Bacterial melanin promotes recovery after sciatic nerve injury in rats
Olga. V. Gevorkyan, Irina B. Meliksetyan, Tigran R. Petrosyan, Anichka S. Hovsepyan
1 Institute of Physiology, National Academy of Sciences of Armenia, Orbeli str. 22, Yerevan, Armenia
2 Department of Kinesiology, Armenian State Institute of Physical Culture, Alex Manukyan 11, Yerevan, Armenia
3 Scientifc and Production Center “Armbiotechnology”, National Academy of Sciences of Armenia, Yerevan, Armenia
Bacterial melanin promotes recovery after sciatic nerve injury in rats
Olga. V. Gevorkyan1, Irina B. Meliksetyan1, Tigran R. Petrosyan2,*, Anichka S. Hovsepyan3
1 Institute of Physiology, National Academy of Sciences of Armenia, Orbeli str. 22, Yerevan, Armenia
2 Department of Kinesiology, Armenian State Institute of Physical Culture, Alex Manukyan 11, Yerevan, Armenia
3 Scientifc and Production Center “Armbiotechnology”, National Academy of Sciences of Armenia, Yerevan, Armenia
Bacterial melanin, obtained from the mutant strain of Bacillus Thuringiensis, has been shown to promote recovery after central nervous system injury. It is hypothesized, in this study, that bacterial melanin can promote structural and functional recovery after peripheral nerve injury. Rats subjected to sciatic nerve transection were intramuscularly administered bacterial melanin. The sciatic nerve transected rats that did not receive intramuscular administration of bacterial melanin served as controls. Behavior tests showed that compared to control rats, the time taken for instrumental conditioned refex recovery was signifcantly shorter and the ability to keep the balance on the rotating bar was signifcantly better in bacterial melanin-treated rats. Histomorphological tests showed that bacterial melanin promoted axon regeneration after sciatic nerve injury. These fndings suggest that bacterial melanin exhibits neuroprotective effects on injured sciatic nerve, contributes to limb motor function recovery, and therefore can be used for rehabilitation treatment of peripheral nerve injury.
nerve regeneration; peripheral nerve injury; sciatic nerve injury; bacterial melanin; motor function; histomorphology; behaviors; neural regeneration
Gevorkyan OV, Meliksetyan IB, Petrosyan TR, Hovsepyan AS (2015) Bacterial melanin promotes recovery after sciatic nerve injury in rats. Neural Regen Res 10(1):124-127.
Introduction
Reconstruction of injured peripheral nerve is one of the main problems of modern reconstruction microsurgery. Nervous system injury causes local reactions in damaged tissue including inflammation, ischemic necrosis, secondary cell destruction, and scar formation. During the first 2 weeks after nervous system injury, axons poorly interact with Schwann cells. Their growth is also blocked by changes in microstructure of denervated distal segments (Röyttä and Salonen, 1988). “Crumpled bands of Bungner” are formed and exist for 18 months after nerve transection (Terenghi et al., 1998). Therefore, suppression of secondary changes after nerve damage is not less important. Evidently, nerve regeneration depends more on the application of cells and/or exogenous peptides (Liu et al., 2013; Ma et al., 2013; Tamaddonfard et al., 2013), than on the most perfect microsurgical technique (Roth et al., 2011; Breshah et al., 2013). That is why application of physiologically active substances, regulating the cascade of degeneration and regeneration processes of nervous tissue, is important for the optimization of regeneration process. Peripheral nerve injury entails sprouting of motoneuron axons, activates growth factors, neurotrophins, and glial reaction (Gupta and Steward, 2003). Mentioned factors (growth factors, neurotrophins, and other peptides) are used for rehabilitation treatment of peripheral nerve injury.
Bacterial melanin was obtained from the mutant strain of Bacillus Thuringiensis at the Institute of Biotechnology in Armenia. Special attention was paid to the role of bacterial melanin in neurodegeneration (Gevorkyan et al., 2007). Bacterial melanin has been shown to exhibit effects on recovery after injuries to different central nervous system structures, such as corticospinal tract, rubrospinal tract, lateral cerebellar nuclei, and sensorimotor cortex (Fanardzhyan et al., 2001; Manvelian et al., 2008; Petrosyan et al., 2009). In rats with pyramidal tract damage, bacterial melanin accelerated motor function recovery and restored motor nerve conduction, as confrmed by electrophysiological experiments (Petrosyan et al., 2012). In experiments using rats with destruction of substantia nigra, bacterial melanin benefted for cell viability, regeneration (Petrosyan et al., 2014a) and functional recovery (Petrosyan et al, 2014b). Therefore, in this study we analyzed functional recovery and histomorphological changes in injured sciatic nerve of rats after intramuscular administration of bacterial melanin.
