Acetylcholinesterase inhibitor modifcations: a promising strategy to delay the progression of Alzheimer’s disease
Acetylcholinesterase inhibitor modifcations: a promising strategy to delay the progression of Alzheimer’s disease
Alzheimer’s disease (AD), a fatal, progressive, neurodegenerative disorder, is the most common cause of old-age dementia, accounting for 50-75% of dementia patients. Early stages of AD are marked by vocabulary shrinkage, spatial disorientation, depression, apraxia, and deterioration of recent forms of declarative memory. In course of time, the patients require close supervision due to the loss of cognitive and functional abilities, and at the terminal stages of the disease, all forms of memory are severely impaired with the patients needing nursing home care (World Alzheimer Report, 2013).
Pathogenesis and hallmarks of AD:Major neuropathological hallmarks of AD are extracellular amyloid beta (Aβ) plaques, derived from aberrant cleavage of the transmembrane amyloid precursor protein (APP), intracellular neurofbrillary tangles, cerebral amyloid angiopathy, glial dysfunction, and neuronal as well as synaptic loss (for review, see Huang and Mucke, 2012). A number of pathogenic mechanisms, often not mutually exclusive, have been described, namely, the cholinergic hypothesis, the amyloid cascade theory, tau hypothesis, calcium hypothesis, oxidative damage, iron dysregulation, abnormal cholesterol metabolism, and mitochondrial hypothesis (for review, see Huang and Mucke, 2012). The seeding prion-like behavior of protein aggregrates has been speculated to cause the progression of AD pathology in the brain. Additionally, environmental factors like diet and physical exercise are suspected to infuence the risk of developing AD.
The amyloid hypothesis is strongly supported by genetics with many familial AD cases resulting from inherited APP or presenilin 1 (PS1) mutations and transgenic models of AD harboring APP mutations developing age-dependent AD-like pathology in animals (for review, see Huang and Mucke, 2012). Also, the apolipoprotein E ε4 (APOEε4) polymorphism, which represents the most important genetic risk factor for the sporadic form of AD, is associated with Aβ accumulation caused by its reduced clearance. Aβ plaques have been speculated to serve as reservoirs of soluble Aβ oligomers injuring surrounding neurites and synapses and to be majorly responsible for aggravated cortical atrophy.Therefore, all major therapeutic strategies against AD target the production, aggregation, or degradation of Aβ (for review, see Huang and Mucke, 2012).
However, significant plaque deposition occurs already before cognitive decline, indicating that the timing of therapeutic intervention might be a crucial factor determining its success. Most treatments are more effective in milder stages compared to the later stages of the disease. Early intervention is indeed eventually cost-effective and aims to prolong the time spent by patients in the milder form of the disease without the need for nursing home placement (World Alzheimer Report, 2013).
Acetylcholinesterase inhibitors as a treatment strategy for AD:Examination of human AD brains consistently revealed abnormalities in the basal and rostral forebrain cholinergic pathways which serve important functional roles in conscious awareness, attention, working memory, and other mnemonic processes. The cholinergic hypothesis of AD, suggesting that cholinergic augmentation should improve cognition, has led to the successful use of acetylcholinesterase inhibitors (AChEI) in AD patients. AChEI, in multiple studies, have proved helpful in restoring cognition. It is not surprising that the AChEIs donepezil (Pfizer), galantamine (Janssen), and rivastigmine (Novartis) are used in symptomatic treatment of mild to moderate Alzheimer’s dementia. AChEI can be either competitive and reversible (galantamine), noncompetitive and reversible (donepezil), pseudo-irreversible (rivastigmine) or noncompetitive (tacrine) with respect to AChE inhibition. Additionally, rivastigmine displays preferential inhibition of the G1 form of AChE, and along with tacrine offers dual inhibition of AChE and butyrylcholinesterase. Galantamine is unique as it acts additionally as an allosterically potentiating ligand of nicotinic acetylcholine receptors, increasing their sensitivity to the neurotransmitter acetylcholine. All these aforementioned second-generation AChEIs have been shown to improve cognition and function, suggesting that the underlying mechanism may be similar, although tacrine use has been discontinued due to its severe hepatotoxicity (for review, see Aisen et al., 2012). AChEIs have been in use since decades and in spite of various other therapeutic strategies attempted and still under consideration e.g., immunotherapy, β and γ secretase inhibitors, to name a few, AChEI use has persisted. The most obvious advantage of this class of drugs is a well established symptomatic therapy with a recognized target. Drugs in this class have a proven track of CNS permeability, known side effect profle, and demonstrated effcacy, making it an obvious choice as the first/only treatment for AD. In fact, all of the current frst-line AD treatments in the US are AChEIs. Importantly, medications prescribed for somatic comorbidities in AD patients must be considered to determine the more suited AChEI in every AD case, for example, donepezil and galantamine are metabolized like many other drugs by the cytochrome P450 system in the liver whereas rivastigmine is not (Clodomiro et al., 2013).
