Results
Gross anatomy of the goat cauda equina
Macroscopic anatomical visualization showed that the goat tail is composed of 11 vertebrae, of which the distal five have no transverse process that may serve to attach the tail muscles. The seventh coccygeal vertebra was relatively small and had a dumbbell shape that was quite different from the first to sixth vertebrae (Figure 2A). Six major erector spinae muscles covered the ventral side of the tail, and two the dorsal. There were intrinsic muscles and ligaments attaching the adjacent vertebra. Intrinsic tail muscles and musculus sacrococcygeus might control the movement of the tail. Gross anatomy indicated that the terminal part of the spinal cord extended into the cauda equina at the level of the posterior superior iliac spine. The region between the posterior superior iliac spine and the proximal end of the tail can be used to fully expose the cauda equina. In the goat, the cauda equina, with a comparatively large diameter, is more clearly visible than in the rat. The cauda equina was divided into several bundles (range 4 to 6) with an intact perineurium. Before the surgery, the goats often swung their tails in a circular arc, which did not appear to affect body stability or balance, which may be in contrast to the rat. All the goats tolerated the surgery and were returned to their herd for free movement. No obvious dysfunction of the hindlimb or bowel or bladder incontinence was found postoperatively.
Histology of the goat cauda equina
The cauda equina was stained with osmium tetroxide. The number and diameter of the nerves could be enumerated. Microscopic images revealed that the goat cauda equina had some similarities to the human cauda equina, in the structure of the myelin sheath and perineurium (Figure 2B).
MRI characteristics of the goat cauda equina
MR DTI reconstruction showed the continuous integrity of the cauda equina axons and myelin, and the distribution of the nerve roots (Figure 2C, D). The nerve bundles were colored red during image reconstruction, and were easy to distinguish. As shown inTable 1, the fractional anisotropy value and apparent diffusion coeffcient of the proximal part seemed not very different from those for the distal cauda equina (Table 1). Changes in fractional anisotropy values and apparent diffusion coefficients can serve as adjunctive diagnostic and prognostic tools in the rehabilitation of cauda equina injury in the future.
Electrophysiological examination of the goat cauda equina before and after injury
Electrical stimulation was applied to different portions of the cauda equina, and the four recording electrodes were used to monitor action potentials. Electrical stimulation on every single cauda equina site produced an electromyography response in the four recording electrodes (Figure 3). When a portion of the cauda equina was cut, the recording electrode also monitored evoked Electromyograhies following electrical stimulation of the left cauda equina. When all cauda equina were transected, no evoked electromyography was recorded in the tail (Figure 3).
Discussion
Fracture of the spine can lead to spinal cord injury involving the cauda equina. The tone of the urinary and anal sphincter is controlled by the cauda equina in humans, and cauda equina injury can lead to severe back pain, saddle anesthesia, bladder and bowel dysfunction, weakness of the lower extremity muscles, and sexual dysfunction. Several attempts have been made to repair the damaged cauda equina. Some studies reported unsatisfactory outcomes (LeBlanc et al., 1969; Gertzbein et al., 1988). However, some studies have reported successful outcomes in experimental studies and in the clinical setting (Cheng et al., 1996; Cheng et al., 2004). It is necessary to evaluate outcomes following cauda equina injury and repair using animal experiments. Previous animal experimental studies focusing on the identification of extrathecal nerve roots in rats have shown promising results (Blaskiewicz et al., 2009; Mackenzie et al., 2012). However, the cauda equina in rats is difficult to observe without surgical microscopes. In contrast, the cauda equina in large animals is amenable to unassisted visual inspection, and the nerve diameters are similar to those in the human. Primates, such as the rhesus monkey, are ideal as models for cauda equina injury and repair. However, primates are expensive and their use is accompanied by major ethical considerations. In comparison, goats are widely available and inexpensive. However, little is known about the anatomy, histology or electrophysiology of the cauda equina in goats.
