Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats
Adem Bozkurt Aras, Mustafa Guven Tarık Akman Adile Ozkan, Halil Murat Sen, Ugur Duz, Yıldıray Kalkan, Coskun Silan, Murat Cosar
1 Department of Neurosurgery, Canakkale Onsekiz Mart University, Canakkale, Turkey
2 Department of Neurology, Canakkale Onsekiz Mart University, Canakkale, Turkey
3 Department of Medical Biochemistry, Ministry of Health, Administration of Public Health, Hakkari, Turkey
4 Deparment of Histology and Embryology, Recep Tayyip Erdoğan University, Faculty of Medicine, Rize, Turkey
5 Department of Pharmacology, Canakkale Onsekiz Mart University, Canakkale, Turkey
Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats
Adem Bozkurt Aras1,*, Mustafa Guven1, Tarık Akman1, Adile Ozkan2, Halil Murat Sen2, Ugur Duz3, Yıldıray Kalkan4, Coskun Silan5, Murat Cosar1
1 Department of Neurosurgery, Canakkale Onsekiz Mart University, Canakkale, Turkey
2 Department of Neurology, Canakkale Onsekiz Mart University, Canakkale, Turkey
3 Department of Medical Biochemistry, Ministry of Health, Administration of Public Health, Hakkari, Turkey
4 Deparment of Histology and Embryology, Recep Tayyip Erdoğan University, Faculty of Medicine, Rize, Turkey
5 Department of Pharmacology, Canakkale Onsekiz Mart University, Canakkale, Turkey
Daidzein, a plant extract, has antioxidant activity. It is hypothesized, in this study, that daidzein exhibits neuroprotective effects on cerebral ischemia. Rat models of middle cerebral artery occlusion were intraperitoneally administered daidzein. Biochemical and immunohistochemical tests showed that superoxide dismutase and nuclear respiratory factor 1 expression levels in the brain tissue decreased after ischemia and they increased obviously after daidzein administration; malondialdehyde level and apoptosis-related cysteine peptidase caspase-3 and caspase-9 immunoreactivity in the brain tissue increased after ischemia and they decreased obviously after daidzein administration. Hematoxylin-eosin staining and luxol fast blue staining results showed that intraperitoneal administration of daidzein markedly alleviated neuronal damage in the ischemic brain tissue. These fndings suggest that daidzein exhibits neuroprotective effects on ischemic brain tissue by decreasing oxygen free radical production, which validates the aforementioned hypothesis.
nerve regeneration; brain ischemia; stroke; daidzein; oxidative stress; apoptosis; superoxide dismutase; malondialdehyde; nuclear respiratory factor 1; neural regeneration
Aras AB, Guven M, Akman T, Ozkan A, Sen HM, Duz U, Kalkan Y, Silan C, Cosar M (2015) Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen Res 10(1):146-152.
Introduction
Cerebral ischemia is a situation that may lead to permanent neurological defcits and serious complications. As a result, it is still a serious clinical problem. While the main target of treatment after ischemia is to maintain reperfusion, this does not always create positive results. On the contrary, starting reperfusion can increase tissue injury (Grace, 1994). As the central nervous system is very susceptible to anoxia, neurological damage can develop after ischemia (Islekel et al., 1999). At this stage, strategies such as hypothermic arrest and selective cerebral perfusion can be used to protect the brain from the developing cerebral ischemia (Bors et al., 1990). One of the basic mechanisms underlying tissue damage is the development of lipid peroxidation caused by release of free radicals (Formica et al., 1995).
In many diseases in humans (e.g., cancer, ischemia, cardiovascular irregularity), free radicals have an effect on tissue damage (Braughler, 1989). The topic of preventing this damage by using antioxidants has occupied a significant place in the literature in recent years (Stavric, 1994). Many exogenous-sourced antioxidants are used widely in foodstuffs currently. These include vitamins, favonoids, polyphenols and other compounds (Stavric, 1994).
Daidzein (DZ) is a polyphenolic compound in the isofavon group. It is found in red clover (Trifolium pratense), alfalfa (Medicago sativa), soya and some legumes (Leguminosae). DZ has been reported to protect against cancers of the chest, prostate and large intestine (Hertog et al., 1993) and to have anti-infammatory (Chan, 2001), cardioprotective (Kim et al., 2009) and antioxidant (Bors et al., 1990) characteristics.
