The human retinal stem niches could overlap a vascular anatomical pattern
The human retinal stem niches could overlap a vascular anatomical pattern
Stem cells are undifferentiated cells which have the unique potential to self-renew and to supply, via intermediate stages of transit amplifying cells (TACs), differentiated cells. Within the stem cell niches not only heterologous cells, but also differentiated progeny of stem cells provide regulation to the stem cells parents. Stem cells can differentiate in various cell types during tissue maintenance or repair.
Mesenchymal stem/stromal cells (MSCs) are the best candidates for regenerative medicine. However, the standard for identifying MSCs indicates their plastic adherence in standard culture conditions. Beyond a great diversity of biomarkers identifying MSCs in in vitro conditions (such as the positive expression of CD73, CD90 and CD105, and negative expression of CD14, CD34 and CD45), their exact identity in vivo is not yet clear, although it was suggested either a pericytic, or a fbroblastic origin (Lv et al., 2014). The most reliable molecules to sort MSCs are Stro-1, SSEA-4 and CD146 (Lv et al., 2014).
Evidence suggests that neural progenitors may exist throughout life in the human retina (Mayer et al., 2005). Different sources for replacement of retinal neurons have been identified. Nevertheless, extrinsic providers of stem/ progenitor cells, such as the bone marrow, could supply the adult retina during regenerative processes. It is intended here to bring arguments for a better focus in researches on the perivascular stem niches of human retina. This perspective results from observational studies in humans and experimental studies are further needed to assess the validity of the different perivascular stem niches.
The retinal stem cells (RSCs) hypothesis resulted from observations in lower vertebrates (Kiyama et al., 2012) of multipotent cells of the ciliary marginal zone (CMZ) which constantly produce throughout life new retinal cells; the CMZ niche is anatomically related to the peripheral retina. All types of retinal neurons can arise from the CMZ (Kiyama et al., 2012). Human RSCs were tested in vitro and were found able to survive, migrate, integrate and to differentiate, especially in photoreceptors (Coles et al., 2004). However, are the CMZ-derived RSCs able, in vivo, to completely supply retina with progenitors, even the central retina?
Retinal pericytes share in rodents some phenotypic features, such as the CD146 expression, with archetypal MSCs (Wittig et al., 2013). Rat aorta pericytes were induced to differentiate into neural-like phenotypes (Montiel-Eulef et al., 2012). A differentiation scheme for pericytes was presented for human adult adipose tissue, according to which pericytes with stem cell properties, including the CD146 expression, differentiate from the inside out, give rise to adventitial CD146+TACs, then to CD146-MSC-like supra-adventitial stromal cells (Zimmerlin et al., 2012). Expression of a stem/progenitor phenotype in vascular smooth muscle cells (VSMCs) is an age-related process associated with arterial remodelling (Ferlosio et al., 2012). Proliferating VSMCs undergo a process of dedifferentiation and this could be equally true for pericytes.
We previously discussed that CD146-positive human retinal ganglion cells could result from regenerative processes driven by stem cells in the retinal perivascular niches (Vrapciu et al., 2014). This is consistent with studies of CD146+pericytes, which determined and strongly support the concept of perivascular stem niche (Crisan et al., 2012; Chen et al., 2013). In order to identify neural progenitor cells for therapies of central nervous system disorders, such as posttraumatic injuries or neurodegenerative disorders, two pericytes subtypes were described in mouse experiments; in type-2, Nestin-GFP+/NG2-DsRed+/CD146+, pericyte-derived Tuj1+/ CD146+/PDGFRbeta+/NG2+cells, the nerve growth factor receptor was exclusively expressed, thus it can identify pericytes with neurogenic potential (Birbrair et al., 2013).
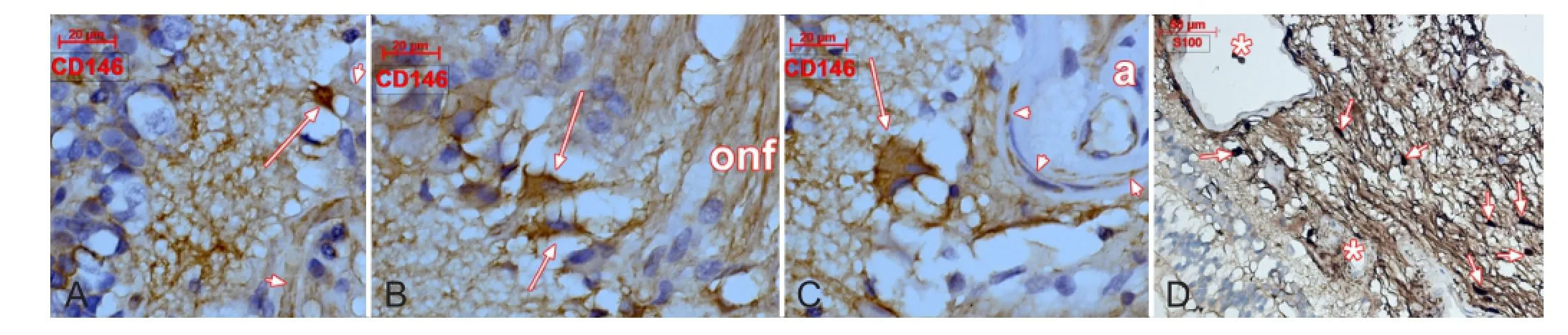
Figure 1 CD146 (A-C) and S100 protein (D) labeling of human retina.
