Expression pattern of neuregulin-1 type III during the development of the peripheral nervous system
Liang-liang Huang, Zhong-yang Liu, Jing-hui Huang, Zhuo-jing Luo
Department of Orthopedics, Xijing Hospital, the Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
Expression pattern of neuregulin-1 type III during the development of the peripheral nervous system
Liang-liang Huang, Zhong-yang Liu, Jing-hui Huang*, Zhuo-jing Luo*
Department of Orthopedics, Xijing Hospital, the Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
Neuregulin-1 type III is a key regulator in Schwann cell proliferation, committing to a myelinating fate and regulating myelin sheath thickness. However, the expression pattern of neuregulin-1 type III in the peripheral nervous system during developmental periods (such as the premyelinating stage, myelinating stage and postmyelinating stage) has rarely been studied. In this study, dorsal root ganglia were isolated from rats between postnatal day 1 and postnatal day 56. The expression pattern of neuregulin-1 type III in dorsal root ganglia neurons at various developmental stages were compared by quantitative real-time polymerase chain reaction, western blot assay and immunofuorescent staining. The expression of neuregulin-1 type III mRNA reached its peak at postnatal day 3 and then stabilized at a relative high expression level from postnatal day 3 to postnatal day 56. The expression of neuregulin-1 type III protein increased gradually from postnatal day 1, reached a peak at postnatal day 28, and then decreased at postnatal day 56. Immunofuorescent staining results showed a similar tendency to western blot assay results. Experimental findings indicate that the expression of neuregulin-1 type III in rat dorsal root ganglion was increased during the premyelinating (from postnatal day 2 to postnatal day 5) and myelinating stage (from postnatal day 5 to postnatal day 10), but remained at a high level in the postmyelinating stage (after postnatal day 10).
nerve regeneration; Schwann cells; dorsal root ganglia; myelin sheath; neuregulin-1 type III; peripheral nervous system; quantitative real-time polymerase chain reaction; western blot; immunofluorescent staining; postmyelinating; rats; NSFC grants; neural regeneration
Funding:This study was supported by grants from the National Program on Key Basic Research Project of China (973 Program), No. 2014CB542206; the National Natural Science Foundation of China, No. 81201389, 30973052; and Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China, No. IRT13051.
Huang LL, Liu ZY, Huang JH, Luo ZJ (2015) Expression pattern of neuregulin-1 type III during the development of the peripheral nervous system. Neural Regen Res 10(1):65-70.
Introduction
Neuregulin-1, a subfamily of the epidermal growth factor family, plays a role in the development of the peripheral nervous system (Birchmeier and Nave, 2008; Newbern and Birchmeier, 2010). The neuregulin-1 gene encodes an entire family of more than 15 transmembrane and secreted isoforms via alternative RNA splicing and promoter usage (Falls, 2003a; Hu et al., 2006). All of these isoforms contain an epidermal growth factor-like domain, which alone is suffcient to bind and activate ErbB receptor tyrosine kinases, and can be classifed into three subgroups (type I, II, III) according to the N-terminal sequence. Types I and II, containing Ig-like domain (types I, II) called “Ig-neuregulins,” can either be directly secreted or can be released as soluble proteins from the cell surface following proteolytic cleavage (Falls, 2003b). Type III, containing a cysteine-rich domain called “cysteine-rich domain-neuregulins,” require proteolytic cleavage for full activity and signal in a juxtacrine fashion (Hu et al., 2006).
Numerous studies have demonstrated that neuregulin-1 acts as a vital regulator at many stages of the Schwann cell lineage through binding to ErbB receptors, including the survival of Schwann cell precursors, Schwann cell proliferation, motility, axon ensheathment and myelination (Garratt et al., 2000a, b; Nave and Salzer, 2006; Birchmeier and Nave, 2008; Limpert and Carter, 2010; Syed et al., 2010; Heermann et al., 2011). Targeted ablation of the neuregulin-1 gene or its receptors resulted in severe deficiency of Schwann cells, which demonstrated the importance of neuregulin-1 in the Schwann cell lineage (Meyer and Birchmeier, 1995; Lyons et al., 2005; Atanasoski et al., 2006; Fricker et al., 2009). Specifc knockout of neuregulin-1 type III demonstrated that type III is the key isoform required for Schwann cell generation (Wolpowitz et al., 2000). Recent studies have confrmed that the level of axon-derived neuregulin-1 type III provides an instructive signal for Schwann cells committing to a myelinating fate and regulating myelin sheath thickness (Michailov et al., 2004; Taveggia et al., 2005; Chen et al., 2006). Knockout of neuregulin-1 type III in adults showed no signifcant differences in G-ratio and axon diameter, indicating that neuregulin-1 type III is unnecessary for maintenance of the myelin sheath in adult animals (Fricker et al., 2011).