DZ is the major circulating isoflavone in humans consuming a high soy diet (Mathey et al., 2006). It is estimated that in humans 30-50% of 4′,7-isofavandiol is produced by intestinal microfora, however, remants of DZ obtained from diet produce equol (Mathey et al., 2006). Equol, a metabolite of DZ, is the primary isofavonoid in circulation, consumed by rodents and is responsible for 80% of plasma isoflavonoids (Lephart et al., 2001). Eating an enriched soya diet has been shown to have neuroprotective effects by preclinical studies (Cederroth et al., 2009). Eating a high soya diet has been shown to reduce the scale of cerebral ischemia after temporary middle cerebral artery occlusion (MCAO) in rats given ovariectomy. This is explained by the isofavones selectively binding to estrogen receptor ligands and mimicking some of the effects of estrogen (Burguete et al., 2006).
In this study, the antioxidant and neuroprotective effects of DZ were researched to prevent cell damage caused by cerebral ischemia.
Materials and Methods
Animals
Before starting this study, permission was granted by Canakkale Onsekiz Mart University Animal Experiments Ethics Committee. In our study, 24 male Sprague-Dawley rats, weighing 250-280 g, aged 8 to 12 weeks, were used. All rats were fed with standard pellet rat food (Bil-Yem Ltd, Ankara, Turkey) and water ad libitum. An automatic photoperiod with white fuorescent light was used to create an environment with 12-hour light-darkness. The temperature was set to 21 ± 2°C and humidity was set to 55-60%. The methods used for animal experiments were organized in accordance with the protocols of the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
Rats were randomly divided into three groups: Control group (n = 8; no ischemia or medication), MCAO group [n = 8; MCAO + 50% ethanol (1 mL, intraperitoneally, as vehicle) and sacrifce at 24 hours], MCAO + DZ group [n = 8; MCAO + DZ (single dose 10 mg/kg, intraperitoneally) and sacrifce at 24 hours].
Surgical procedure
All rats were anesthetized with 5 mg/kg xylazine (Bayer, Istanbul, Turkey) and 50 mg/kg ketamine hydrochloride (Parke Davis, Istanbul, Turkey) with spontaneous respiration and at room temperature. The rats were placed in the supine position on an operating table under sterile conditions and the right paramedian of the neck was incised through skin and subdermis. Described by Hata et al. (1998) and modified by Kawamura et al. (1994), the intraluminal flament technique was used for MCAO and focal cerebral ischemia was induced. To induce MCAO, 4/0 nylon monoflament suture material (Ethilon Inc., Somerville, New Jersey, USA) was advanced 16-18 mm distal of the carotid bifurcation through a small incision made in the common carotid artery. At 5 minutes after MCAO, rats from the MCAO + 50% ethanol group were given 10 mg/kg DZ intraperitoneally. The drug DZ (≥ 98% synthetic; Sigma-Aldrich, St. Louis, MO, USA) was dispersed in ethanol and the fnal concentration of ethanol was 50%. All rats were given a high dose anesthetic (ketamine 50 mg/kg) at the 24thhour and sacrifced. Immediately after sacrifice, craniotomy was performed and the brain was removed. The removed tissue was stored in 10% neutral formaldehyde solution. The tissue was sent to the laboratory for histopathological investigation of the right hemisphere with MCAO and measurement of malondialdehyde (MDA), superoxide dismutase (SOD) and nuclear respiratory factor 1 (NRF-1) levels.
Measurement of SOD, MDA and NRF-1 levels in brain tissue
Tissue SOD activity assay kit (Catalogue No. 706002; Cayman Chemical Company, Ann Arbor, MI, USA) was used for spectrophotometric measurement. Results are given as U/mg protein.
MDA and NRF-1 levels were measured with the ELISA method according to the manufacturer’s instructions using a rat MDA ELISA kit (Cat. No. CK-E30266; Hangzhou Eastbiopharm Co., Ltd., Hangzhou, China) and a rat NRF-1 ELISA kit (Cat. No. CK-E90555; Hangzhou Eastbiopharm Co., Ltd.). The MDA and NRF-1 results are divided by protein amount and given as nmol/mg protein and ng/mg protein, respectively.
Histopathological investigation
The brain tissues were labeled and left in 10% neutral formaldehyde solution. After being left in fixative for 24 hours, they were washed for 6-8 hours in running water and passed through ethanol-xylene series with an automatic tissue processor (Citadel 2000, Thermo Fisher Scientifc Shandon, Loughborough, England) before being submerged in liquid paraffn. Tissues were cut into sections 4-6 µm thick for routine hematoxylin-eosin and luxol fast blue staining and to 3-4 µm thickness for immunohistochemical staining (caspase-3 and caspase-9). Appropriate areas observed under a light microscope (Eclipse E-600 Nikon, Japan) at different magnifcations were examined and photographed. In the evaluation of hematoxylin-eosin and luxol fast blue stained sections, the dead neurons which were characterized by karyolitic, karyorectic nucleus and vacuolated cytoplasm were counted in 15 different random areas under × 20 objective magnifcation. Each area was 1,200 micrometer square with remaining neuronal cells in the area counted to determine cell density. In the evaluation of hematoxylin-eosin and luxol fast blue staining, a graded scale (+ to ++++) was used for measurement of edema, red neurons, vacuolization and neuronal degeneration. Percentile values were obtained from hematoxylin-eosin and luxol fast blue staining. Blind grading was used for measurement of edema by a pathologist.