We found S100 protein+cells in the optic nerve head(ONH) (Figure 1), which suggests the presence of neural cells. Neurons were not previously assessed in the ONH. They could be the result of a perivascular stem niche activation related to an ongoing process of regeneration. This is supported by CD146+cells with neuronal morphologies which were found nearing vessels within the inner layers of central retina and the ONH (Figure 1). Thus, along vessels deriving from the central artery of retina a perivascular niche could supply with newly formed neurons the ganglion cell layer of retina during reparatory/regenerative processes. Tissue samples of a previously described lot (Vrapciu et al., 2014) were used for CD146 (clone N1238, Novocastra-Leica, Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, U.K., 1:50) and S100 (polyclonal, Dako, Glostrup Denmark, 1:400) protein labeling.
Vascular/progenitor stem cells able to differentiate into endothelial and smooth muscle cells can be equally native vascular wall residents and bone marrow-derived cells. Pericytes are able to repair endothelia, in a centripetal/luminal direction but are also able to replenish, in a centrifugal manner, the perivascular tissue-specifc differentiated cells. The vascular-related stem/progenitor niche could be regarded anatomically, being superposed on the vascular architecture of an organ/tissue. In this regard, the eye, which is supplied from two vascular systems (i.e., the ciliary arteries and the central retinal artery), possesses two different vascular/perivascular stem niches, which may not be exclusive. The ciliary arteries niche can be viewed as a stem/progenitor anatomic supplier of the CMZ. The central artery of retina which courses through the optic disc, or the ONH, further branches within the nerve fbers retinal layer being thus anatomically able to support retinal ganglion cells replenishment from the vascular/perivascular stem niche. However, regenerative processes in the outer layers of the central retina could also rely on the ciliary arteries system. So, the stem/progenitor niche of the central retinal artery could relate to retinal ganglion cells regeneration and the arteries of the uvea could be regarded, but not exclusively, as stem/progenitor providers of the outer retinal layers.
Further studies are mandatory to assess the stem potentiality of the choroid which is anatomically able to ensure regeneration of the central retina, in a similar manner to the CMZ which is able to supply stem/progenitor cells in the peripheral retina.
Mugurel Constantin Rusu*, Alexandra Diana Vrapciu Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; MEDCENTER, Center of Excellence in Laboratory Medicine and Pathology, Bucharest, Romania; International Society of Regenerative Medicine and Surgery (ISRMS), Romania (Rusu MC)Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (Vrapciu AD)
*Correspondence to: Mugurel Constantin Rusu, M.D., Ph.D., anatomon@gmail.com.
Accepted: 2014-12-14
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10:67-84.
Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, Corselli M, Crisan M, Saparov A, Tobita K, Peault B, Huard J (2013) Human pericytes for ischemic heart repair. Stem Cells 31:305-316.
Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D (2004) Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A 101:15772-15777.
Crisan M, Corselli M, Chen WC, Peault B (2012) Perivascular cells for regenerative medicine. J Cell Mol Med 16:2851-2860.
Ferlosio A, Arcuri G, Doldo E, Scioli MG, De Falco S, Spagnoli LG, Orlandi A (2012) Age-related increase of stem marker expression infuences vascular smooth muscle cell properties. Atherosclerosis 224:51-57.
Kiyama T, Li H, Gupta M, Lin YP, Chuang AZ, Otteson DC, Wang SW (2012) Distinct neurogenic potential in the retinal margin and the pars plana of mammalian eye. J Neurosci 32:12797-12807.
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) The surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408-1419.
Mayer EJ, Carter DA, Ren Y, Hughes EH, Rice CM, Halfpenny CA, Scolding NJ, Dick AD (2005) Neural progenitor cells from postmortem adult human retina. Br J Ophthalmol 89:102-106.
Montiel-Eulefi E, Nery AA, Rodrigues LC, Sanchez R, Romero F, Ulrich H (2012) Neural differentiation of rat aorta pericyte cells. Cytometry A 81:65-71.
Vrapciu AD, Rusu MC, Voinea LM, Corbu CG (2014) CD146- and CD105-positive phenotypes of retinal ganglion cells. Are these in situ proofs of neuronal regeneration? Med Hypotheses 83:497-500.
Wittig D, Jaszai J, Corbeil D, Funk RH (2013) Immunohistochemical localization and characterization of putative mesenchymal stem cell markers in the retinal capillary network of rodents. Cells Tissues Organs 197:344-359.
Zimmerlin L, Donnenberg VS, Donnenberg AD (2012) Pericytes: a universal adult tissue stem cell? Cytometry A 81:12-14.
10.4103/1673-5374.150651 http://www.nrronline.org/ Rusu MC, Vrapciu AD (2015) The human retinal stem niches could overlap a vascular anatomical pattern. Neural Regen Res 10(1):39-40.
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