Materials and Methods
Animals
Twelve adult white mongrel male rats weighing 180-250 g were used in this study. They were housed with their littermates in plastic boxes covered by a wire lid and maintained on a standard light-dark cycle with food and water available ad libitum. All rats were randomly and evenly divided into a control group and a bacterial melanin-injected group. Theywere initially trained to induce instrumental conditioned reflex (ICR) and then were subjected to unilateral sciatic nerve transection. Care and use of rats were in accordance with institutional guidelines and national and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 86-23, 1985). The study was approved by the Ethical Committee of Armenian National Academy of Sciences. All efforts were made to minimize the number of rats used in this study and their suffering.
Elaboration of ICR
The ICR was developed as follows: rats were trained to balance on a slowly rotating (9 rounds/min) horizontal bar (diameter 2 cm and length 30 cm) located at a height of 90 cm above a soft pillow. Training to the balancing refex was assessed in terms of the time spent by the rat on the rotating bar, on which the rat balanced exclusively using the hindpaws, which they alternated. At the moment the rats were placed on the bar, they clung to it with their forepaws, but after establishment of body balance they lay calmly on the bar or hung freely from it. Trials were repeated 10 times daily, with intervals of 60 seconds. Initial training to ICR was conducted in 4 days. The criterion for performance of the refex was the time spent for balancing on the rotating bar being at least 250 seconds (Kennedy, 1990; Fanardzhyan et al., 2002). After elaboration of ICR, when strengthening of the conditioned refex was completed, rats underwent unilateral sciatic nerve transection. ICR tests were resumed 1 day after unilateral sciatic nerve transection.
Surgery
When training to ICR was performed, all rats were anesthetized with Nembutal (35-40 mg/kg, intraperitoneally) and were subjected to unilateral sciatic nerve transection. The right sciatic nerve was exposed in the upper thigh region and the transection was performed with microscissors. Immediately after transection, an anastomotic procedure was conducted under a microsurgical microscope (Carl Zeiss AG, Switzerland). The anastomoses were performed by suturing. The epineurium of both nerve endings were approximated using 10-0 prolene sutures.
Administration of bacterial melanin
On the day after surgery, six rats in the bacterial melanin-injected group were intramuscularly administered bacterial melanin solution (obtained from the mutant strain of Bacillus Thuringiensis at the Institute of Biotechnology in Armenia and prepared into 6 mg/mL solution). Intramuscular injection of bacterial melanin solution was performed in femoral region of operated limb on the second day after unilateral sciatic nerve transection. The volume of bacterial melanin solution for intramuscular administration was determined according to the optimally tolerated dose of 170 g/kg. In the control group, bacterial melanin administration was not performed, but ICR tests were resumed.
Histomorphological observation
At 55 days after unilateral sciatic nerve transection, behavioural experiments were performed. After deep anesthesia with 45-50 mg/kg Nembutal, rats were decapitated. After unilateral sciatic nerve transaction, the anastomotic area was harvested. Frozen longitudinal sections (50 µm thick) were transferred into a freshly prepared mixture for identifcation of Ca2+-dependent acid phosphatase activity (Gevorkyan et al., 2007). The initial mixture contained 20 mL 0.38% lead acetate solution, 5 mL acetate buffer (pH 5.6), and 5 mL 2% β sodium glycerophosphate solution. This mixture was adjusted with 3% calcium chloride solution (non-fused) to 100 mL, and then fltered. Incubation of sections in this mixture was carried out in a thermostat at 37°C for 1-3 hours. Then the sections were rinsed with distilled water three to five times for 5 minutes each, developed in the sodium sulphide solution, washed repeatedly, and fnally mounted in the balsam. Finally, the sections were carefully inspected at different microscopic (Carl Zeiss Microscope OPTON, Switzerland) magnifcations (Figure 1).
Statistical analysis
Values indicated throughout the results are presented as the mean ± SD. Whenever possible, significance of the results was assessed using Student’s t-test (computed in Microsoft EXCEL 2010, Microsoft).