Sections cut for immunohistochemical staining were left for 20 minutes in xylene, and after being passed through an alcohol series (70-99%), they were left for 10 minutes in 3% H2O2solution. After washing with PBS, they were heated four times 5-10 minutes for each in citrate buffer solution and then left in secondary blocking material for 20 minutes. They were left for 60-75 minutes in each preparation of primary antibody at different dilutions (1:200-1:250) at room temperature [anti-caspase 3 rabbit polyclonal antibody (ab4051), Abcam plc, Cambridge, UK and anti-caspase 9 rabbit monoclonal antibody (ab32539), Abcam plc]. Biotinylated goat anti-polyvalent antibody (UltraVision Large Volume Detection System, horseradish peroxidase (readyto-use), TP-125-HL, Thermo Scientifc Inc., Waltham, MA, USA) was used without dilution as secondary antibodies. Sections were incubated for 20 minutes at room temperature (20-22°C). Chromogenous 3,3′-diaminobenzidine (DAB) solution was used for negative staining while Mayer’s hematoxylin (Thermo Scientific Inc. Waltham, MA, USA) was used for staining. PBS was used for negative controls. The preparations were covered with appropriate material and photographed under a light microscope (Eclipse E-600 Nikon, Japan). At least 1,000 cells were analyzed in each case.The percentage of cells stained in each case was evaluated semiquantitatively on a 5% incremental scale ranging from 0 to 95%. A numeric intensity score between 1 and 4 was assigned to each case on a scale from 1 to 4. The results of immunohistochemical staining were divided into four categories according to immunoreactive area percentile values: (+) slight, (++) moderate, (+++) intense and (++++) very intense.
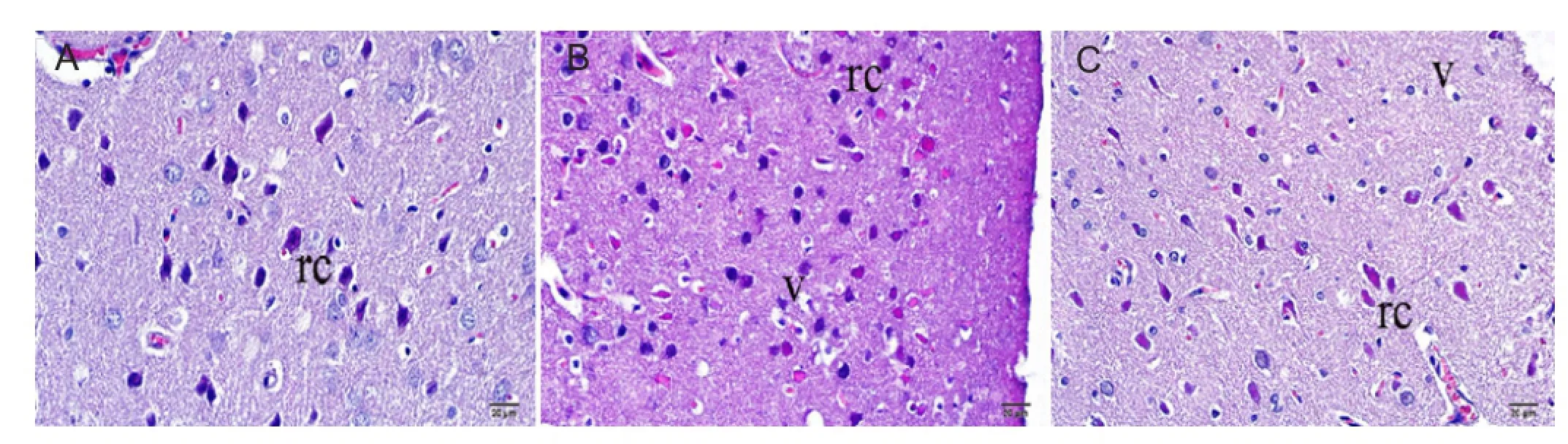
Figure 2 Representative photomicrographs showing neurons, astrocytes, and microglial cells stained with hematoxylin and eosin in the ischemic brain area of rats after 24 hours of middle cerebral artery occlusion (MCAO).