At present, the expression pattern of neuregulin-1 type III in the peripheral nervous system during development has never been studied in vivo. Of interest is whether neuregulin-1 type III would be up-regulated during the myelinating stage and down-regulated in the following stages.
We examined the patterns of expression of neuregulin-1 type III during specific developmental periods in an attempt to gain a better understanding of interactions between glial cells and neurons that are responsible for myelination and myelin sheath maintenance. We analyzed the mRNA and protein expression levels of neuregulin-1 type III of intact rat dorsal root ganglia isolated at developmental time points ranging from postnatal day 1 to postnatal day 56 by quantitative real-time polymerase chain reaction and western blot assay. Additionally, we used immunofuorescent staining to detect the pattern of neuregulin-1 type III protein expression.
Materials and Methods
Experimental animals
Postnatal Sprague-Dawley rats were provided by the Experimental Animal Center of the Fourth Military Medical University of Chinese PLA (Xi’an, Shaanxi Province, China). All the experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1985).
Isolation of rat dorsal root ganglia
Dorsal root ganglias were isolated from postnatal Sprague-Dawley rats (provided by the Experimental Animal Center of the Fourth Military Medical University of Chinese PLA) at various developmental time points ranging from postnatal day 1 to postnatal day 56 (at 1, 3, 7, 14, 28, 56 days) with 20 rats per time point, which encompasses the proliferating stage, the premyelinating stage, the myelinating stage and the postmyelinating stage (Reinhard et al., 2009).
Total RNA isolation
Total RNA was isolated from dorsal root ganglias using the PARIS kit according to the manufacturer’s instructions (Ambion Inc., Austin, Texas, USA). The RNA was placed on ice and a Beckman DU-600 spectrometer (Beckman coulter, San Francisco, California, USA) was used to measure RNA concentration and purity by comparing the A260nm/A280nmratio. The RNA sample was then immediately stored at −80°C.
Quantitative real-time polymerase chain reaction
The total RNA isolated from dorsal root ganglias at various developmental time points was used for quantitative real-time polymerase chain reaction. The cDNA was synthesized using Superscript III reagents according to the manufacturer’s instructions (Invitrogen Corp., Carlsbad, CA, USA). The cDNA products were stored at −80°C until samples from all time points were ready to be analyzed. Quantitative real-time polymerase chain reaction was performed using an Eppendorf Master Cycler ep realplex Thermal Cycler and the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The primer sequences used in this study are shown inTable 1. Quantitative real-time polymerase chain reaction conditions were as follows: denaturation at 95°C for 30 seconds; primer annealing at 59°C for 30 seconds; and elongation at 72°C for 40 seconds. Quantification of PCR products was performed using the 2-ΔΔCtmethod (Sun et al., 2003). Quantities of mRNA were normalized to the housekeeping gene GAPDH. Assays were performed three times using triplicate wells.
Western blot assay
Dorsal root ganglias were isolated from rats at various developmental time points. Dorsal root ganglia homogenates were prepared by homogenizing dorsal root ganglias in 10 volumes of ice cold lysis buffer (50 mM Tris pH 7.4; 150 mM NaCl; 1% NP-40; 1 mM EDTA) containing protease inhibitors (100 µM Antipain; 5 µg/mL Leupeptin; 5 µg/mL Pepstatin). Lysates were incubated for 30 minutes at 4°C and centrifuged at 10,000 × g for 10 minutes at 4°C. Supernatants were collected and protein was quantifed using the Bradford protein assay (Bio-Rad). Samples of 10 µg from each lysate were loaded on a 10% sodium dodecyl sulfate-polyacrylamide slab gel and transferred to nitrocellulose membranes (45 µm, Invitrogen Corp., Carlsbad, CA, USA) in 25 mM Trizma-base, 192 mM glycine and 20% methanol, pH 8.3, using a Mini Trans-Blot system (Bio-Rad) for 1 hour at 100 V. After blotting, membranes were blocked at 4°C overnight with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween20 (TBST). Blots were then incubated at 4°C for 1 hour with rabbit anti-neuregulin-1 type III polyclonal antibody (1:1,000; Abcam Inc., UK). After rinsing membranes with TBST, secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (1:2,000; Sigma, St. Louis, MO, USA) were incubated for 1 hour. Membranes were incubated in NEN luminal reagent (NENTMLife Science Products) and exposed to X-ray flm. The developed flms were transilluminated and photographed with a Nikon 990 digital camera (Nikon Corporation, Chiyoda-ku, Tokyo, Japan). Densitometry of individual bands was performed using ImageJ software 1.46 m (http:// rsb.info.nih.gov/ij/). Values were normalized to the optical density of GAPDH. Results are representative of at least three trials from different dorsal root ganglia preparations for each developmental time point and for each antibody.