Results
Behavioral tests
After unilateral sciatic nerve transection, ICR tests were resumed on the next day. ICR tests showed that all rats on the third day after sciatic nerve transection started using the operated hindlimb in locomotion. Both control rats and bacterial melanin-treated rats used the operated limb in locomotion, putting it on the foor and holding the paw vertically to the floor. The hindlimb and paw phalanxes were paralyzed in all rats. At that time, the movement of bacterial melanin-treated rats was faster than that of control rats. The control rats were trying to put their knees on the rotating bar, but without success, and in 5-10 seconds they fell down. Rats injected with bacterial melanin were able to put their knees on the rotating bar, but in 10 seconds the limb slid from the bar and hanged down. However, bacterial melanin-treated rats kept the balance on the rotating bar for a considerably longer period (30-50 seconds) than control rats; moreover, during the whole testing period, bacterial melanin-treated rats were trying to put back the hanging limb. Not always the rats succeeded in their efforts to hold the operated limb on the bar, and after balancing on the bar for 100-120 seconds, they fell down. Paralyzed paw and phalanxes of bacterial melanin-treated rats became active after the 23rdexperimental day, and after 54 testing days (testing the animal 10 times daily), balancing movements of the operated hindlimb were close to normal (250 seconds). At that time, the rats were able to hold the limb on the bar and put the phalanxes on it, only for some short episodes the limb slid from the bar, but the rats were able to put the limbback to keep the balance till the testing period was over (250 seconds). The time spent for ICR recovery for the control rats, or the balancing time spent for their injured hind limb in 54 testing days gradually increased, but the activity of the paw and phalanxes was not changed. The paw and phalanxes gradually became atrophied and were almost shriveled at the end of the experiments. Thus, the criteria for the recovery of hindlimb’s motor function after sciatic nerve transaction were not only the rat’s ability to hold the injured limb with paw and phalanxes on the rotating bar but also to keep balance by moving them. Only the rats injected with bacterial melanin managed to complete the task, whereas in control rats the balancing movements of injured limb did not recover for the whole period of study (55 days). At that time point, control rats managed to keep balance for 166 ± 19.6 seconds, whereas all bacterial melanin-treated rats were able to complete the task and keep balance on the rotating bar for 250 ± 11.2 seconds (P < 0.05).
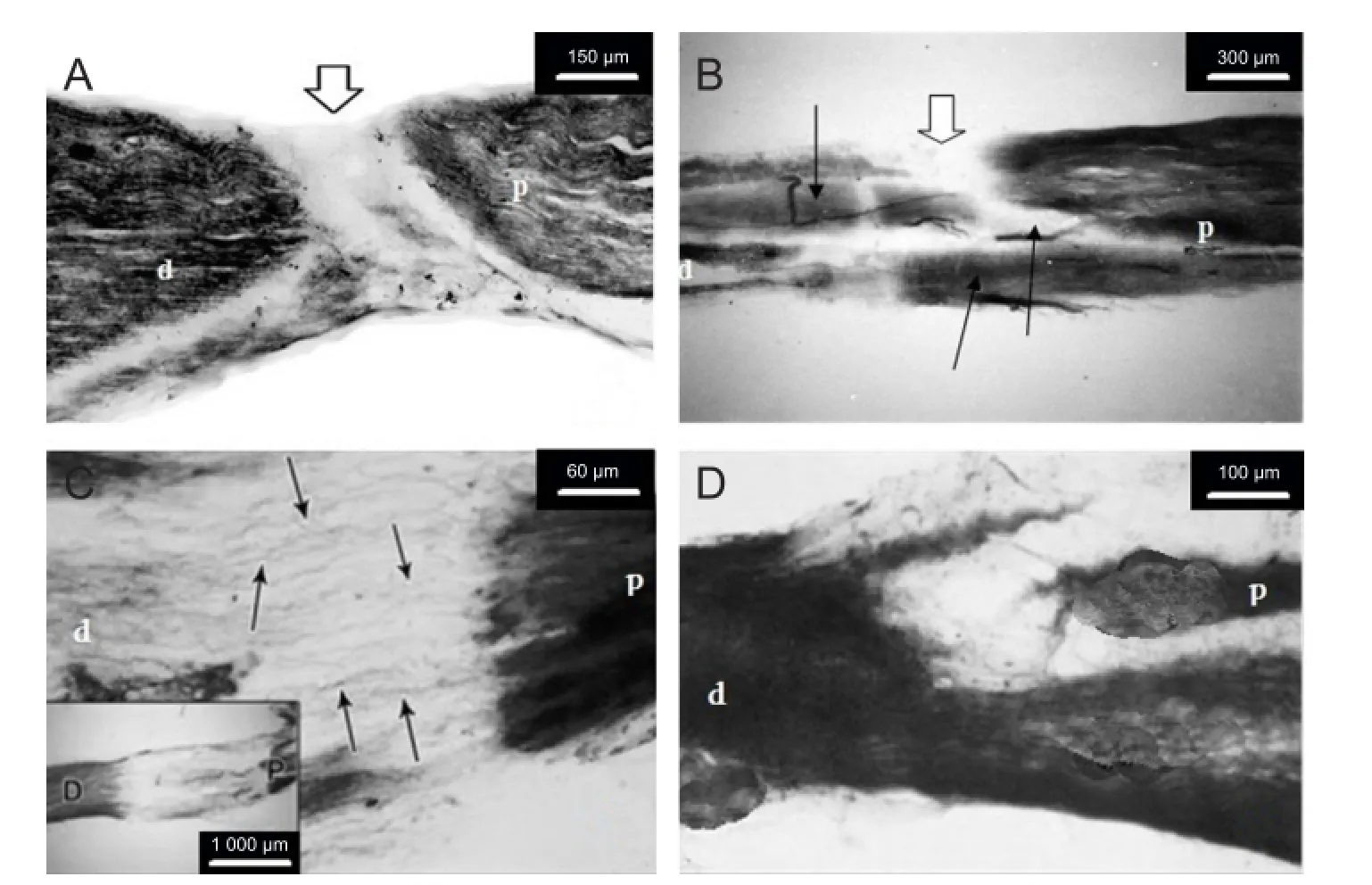
Figure 1 Longitudinal sections of the sciatic nerve of control (A, C) and bacterial melanin-treated (B, D) rats prepared 55 days after unilateral sciatic nerve transection.