Previous studies have examined the electrophysiological characteristics of extrathecal nerve roots in the rat model (Blaskiewicz et al., 2009; Mackenzie et al., 2012). Stimulus-evoked electromyography is often used in spine surgery and peripheral nerve surgery (Bose et al., 2002; Holland, 2002; Luo et al., 2012). Furthermore, it is an effective technique for the diagnosis and prognosis of nerve injury. Accordingly, the recruitment patterns of the muscles controlling the goat tail were examined in this study using stimulus-evoked electromyography.
Electrophysiology showed that the muscles in the tail were not innervated exclusively by a single cauda equina nerve. Even with little cauda equina remaining, the function of the tail was partially preserved, and electromyography activity in the tail muscles could also be observed. Therefore, we postulate that the cauda equina nerves cross-innervate the tail muscles. Thus, if a part of the cauda equina is damaged, the function of the target muscles could still be restored. The cauda equina could serve as a nerve donor for nerve defect repair. Electrophysiology is useful for evaluating the recovery process following cauda equina repair after transection, and can be used to assess the functional recovery of tail muscles.
Using osmium tetroxide staining, the number and size of cauda equina nerves were clearly visible under the light microscope and calculated in the digital image. This stainingtechnique can be used to assess morphological recovery. The nerve fbers in the proximal and distal parts of the nerve can be compared to evaluate nerve regeneration at different time points. This approach can help estimate recovery time after cauda equina injury.
MRI is an effective imaging technology in the diagnosis and management of spinal injury. MRI can help evaluate the arrangement and integrity of the spinal cord compared with CT angiography (Goldberg and Kershah, 2010). Hemorrhage and cord swelling detected by MRI can help predict the prognosis of spinal cord injury (Miyanji et al., 2007). Changes in water content due to altered axoplasmic fow secondary to neurotmesis produce signal changes on MRI sequences in peripheral nerve injury (Filler et al., 1996). MR DTI can provide additional insight into axon and myelin integrity (Kim et al., 2006). A number of studies have successfully used DTI in rats (Gullapalli et al., 2006; Ellingson et al., 2008; Kim et al., 2009; Zhang et al., 2009). Although less sensitive than electromyography, MRI may be a useful adjunctive diagnostic tool, with a sensitivity of 84% and specifcity of 100% for detecting denervation (McDonald et al., 2000). MRI was also used in our pilot study to provide detailed information on the cauda equina in goats, which had not been reported before. Difference in fractional anisotropy and apparent diffusion coeffcient values at different time points can be quantifed for comparison, and can serve as adjunctive diagnostic and prognostic tools. Images constructed by MRI provide a clear visualization of the cauda equina in any plane, and can be helpful for diagnosing cauda equina injury and for evaluating postoperative repair outcome and the integrity of the axonal structure in vivo following surgical reconstruction. Our results demonstrate the feasibility of using MRI in the diagnosis and prognosis of cauda equina injury.
Our research shows that the target organ of the cauda equina in goats is the tail muscle, and that MRI-DTI is effective for the diagnosis and prognosis of cauda equina injury. The tail muscles were found to be cross-innervated by cauda equina nerves by electrophysiology. The functional recovery of tail muscles can be evaluated using electrophysiological testing by comparing the amplitude and interval of electromyography activity at different time points.
Although this pilot study is descriptive and qualitative, not quantitative, it provides fundamental histological, electrophysiological and MRI data on the cauda equina in goats. The degeneration and regeneration of the nerves and target tail muscles can be evaluated using the methods used in this study. Furthermore, our animal model is easy to establish and reproduce. Our findings demonstrate the feasibility of surgical repair of the cauda equina. It is ideally suited for morphological and functional analysis during the entire neurological recovery process.
Author contributions:WTC, PXZ, FX and BGJ designed the study. WTC, PXZ, FX, CYQ, JM, BC, YLY and JXD performed experiments. WTC, PXZ, XFY and BGJ analyzed the data. WTC, PXZ, FX and BGJ wrote the paper. All authors approved the final version of the manuscript.
Conficts of interest:None declared.