Modifications of AChEI:The prescription of higher and possibly more effective doses of AChEI is limited by considerable side effects in particular nausea, vomiting, loss of appetite, dizziness, and diarrhea, hindering long term treat-ments in human (Maelicke et al., 2010). However, the effcacy of acetylcholinesterase inhibitors is dose dependent as exemplifed by the higher doses of rivastigmine and donepezil (for references, see Aisen et al., 2012). Substantial research aims to develop strategies to circumvent the unwanted side effects and to improve the bioavailability and efficacy of AChEI as a therapeutic drug for AD. Extended-release oral capsules, intranasal delivery, and skin patch formulations are approaches being studied to ensure better compliance of both the patient and the caregiver. Biomembrane penetration of tacrine in domestic pigs to lessen hepatotoxicity, intranasal and transdermal approaches of physostigmine, and a galantamine formulation suitable for intranasal delivery have been reported (Costantino et al., 2008; Maelicke et al., 2010). Transdermal administration of rivastigmine improved safety and tolerability, facilitating delivery of higher doses and reducing caregiver burden (Costantino et al., 2008; Aisen et al., 2012). A 13.3 mg daily patch provides higher cognitive effcacy and leads to fewer treatment discontinuations without additional serious adverse effects than a previous patch rendering 9.5 mg daily (Cummings et al., 2012). Furthermore, an extended release form of galantamine, resulting in fewer episodes of nausea and emesis, is commercially in use since 2005 (Aisen et al., 2012). Also, intranasal galantamine-lactate administration reduces emetic responses in ferrets (for review, see Costantino et al., 2008).
The naturally occuring alkaloid physostigmine, similar to galantamine, is a reversible dual action AChEI which has a limited oral effcacy due to its low short half-life and bioavailability. Intranasal and transdermal delivery routes for physostigmine were initially investigated. Transdermal physostigmine provided a mean absolute bioavailability of 36% as compared with only 3% for oral delivery in humans, and intranasal physostigmine was speculated to provide complete bioavailability (for review, see Costantino et al., 2008). However, the effectiveness of physostigmine for the symptomatic treatment of AD could not be improved as it showed no convincing beneft in patients and adverse effects remained leading to a high rate of withdrawal. Rivastigmine, however, was modeled on the structure of physostigmine and is proven to be successful, as it is FDA approved and shows convincing results in AD patients (Pinho et al., 2013).
Galantamine an AChE inhibitor and allosteric nicotinic receptor ligand:The chronic low-level stimulation of nicotinic receptors, as achieved by galantamine treatment, can up-regulate their expression, slow down neurodegeneration, and confer protection against Aβ toxicity (for review, see Maelicke, 2006). Furthermore, galantamine exerts neuroprotective efects in vitro and in vivo by modulating microglial nicotinic AChRs enhancing Aβ phagocytosis by cultured rat microglia and Aβ clearance in the brain of Aβ-injected rats or APdE9-transgenic mice (Takata et al., 2010). Galantamine has been recently reported to delay behavioral decline and the progression of plaque deposition and gliosis in the 5XFAD mouse model (Bhattacharya et al., 2014). Analysis of data on 2,033 patients, pooled from multiple studies, suggests that chronic galantamine treatment leads to reduced behavioral symptoms as measured by the Neuropsychiatric Inventory (NPI), particularly on symptoms of agitation, anxiety, disinhibition, and aberrant movements (Kavanagh et al., 2011). Importantly, long-term galantamine treatment delays a patient’s nursing home placement and caregiver burden, making it a cost-effective treatment. However, like other AChEI, gastrointestinal side effects frequently result in discontinuation of its treatment, making it necessary to develop modifications offering better bioavailability and a non-intestinal delivery route.