Immunohistochemistry
Dorsal root ganglias were isolated from rats at various developmental time points, post-fxed, and sectioned. Sections were subjected to immunofluorescent staining following a standard procedure. In brief, sections were incubated with 0.3% (v/v) Triton-X for 30 minutes and then blocked in 10% horse serum for 2 hours. The sections were then incubated with rabbit anti-neuregulin-1 type III polyclonal antibody (1:400; Abcam Inc.) and mouse anti-NF200 monoclonal antibody (1:200; Sigma) at 4°C for 24 hours. Next, the primary antibodies were probed with either indocarbocyanine-conju-gated goat anti-rabbit monoclonal antibody (1:1,000; Abcam Inc.) or fuorescein-conjugated goat anti-mouse monoclonal antibody (1:1,000; Abcam Inc.), and DAPI (10 µg/mL; Sigma), a fluorescent nuclear dye. All of the incubation steps, except the overnight incubation, were performed at room temperature. Samples were rinsed three times in PBS (pH 7.4) between each step. Sections were then examined under a laser confocal microscope (FV1000; Olympus, Tokyo, Japan). All images were taken under the same conditions and parameters. Red fluorescence intensity represented the expression level of neuregulin-1 type III protein.
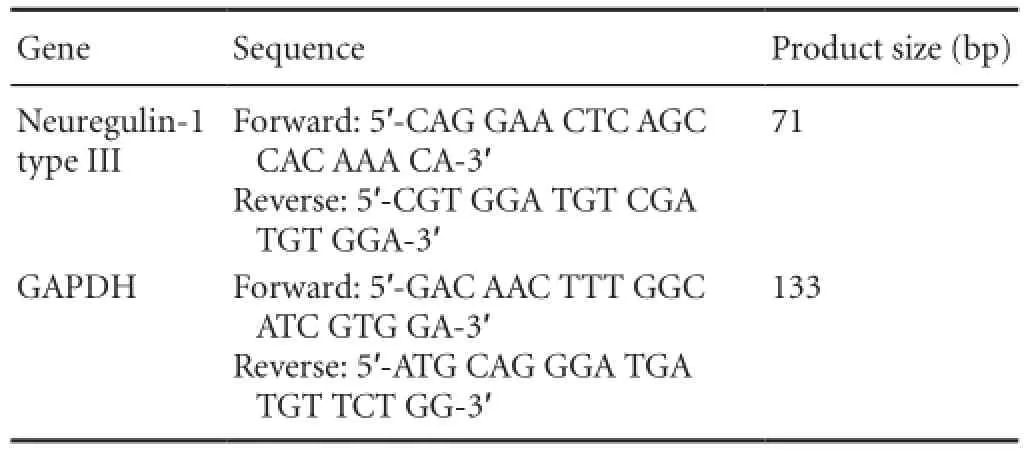
Table 1 Primer sequences used for quantitative real-time polymerase chain reaction
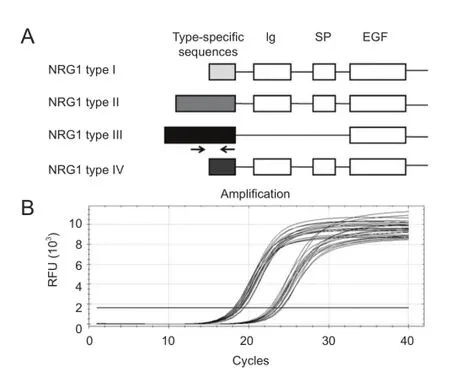
Figure 1 Diagram of neuregulin-1 (NRG1) gene structure and amplifcation curves of quantitative real-time polymerase chain reaction.
Statistical analysis
All data were presented as the mean ± SD. One-way analysis of variance was used for statistical comparison of the means. Signifcant results were analyzed by Tukey’s post hoc testing using GraphPad Prism for Windows, version 5.0 software (GraphPad Software, San Diego, CA, USA). A P value of less than 0.05 was considered statistically signifcant.