Blaskiewicz DJ, Smirnov I, Cisu T, DeRuisseau LR, Stelzner DJ, Calancie B (2009) Cauda equina repair in the rat: part 1. Stimulus-evoked EMG for identifying spinal nerves innervating intrinsic tail muscles. J Neurotrauma 26:1405-1416.
Bose B, Wierzbowski LR, Sestokas AK (2002) Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine 27:1444-1450.
Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273:510-513.
Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH (2004) Spinal cord repair with acidic fbroblast growth factor as a treatment for a patient with chronic paraplegia. Spine 29:E284-288.
Conzen M, Sollmann H (1985) Reinnervation after microsurgical repair of transected cauda equina fbres. An electromyographic study. Neurochirurgia 28:6-7.
Ellingson BM, Kurpad SN, Li SJ, Schmit BD (2008) In vivo diffusion tensor imaging of the rat spinal cord at 9.4T. J Magn Reson Imaging 27:634-642.
Filler AG, Kliot M, Howe FA, Hayes CE, Saunders DE, Goodkin R, Bell BA, Winn HR, Griffiths JR, Tsuruda JS (1996) Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg 85:299-309.
Gertzbein SD, Court-Brown CM, Marks P, Martin C, Fazl M, Schwartz M, Jacobs RR (1988) The neurological outcome following surgery for spinal fractures. Spine 13:641-644.
Goldberg AL, Kershah SM (2010) Advances in imaging of vertebral and spinal cord injury. J Spinal Cord Med 33:105-116.
Gullapalli J, Krejza J, Schwartz ED (2006) In vivo DTI evaluation of white matter tracts in rat spinal cord. J Magn Reson Imaging 24:231-234.
Holland NR (2002) Intraoperative electromyography. J Clin Neurophysiol 19:444-453.
Kim JH, Budde MD, Liang HF, Klein RS, Russell JH, Cross AH, Song SK (2006) Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis 21:626-632.
Kim JH, Haldar J, Liang ZP, Song SK (2009) Diffusion tensor imaging of mouse brain stem and cervical spinal cord. J Neurosci Methods 176:186-191.
Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S (1995) A model for acute, chronic, and delayed graded compression of the dog cauda equina. Presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine 20:2758-2764.
LeBlanc HJ, Gray LW, Kline DG (1969) Stab wounds of the cauda equina. Case report. J Neurosurg 31:683-685.
Luo W, Zhang F, Liu T, Du XL, Chen AM, Li F (2012) Minimally invasive transforaminal lumbar interbody fusion aided with computer-assisted spinal navigation system combined with electromyography monitoring. Chin Med J (Engl) 125:3947-3951.
Mackenzie SJ, Smirnov I, Calancie B (2012) Cauda equina repair in the rat: part 2. Time course of ventral root conduction failure. J Neurotrauma 29:1683-1690.
McDonald CM, Carter GT, Fritz RC, Anderson MW, Abresch RT, Kilmer DD (2000) Magnetic resonance imaging of denervated muscle: comparison to electromyography. Muscle Nerve 23:1431-1434.
Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG (2007) Acute cervical traumatic spinal cord injury: MR imaging fndings correlated with neurologic outcome--prospective study with 100 consecutive patients. Radiology 243:820-827.
Wang ZY, Zhang PX, Han N, Kou YH, Yin XF, Jiang BG (2013) Effect of modifed formula radix hedysari on the amplifcation effect during peripheral nerve regeneration. Evid Based Complement Alternat Med 2013:647982.
Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Griffn JW, Sheikh KA, Miller MI, Mori S, Calabresi PA (2009) Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci 29:3160-3171.
Copyedited by Patel B, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to: Pei-xun Zhang, M.D., Ph.D., zhangpeixun@126.com. Feng Xue, M.D., Ph.D., drxuefeng@gmail.com. Bao-guo Jiang, M.D., Ph.D., jiangbaoguo@vip.sina.com.
10.4103/1673-5374.150707
http://www.nrronline.org/
Accepted: 2014-12-15
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