Memogain-a promising galantamine prodrug:A relatively recent improved derivative of galantamine is memogain, which can be administered through the nasal route (maelicke et al., 2010). The prodrug memogain is more hydrophobic than galantamine resulting in a higher enrichment in the brain compared to blood. Enzymatic cleavage of memogain liberates galantamine as the active compound. In fact, less gastrointestinal and other peripheral side effects of memogain have been observed compared to equal doses of galantamine in ferrets (Maelicke et al., 2010). Also, a threeto fvefold increase of potency of memogain over galantamine in reversing scopolamine-induced amnesia is revealed by performance recovery in a T-maze task in mice (Maelicke et al., 2010). Therefore, memogain is substantially more specific and potent compared to galantamine, resulting in a higher bioavailability, a desirable feature of a prodrug. We recently showed that memogain can be easily and successfully administered nasally in the 5XFAD mouse model.
Intranasal dosing provides an extremely convenient alternative compared to oral or intravenous delivery. The nasal mucosa has a large surface area for absorption. Patient preference in chronic treatment is substantially higher compared to other delivery routes as exemplifed by insulin, calcitonin, and histamine delivery. Intranasal delivery has proven to be substantially effcient in a number of diverse therapeutic areas namely, breakthrough cancer pain, migraine, cluster headaches, cardiovascular disorders, sedative agents, diabetes, etc. It is preferred because of patient convenience and preference, rapid drug onset, avoidance of gastro-intestinal side-effects, and more consistent delivery for disease states associated with gastric dysmotility. Also, the requirement of a sterile product/dosing technique is not required.
In our study, chronic nasal memogain application ameliorated affected behaviors even before cognitive decline. In addition, it effciently lowered the plaque load (Figure 1) at lower doses than orally applied galantamine without affecting general health as reflected by body weight and motor capabilities of the test mice. Memogain could, therefore, be an effective treatment strategy against AD and its potential should be further examined in clinical trials.
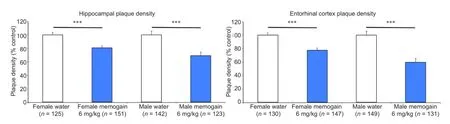
Figure 1 Chronic nasal treatment with memogain signifcantly reduces the number of plaques in the brains of 5XFAD mice.
Conclusion:To sum up, benefcial effects of AChEI on cognition have been proven both in animal models and in humans. However, an effect of acetylcholinesterase inhibitors on plaque formation, although previously speculated, had not been systematically investigated. Our recent work indicates that galantamine apart from augmenting cholinergic function and acting as an allosterically potentiating ligand on nicotinic receptors, retards the deposition of plaques and associated gliosis, and prevents behavioral decline related to AD. Memogain, a modified pro-drug of galantamine, may be particularly useful in targeting early stages of the disease, and at the same time, provides the ease of nasal application and circumvention of gastrointestinal side effects. In conclusion, memogain represents a potential example candidate in present AD treatment strategy which should be explored further. This example illustrates that currently identifed drugs with small therapeutic effects may be engineered to generate more potent medications. Although a cure for AD appears not feasible, combination treatments with drugs targeting Aβ production, toxicity and/or clearance may potentially retard the progression of symptoms effectively. Therefore, further modifcations with better effcacy and safety profles than their active drug should be developed and investigated. It is noteworthy that newly developed AChEIs need to be more effective than the FDA approved donepezil, rivastigmine, and galantamine to obtain approval. In addition to investigating novel alternative strategies to interfere with AD progression, modifying the only currently approved and reliable treatment strategies could be time and cost effective.