Figure 3 Representative photomicrographs showing neurons, astrocytes, and microglial cells stained with luxol fast blue in the ischemic brain area of rats after 24 hours of middle cerebral artery occlusion (MCAO).
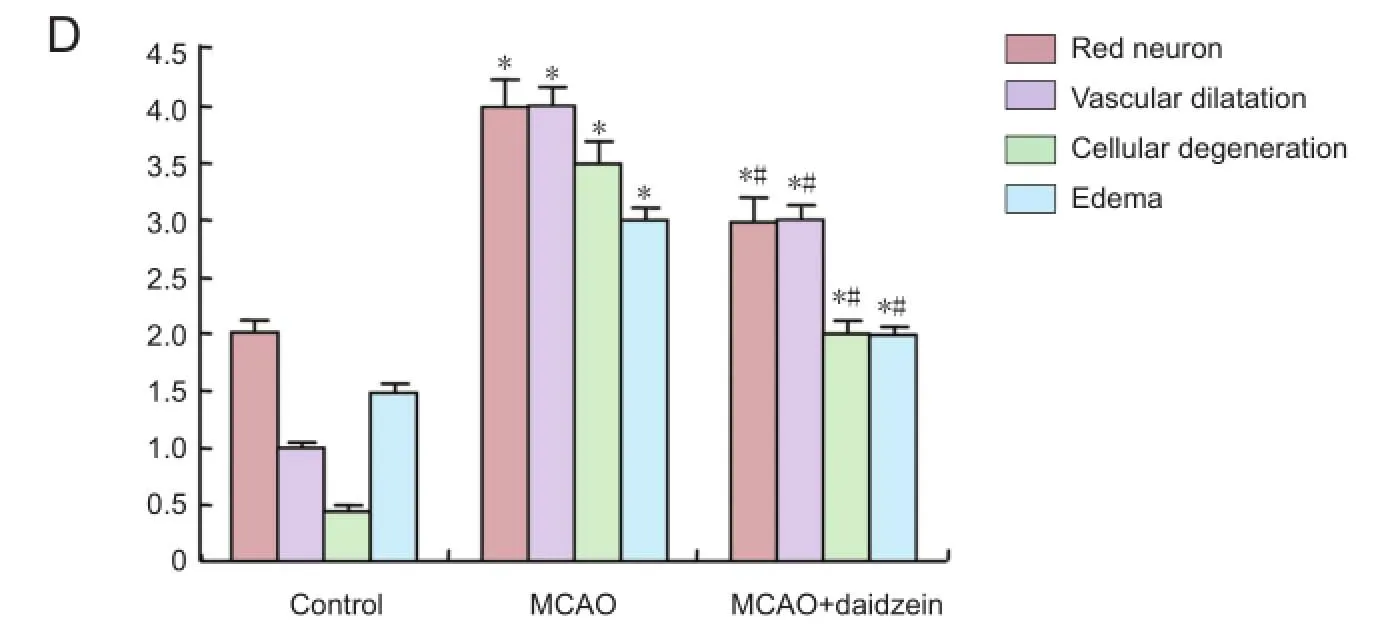
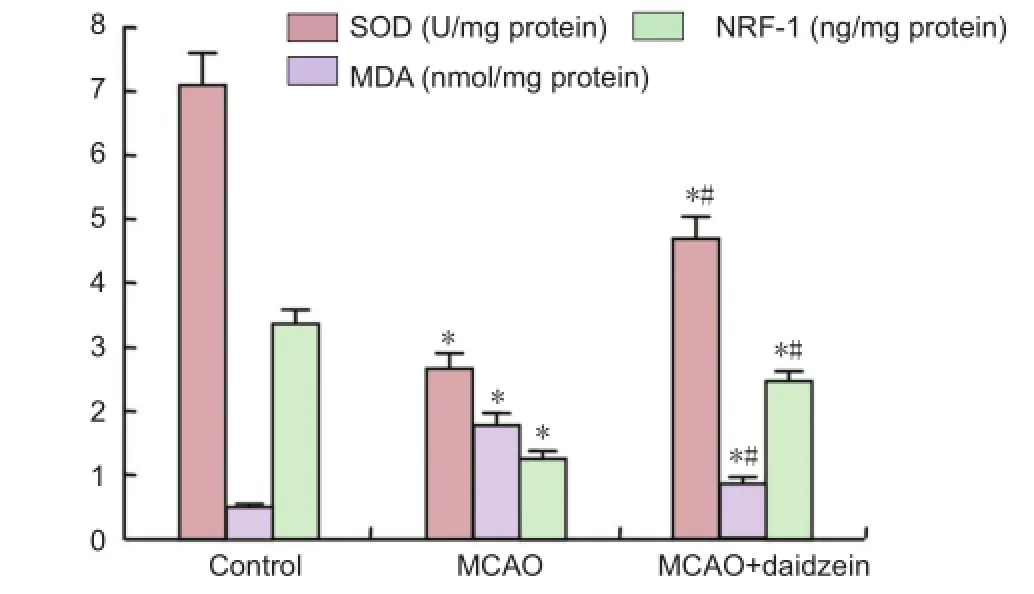
Figure 1 Superoxide dismutase (SOD) activity, malondialdehyde (MDA) and nuclear respiratory factor-1 (NRF-1) levels in ischemic cerebral tissue after 24 hours of middle cerebral artery occlusion (MCAO).