Histomorphological observation
Histomorphological data revealed absence of regeneration processes in transection area of control rats (Figure 1A). As a rule, the distal segment of the anastomized nerve has a stub end, because of necrosis and destruction of afferent and efferent fbers. After sciatic nerve transection, secondary degeneration occurred, characterized by diameter irregularity of nerve fbers and structural changes in the form of swelling and fber delamination.
In the bacterial melanin-treated group, axonal thickening, convolution, vacuolization and fragmentation (in some areas) were observed. Along with degeneration processes, at the proximal nerve segment, slight proliferation of Schwann cells was observed. Cell mass formed around the transected nerve ending, which was of small size and narrowed progressively, but it never reached the distal segment and did not grow into it. Bacterial melanin preserved the enzyme activity along almost the whole length of the nerve, with an insignificant prevalence in the proximal segment (Figure 1B). In the area of compression, random alternation in the activity of kreatine phosphate was revealed, which was manifested with weak or strong staining of nerve fbers. In middle and distal parts, blood vessels dilated. Histomorphological data of this study indicate that bacterial melanin can induce regeneration of damaged peripheral nerve.
Discussion
Results from this study suggest that bacterial melanin has neuroprotective effects and promotes regeneration and motor function recovery. Similar effects have been observed in a study on the effects of melanocyte-stimulating hormone (Luneberg and Flohr, 1989). In this study, recovery of ICR after unilateral sciatic nerve transection also started, but limb movements did not recover, in control rats, and so the control rats were not able to complete the ICR task. Different time periods spent for ICR recovery in sciatic nerve transected rats treated with or without bacterial melanin provide evidence that bacterial melanin can accelerate the recovery process after sciatic nerve injury. It remains possible that bacterial melanin can accelerate the process of axon sprouting or nerve invasion (Murakami et al., 1987). Histomorphological results regarding the sections of experimental rats also showed that bacterial melanin administrationwith the aim of promoting clinical recovery after peripheral nerve damage probably activates a series of trophic processes contributing to nerve regeneration (Griffin et al., 1992; Deckert-Schulter et al., 1992; Raivich et al., 1999). The main challenge in the facilitation of nerve regeneration is to prevent scar tissue formation in the injured area (Castañeda and Kinne, 2002). Results from this study showed that bacterial melanin stimulated vascularization and led to capillary dilation. Nerve sections of bacterial melanin-treated rats contained more newly generated nerve fbers without scar tissue formation than the sections of control rats. These fndings suggest that bacterial melanin can improve the directional growth of regenerating axon sprouts and therefore can be used for rehabilitation treatment of peripheral nerve injury.
Author contributions:OVG designed the research and performed behavioral experiments and operations. IBM performed histomorphological experiments. TRP performed behavioral experiments, analyzed data and drafted the manuscript. ASH provided technical and material support. All authors approved the final version of this manuscript.
Conficts of interest:None declared.
Breshah MN, Sadakah AA, Eldrieny EA, Saad KA (2013) Functional and histological evaluation of rat sciatic nerve anastomosis using cyanoacrylate and fbrin glue. Tanta Dent J 10:67-74.
Castañeda F, Kinne RK (2002) Omental graft improves functional recovery of transected peripheral nerve. Muscle Nerve 26:527-532.