This work was in part supported by the German Ministry for Education and Research (BMBF) special network program KMU-Innovativ-2.
Soumee Bhattacharya, Dirk Montag*Leibniz Institute for Neurobiology, Neurogenetics Special Laboratory, Magdeburg, Germany
*Correspondence to: Dirk Montag, Ph.D., montag@lin-magdeburg.de.
山东省烟台市爱农农资有限公司洪磊认为相似商标也给执法带来了难度。他说:“由于一些品牌在工商部门确实注册了真实的商标,给执法增添了不少难度,所以还是应该在商标注册的环节严格把关才是解决这一问题的关键。”龙福则认为,既然相似商标容易对农民产生误导,那么这就要求经销商要立足诚信经营,告诉农民不同品牌之间的区别,让农民更理性地去选择。
Accepted: 2014-12-12
Aisen PS, Cummings J, Schneider LS (2012) Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harbor Perspect Med 2:a006395.
Bhattacharya S, Haertel C, Maelicke A, Montag D (2014) Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer’s disease. PLoS One 9:e89454.
Clodomiro A, Gareri P, Puccio G, Frangipane F, Lacava R, Castagna A, Manfredi VG, Colao R, Bruni AC (2013) Somatic comorbidities and Alzheimer’s disease treatment. Neurol Sci 34:1581-1589.
Costantino HR, Leonard AK, Brandt G, Johnson PH, Quay SC (2008) Intranasal administration of acetylcholinesterase inhibitors. BMC Neurosci 9 Suppl 3:S6.
Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo JL, Kressig RW, Downs P, Caputo A, Strohmaier C (2012) Randomized, double-blind, parallel-group, 48-week study for effcacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm²) in Alzheimer’s disease. Dement Geriatr Cogn Disord 33:341-353.
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204-1222.
Kavanagh S, Gaudig M, Van Baelen B, Adami M, Delgado A, Guzman C, Jedenius E, Schäuble B (2011) Galantamine and behavior in Alzheimer disease: analysis of four trials. Acta Neurol Scand 124:302-308.
Maelicke A (2006) Allosteric sensitisation of brain nicotinic acetylcholine receptors as a treatment strategy in Alzheimer’s dementia. In: Therapeutic Strategies in Dementia (Ritchie CW, Dave Ames D, Masters CL, Cummings JL, eds). Atlas Medical Publishing Ltd, Chapter 13.
Maelicke A, Hoeffe-Maas A, Ludwig J, Maus A, Samochocki M, Jordis U, Koepke AK (2010) Memogain is a galantamine pro-drug having dramatically reduced adverse effects and enhanced efficacy. J Mol Neurosci 40:135-137.
Pinho BR, Ferreres F, Valentão P, Andrade PB (2013) Nature as a source of metabolites with cholinesterase-inhibitory activity: an approach to Alzheimer’s disease treatment. J Pharm Pharmacol 65: 1681-1700.
Takata K, Kitamura Y, Saeki M, Terada M, Kagitani S, Kitamura R, Fujikawa Y, Maelicke A, Tomimoto H, Taniguchi T, Shimohama S (2010) Galantamine-induced amyloid-β clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem 285:40180-40191.
World Alzheimer Report, Alzheimer’s Disease International, London, September 2013.
10.4103/1673-5374.150648 http://www.nrronline.org/ Bhattacharya S, Montag D (2015) Acetylcholinesterase inhibitor modifications: a promising strategy to delay the progression of Alzheimer’s disease. Neural Regen Res 10(1):43-45.
- 中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research (NRR) Instructions for Authors (2015)
- Hypersensitivity of vascular alpha-adrenoceptor responsiveness: a possible inducer of pain in neuropathic states
- Neural regeneration after peripheral nerve injury repair is a system remodelling process of interaction between nerves and terminal effector
- Acute carbon monoxide poisoning and delayed neurological sequelae: a potential neuroprotection bundle therapy
- Prediabetes and type 2 diabetes implication in central proliferation and neurogenesis
- Clinical strategies to enhance nerve regeneration