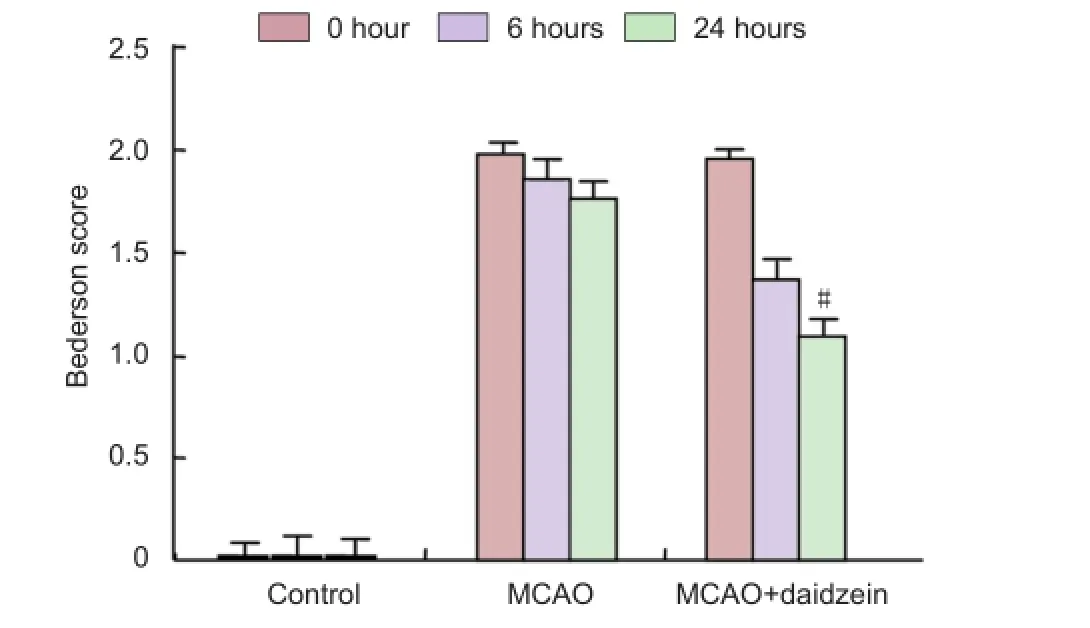
Figure 5 Effect of daidzein on neurological functinon of rats with cerebral ischemia injury before and after 6 and 24 hours of middle cerebral artery occlusion (MCAO).

Figure 4 Effects of daidzein on caspase-3 and -9 immunoreactivity in the ischemic brain area after 24 hours of middle cerebral artery occlusion (MCAO).
Functional assessment
Neurological status was scored at 0, 6 and 24 hours after ischemia. The degree of functional defcit was scored using the Bederson’s scale (Bederson et al., 1986): a score of 0, no defcit; 1, mild forelimb weakness; 2, severe forelimb weakness, consistently turns to side of defcit when lifted by tail; 3, compulsory circling; 4, unconscious; and 5, dead. The individual who evaluated functional defcits was blind to whether vehicle or drugs were administered.
Statistical analysis
A computer program (SPSS 19.0, SPSS, Chicago, IL, USA)was used for statistical analysis. Statistical analysis data are expressed as the mean ± SD. The comparisons among three groups were made by one-way analysis of variance followed by Bonferroni post-test. As histopathological results were nonparametric, they were evaluated with the Kruskal-Wallis test. Statistical signifcance was accepted as P < 0.05.
Results
Anti-oxidative effect of DZ on cerebral ischemia
As shown inFigure 1, SOD activity and NRF-1 level in the MCAO and MCAO + DZ groups were signifcantly reduced compared to the control group (P < 0.05). The SOD activity and NRF-1 level in the MCAO + DZ group were signifcantly increased than in the MCAO group (P < 0.05). The MDA levels in the MCAO and MCAO + DZ groups were signifcantly increased compared to the control group (P < 0.05). The MDA level in the MCAO + DZ group was signifcantly decreased than in the MCAO group (P < 0.05).