Deckert-Schulter M, Schluter D, Hof H, Wiestler OD (1992) Rapid progress in the Encephalitis. Semin Immunol 4:111-119.
Fanardzhyan VV, Gevorkyan OV, Mallina RK, Melik-Musyan AB, Meliksetyan IB (2002) Dynamics of changes in operant reflexes in rats after transection of the corticospinal tract and removal of the sensorimotor region of the cerebral cortex. Neurosci Behav Physiol 32:477-484.
Gevorkyan OV, Meliksetyan IB, Ovsepyan AS, Sagiyan AS (2007) Effects of BT-melanin on recovery of operant conditioned reflexes in rats after ablation of the sensorimotor cortex. Neurosci Behav Physiol 37:471-476.
Griffn DE, Levine B, Tyor WR, Irani DN (1992) The immune response in viral feld of infectious and autoimmune disorders of the nervous system. Neuropathol Appl 18:424-433.
Gupta R, Steward OJ ( 2003) Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. Comp Neurol 461:174-186.
Kennedy PR (1990) Corticospinal, rubrospinal and rubroolivary projections: a unifying hypothesis. Trends Neurosci 13:474-479.
Liu B, Liu Y, Yang G, Xu ZM, Chen JJ (2013) Ursolic acid induces neural regeneration after sciatic nerve injury. Neural Regen Res 8:2510-2519.
Luneberg U, Flohr H (1989) Vestibular Compensation: Facts, Theories and clinical perspectives, pp161-174. Elsevier, Paris.
Ma SZ, Peng CL, Wu SQ, Wu DJ, Gao CZ (2013) Sciatic nerve regeneration using a nerve growth factor-containing fbrin glue membrane. Neural Regen Res 8:3416-3422.
Manvelian LP, Gevorkian OV, Petrosian TR (2008) Recovery of instrumental conditional refexes in rats after pyramidotomy and action of bacterial melanin. Zh Evol Biokhim Fiziol 44:268-273.
Meiksetyan IB (2007) Revealing the activity of Ca2+- dependent acidic phosphatase in the rat brain cellular structures. Morphol Russian (St. Petersburg) 131:77-80.
Murakami F, Higashi S, Katsumaru J, Oda Y (1987) Formation of new corticorubral synapses as a mechanisms for classical conditioning in the cat. Brain Res 437:319-382.
Petrosyan TR, Gevorkyan OV, Meliksetyan IB, Hovsepyan AS, Sagiyan AS, Manvelyan LR (2009) Recovery processes in Rats after Unilateral Pyramidotomy, Destruction of Rubrospinal Tract and Action of Bacterial Melanin. In: International Symposium “Immune System of the Brain: Neurochemical and Neuroendocrine Aspects”, dedicated to the 80th Anniversary of Academician Armen Galoyan, Yerevan. pp90.
Petrosyan TR, Gevorkyan OV, Meliksetyan IB, Hovsepyan AS, Manvelyan LR (2012) Neuroprotective action of bacterial melanin in rats after corticospinal tract lesions. Pathophysiology 19:71-80.
Petrosyan TR, Gevorkyan OV, Chavushyan VA, Meliksetyan IB, Hovsepyan AS, Manvelyan LR (2014a) Effects of bacterial melanin on motor recovery and regeneration after unilateral destruction of substantia nigra pars compacta in rats. Neuropeptides 48:37-46.
Petrosyan TR, Gevorkyan OV, Hovsepyan AS (2014b) Effects of bacterial melanin on movement, posture, and skilled balancing defcits after unilateral destruction of substantia nigra pars compacta in rats. J Mot Behav 46:67-72.
Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30:77-105.
Roth J, Shtokman J, Shamir MH, Nissan M, Shchetinkov L, Trejo LL, Rochkind S (2011) Regeneration of the transected rat sciatic nerve after suturing or adhesion with cyanoacrylate glue. J Neurosurg 114:245-252.
Röyttä M, Salonen V (1988) Long-term endoneurial changes after nerve transection. Acta Neuropathol 76:35-45.
Tamaddonfard E, Farshid AA, Ahmadian E, Hamidhoseyni A (2013) Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran J Basic Med Sci 16:83-90.
Terenghi G, Calder JS, Birch R, Hall SM (1998) A morphological study of Schwann cells and axonal regeneration in chronically transected human peripheral nerves. J Hand Surg Br 23:583-587.
Copyedited by Varejao AS, Martinez AM, Li CH, Song LP, Zhao M
*Correspondence yo: Tigran R. Petrosyan, tigpetrosyan@mail.ru.
10.4103/1673-5374.150719
http://www.nrronline.org/
Accepted: 2014-12-16
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