Effect of DZ on neuronal damage in ischemic cerebral tissue
As shown inFigures 2and3, hematoxylin-eosin and Luxol fast blue staining showed that cells and tissues with normal histomorphology in the brain tissues were observed in the control group. When the general appearance of these tissues was examined, the neuronal cells in the outer granular layer of the cortex were smaller with pyramidal or triangular shapes and had large and round nuclei, with nucleoli stained basophilic. The neuronal cells found intensely in the cortical layer had basophilic cytoplasma with some slight eosinophilic staining and very few were found in the lamina granulosa. In some examinations, increased numbers of neurons in the lamina pyramidal externa were found with eosinophilic structures. Numbers of red neurons were not too much and they were homogenously distributed in all areas of tissue (Figures 2A,3A,3D).
In the MCAO group, focal ischemia lesions were observed widely in the cerebral cortex and subcortex. Some cells in the outer granular layer were shriveled with folds into the cytoplasm and cell walls, and the areas where neuroglial cells were found with severe vacuolization were observed. Additionally, swollen areas surrounded the vacuolar areas. The microglial cells of the neuroglial cells in these areas showed intense basophilic staining and increased vacuolization was observed around them. Luxol fast blue staining of the brain tissue from the MCAO group showed that the pyramidal neurons close to the subcortex were stained lighter blue compared to the control group. While some slight basophilic staining was seen, neuronal nuclei in the external pyramidal layer were large, shriveled and stained slightly basophilic. Compared to the control group, the number of red neurons was obviously increased, with these neurons observed clustered together more frequently in the cortex (Figures 2B,3B,3D).
In the MCAO + DZ group, hematoxylin-eosin staining showed increased neuronal damage after ischemia, tissue fuid similar to edema in the dilated areas was observed, and chromatin intensity in the nuclei was greater compared to the control group. Severe acidophilic neuronal cytoplasm and karyorrhexis in red neurons with piknotic structure with damaged neuronal histomorphometric appearance were observed. The number of red neurons in the outer granular layer in the MCAO + DZ group was slightly less than in the control group and greater than in the MCAO group. In the MCAO + DZ group, red neuronal cell counts were obviously increased and the vacuoles around the cells were much larger compared to the control group, and cell degeneration was occasionally observed. However, the changes in the MCAO+DZ group were less than those observed in the MCAO group (Figures 2C,3C,3D).
Anti-apoptotic effect of DZ on cerebral ischemia
After MCAO, along with denser positive areas in the nuclei of neurons, caspase-3-immunoreactive areas located in the periphery of the nuclei were identifed as more lightly dyed with pale, ring shaped, and diffuse appearance.
Very intense caspase-3 immunoreactivity was observed in the MCAO and MCAO + DZ groups. Especially in the cortex and subcortex, increased caspase-3 immunoreactivity was observed. The caspase-3 immunoreactivity was widely spread along the ischemic zone and the nuclei of intense apoptotic cells located in this region had karyolysis structure and immunoreactivity. The caspase-3 immunoreactivity was intense in the white matter and granular cells. The caspase-3 immunoreactivity was intense especially in the cells located in the globus pallidus externus and internus. The morphology of the necrotic cells in the ischemic area was positively related to the vacuolization in the cells. The caspase-3 staining in the granular cells was identifed with different levels of caspase-3 immunoreactivity in different regions of the hippocampus. Microglial cell clusters surrounding the injury areas in the MCAO and MCAO + DZ groups showed caspase-3 immunoreactivity in the nuclei. The caspase-3 immunoreactivity in the MCAO + DZ group was less than in the MCAO group, but it was similar to the control group (Figure 4).
In the MCAO and MCAO + DZ groups, caspase-9 immunoreactivity was intense in the nuclei of pyramidal cells, but it was weak in the cytoplasm. Caspase-9 immunoreactivity was also detected in the endothelial cells of capillary vessels and in the swollen cells. Caspase-9 immunoreactivity in the hippocampal CA1, CA2 and CA3 regions in the MCAO + DZ group was similar to the control group (Figure 4).
Functional assessment
After cerebral ischemia, rats exhibited a variety of neurological defcits. No neurological defcits were observed in the rats of the control group or in the hemisphere contralateral to the ischemic side. The neurological scores were signifcantly decreased at 24 hours after DZ treatment compared to the MCAO group (P < 0.05) (Figure 5).
Discussion
Stroke is the leading cause of adult disability and mortalityand is the third largest cause of death worldwide (Bonita et al., 2004). Cerebral ischemia causes a rapid onset of neurological injury due to a decrease in blood circulation in affected areas that leads to decreased oxygen and glucose supplies, resulting in cellular damage and neurological function impairment. During ischemia, disruption of the brain’s energy metabolism, loss of aerobic glycolysis, accumulation of intracellular sodium and calcium, increase in lactate levels, production of free radicals, cell swelling, and overactivation of lipase and protease occur, resulting in cell death (Durukan et al., 2007; Brouns et al., 2009). During ischemic damage, reactive oxygen compounds are inactivated by antioxidants such as SOD, catalase, glutation peroxidase, vitamin C and E (Chan, 2001). However, neurons are extremely susceptible to oxidative damage and have limited antioxidant capacity. As a result, in recent years many studies have been performed on molecules with antioxidant and neuroprotective effects to reduce the permanent damage in ischemia models.
DZ is a polyphenolic compound in the isoflavon group. DZ protects embryonic rat primary cortical neurons from ischemia-like injury in vitro at doses typical of circulating concentrations in human populations (Schreihofer et al., 2009). Moreover, DZ administration in vivo reduced ischemia/reperfusion-induced myocardial damage via inhibition of nuclear factor-kappaB activation (Kim et al., 2009). In this study, we investigated the neuroprotective and antioxidant effects of DZ in rats with ischemic stroke histopathologically and biochemically.
SOD is a metalloenzyme that protects cells from the toxic effects of endogenous superoxide radicals. SOD catalyses superoxides to create hydrogen peroxide and is the primary protector from oxyradicals (Cosar et al., 2008). SOD, a free radical scavenger, is an endogenous antioxidant enzyme naturally found in the mitochondria. Cosar et al. (2008) found that treatment with fsh n-3 fatty acids led to a fall in SOD activity in a diabetic rat model of cerebral ischemia. According to Deng et al. (2000), SOD activity decreased after focal cerebral ischemia, but it increased again after treatment with 3,6-dimethamidodibenzopyriodonium gluconate. Results from this study showed that after ischemia, DZ treatment increased SOD activity, indicating a reduction in oxidative stress. This supports the antioxidant property of DZ.
Tissue MDA levels refect a decrease in the tissue damage and energy metabolism linked to lipid peroxidation where free radicals play a role (Cosar et al., 2008). Many studies have shown that tissue MDA levels increase in cerebral tissue after ischemia (Braughler et al., 1989). Yavuz et al. (1997) reported that tissue MDA levels increased after cerebral ischemia and decreased by a statistically signifcant amount with 2-chloroadenosine treatment. In this study, in accordance with the literature, DZ treatment given after ischemia reduced MDA levels.
NRF-1 is a transcription factor that plays a role in mitochondrial respiration, DNA transcription and replication and activates the expression of important metabolic genes regulating cellular growth and nuclear genes. NRF-1 also plays an important role in regulating enzymes that arise during antixodiant production and oxidative stress (Kumari et al., 2012). In a recent study, NRF-1 level increased in a neonatal rat of cerebral hypoxia-ischemia (Kumari et al., 2012). This study simulated the human brain embolism exactly. Therefore, in this study, intraluminal flament was not retracted and reperfusion was not allowed. As a result, NRF-1 level decreased in the MCAO group. A signifcant increase in NRF-1 level was observed in the MCAO + DZ group. In accordance with the literature, where NRF-1 level in the ischemia-reperfusion groups decreased with reperfusion time, in this study, NRF-1 level increased after use of neuroprotective agent DZ (Kumari et al., 2012; Mehta et al., 2012).
During ischemic stroke, apoptosis may start by two pathways, intrinsic and extrinsic. Cytochrome c is released from mitochondria and caspase 3 stimulation forms the intrinsic pathway (Yavuz et al., 1997). It is known that caspase-3 and caspase-9 are important in apoptosis resulting from ischemia in neuronal cells. Studies on apoptotic neuronal cells have reported that caspase-3 may be activated through both intrinsic and extrinsic signal pathways. Therefore, it is known that caspase-3 plays a key role in stroke apoptosis (Porter et al., 1999). Activated caspase-3 causes fragmentation of DNA (Abas et al., 2010). Some studies have shown a relationship between DNA fragmentation and ischemic infarction (Li et al., 1997). In this study, in the MCAO + DZ group, caspase-3 immunoreactivity was less compared to the MCAO group and was similar to that in the control group. Thus our study shows that DZ has antioxidant and neuroprotective effects on the modulation pathways of the apoptosis mechanism.
DZ protected rats from ischemia-induced brain injury, which may be due to the reduction of oxidative stress. These fndings suggest that DZ may be a clinically viable protective agent against cerebral ischemia where cellular damage is a consequence of oxidative stress. In addition, DZ may potentially be a new agent to be used in the prevention of focal cerebral ischemia, while also being inexpensive and easily available.
Author contributions:All authors participated in the design and conception of the study, definition of intellectual content, and experiment conduction and approved the final version of this article. ABA, MG, TA and HMS were responsible for literature search. ABA, MG, TA, HMS and UD contributed to data acquisition. ABA, MG, HMS, UD and YK were responsible for data analysis. ABA, MG and MC participated in statistical analysis. ABA, MG and MC participated in manuscript preparation. ABA, MG, TA, HMS, UD and MC were responsible for manuscript editing. ABA, MG, AO, UD and MC contributed to manuscript review. ABA, MG, UD and MC were the guarantors of the study.
Conficts of interest:None declared.
Abas F, Alkan T, Goren B, Taskapilioglu O, Sarandol E, Tolunay S (2010) Neuroprotective effects of postconditioning on lipid peroxidation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk Neurosurg 20:1-8.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472-476.
Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F (2004) The global stroke initiative. Lancet Neurol 3:391-393.
Bors W, Heller W, Michel C, Saran M (1990) Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 186:343-355.
Braughler JM, Hall ED (1989) Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med 6:289-301.
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111:483-495.
Burguete MC, Torregrosa G, Perez-Asensio FJ, Castello-Ruiz M, Salom JB, Gil JV, Alborch E (2006) Dietary phytoestrogens improve stroke outcome after transient focal cerebral ischemia in rats. Eur J Neurosci 23:703-710.
Cederroth CR, Nef S (2009) Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol 304:30-42.
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2-14.
Cosar M, Songur A, Sahin O, Uz E, Yilmaz R, Yagmurca M, Ozen OA (2008) The neuroprotective effect of fsh n-3 fatty acids in the hippocampus of diabetic rats. Nutr Neurosci 11:161-166.
Deng XL, Qian ZY, Liu NF, Ma XY, Wang HF, Hou ZJ (2000) Antagonistic effect of 3,6-dimethamidodibenzopyriodonium gluconate on lipid peroxidation in cerebral cortical neuronal cultures and rat brains during focal cerebral ischemia reperfusion. Acta Pharmacol Sin 21:460-462.
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87:179-197.
Formica JV, Regelson W (1995) Review of the biology of Quercetin and related biofavonoids. Food Chem Toxicol 33:1061-1080.
Grace PA (1994) Ischaemia-reperfusion injury. Br J Surg 81:637-647.
Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA (1998) A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab 18:367-375.
Hertog MGL, Hollman PCH, van de Putte B (1993) Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J Agric Food Chem 41:1242-1246.
Islekel S, Islekel H, Guner G, Ozdamar N (1999) Alterations in superoxide dismutase, glutathione peroxidase and catalase activities in experimental cerebral ischemia-reperfusion. Res Exp Med (Berl) 199:167-176.
Kawamura S, Li Y, Shirasawa M, Yasui N, Fukasawa H (1994) Reversible middle cerebral artery occlusion in rats using an intraluminal thread technique. Surg Neurol 41:368-373.
Kim JW, Jin YC, Kim YM, Rhie S, Kim HJ, Seo HG, Lee JH, Ha YL, Chang KC (2009) Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kappaB activation. Life Sci 84:227-234.
Kumari S, Anderson L, Farmer S, Mehta SL, Li PA (2012) Hyperglycemia alters mitochondrial fssion and fusion proteins in mice subjected to cerebral ischemia and reperfusion. Transl Stroke Res 3:296-304.
Lephart ED, Adlercreutz H, Lund TD (2001) Dietary soy phytoestrogen effects on brain structure and aromatase in Long-Evans rats. Neuroreport 12:3451-3455.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489.
Mathey J, Lamothe V, Coxam V, Potier M, Sauvant P, Bennetau-Pelissero C (2006) Concentrations of isofavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isofavones. J Pharm Biomed Anal 41:957-965.
Mehta SL, Kumari S, Mendelev N, Li PA (2012) Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci 13:79.
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99-104.
Schreihofer DA, Redmond L (2009) Soy phytoestrogens are neuroprotective against stroke-like injury in vitro. Neuroscience 158:602-609.
Stavric B (1994) Role of chemopreventers in human diet. Clin Biochem 27:319-332.
Yavuz O, Turkozkan N, Bilgihan A, Dogulu F, Aykol S (1997) The effect of 2-chloroadenosine on lipid peroxide level during experimental cerebral ischemia-reperfusion in gerbils. Free Radic Biol Med 22:337-341.
Copyedited by Jackson C, Li HF, Li CH, Song LP, Zhao M
*Correspondence to: Adem Bozkurt Aras, guven2340@hotmail.com.
10.4103/1673-5374.150724
http://www.nrronline.org/
Accepted: 2014-11-16
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