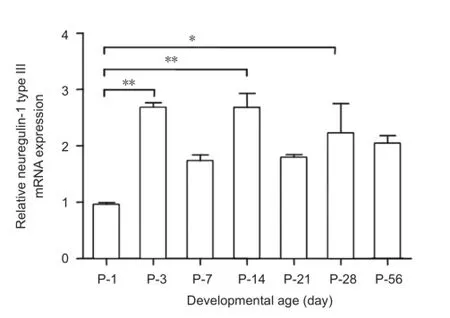
Figure 2 Relative neuregulin-1 (NRG1) type III mRNA expression at different developmental time points.

Figure 3 Neuregulin-1 (NRG1) type III protein expression isolated from dorsal root ganglia at different developmental time points.
Results
The mRNA levels of neuregulin-1 type III at different developmental time points

Figure 4 Immunofuorescent staining of neuregulin-1 (NRG1) type III isolated from dorsal root ganglia at different developmental time points (A-G) under laser confocal microscopy.
The specifc primers designed for detection of neuregulin-1 type III mRNAs are shown as arrows inFigure 1A. The amplification curves of neuregulin-1 type III and GAPDH by quantitative real-time polymerase chain reaction are shown inFigure 1B. The mRNA levels of neuregulin-1 type III at different developmental time points from postnatal day 1 to postnatal day 56 were compared inFigure 2. The expression of neuregulin-1 type III mRNA reached its peak at postnatal day 3 and then stabilized at a relatively high expression level from postnatal day 3 to postnatal days 56. The mRNA level of neuregulin-1 type III at postnatal days 3, 14 and 28 was higher (2.6-fold, 2.7-fold and 2.2-fold, respectively) than that at postnatal day 1 (Figure 2). The mRNA level of neuregulin-1 type III at postnatal days 7, 21 and 56 appeared higher than that at postnatal day 1, but did not reach signifcance (P > 0.05). There was no signifcant difference of neuregulin-1 type III mRNA level from postnatal day 3 to postnatal day 56 (P > 0.05).
The protein levels of neuregulin-1 type III at different developmental time points
The expression of neuregulin-1 type III protein in dorsal root ganglias isolated at different developmental time points from postnatal day 1 to postnatal day 56 was investigated by western blot assay. As shown inFigure 3, the densitometric results depicted the immunoreactivity of neuregulin-1 type III. The expression of neuregulin-1 type III increased gradually from postnatal day 1 to postnatal day 28, reached a peak at postnatal day 28, and then decreased at postnatal day 56. The expression of neuregulin-1 type III at postnatal days 14, 21, 28 and 56 was significantly higher (2.0-, 2.2-, 3.0- and 2.5-fold, respectively) when compared to that at postnatal day 1 (Figure 3). In addition, the expression of neuregulin-1 type III at postnatal day 28 was also significantly higher than that at postnatal day 3 (P < 0.01). From postnatal day 1 to postnatal day 7, the expression of neuregulin-1 type III protein in dorsal root ganglias showed an obvious increasing tendency, but did not reach significance (P > 0.05). There was no signifcant difference in neuregulin-1 type III protein level between the other time points (P > 0.05).
Immunofuorescent staining of neuregulin-1 type III at different developmental time points
To further confirm the expression pattern of neuregulin-1 type III at different developmental time points, we utilized immunofluorescent staining. Neurofilament was used as a positive control and cell nuclei were labeled with DAPI. All images were taken under the same conditions and parameters. As shown inFigure 4, the red fluorescence intensity represented the expression level of neuregulin-1 type III protein, which tended to increase gradually from postnatal day 1 to postnatal day 28, reached a peak at postnatal day 28, and then decreased at postnatal day 56. Thus, immunofuorescent staining results showed a similar tendency to the western blot assay results. In addition, big dorsal root ganglia neurons displayed higher fluorescence intensity than the small neurons.
Discussion
Neuregulin-1 is known to be involved in the development of the nervous system (Chen et al., 2003); however, different isoforms exhibit distinct cell specific function (Wen et al.,1992; Marchionni et al., 1993; Canoll et al., 1996). Neuregulin-1 type III is the key isoform required for Schwann cell proliferation, committing to a myelinating fate and regulating myelin sheath thickness (Garratt et al., 2000a; Taveggia et al., 2005; Birchmeier and Nave, 2008; Heermann et al., 2011). However, limited information is available from previous studies regarding the expression pattern of neuregulin-1 type III in the peripheral nervous system during developmental periods (such as the premyelinating stage, myelinating stage and postmyelinating stage). In this study, we have examined the expression of neuregulin-1 type III in dorsal root ganglia neurons at various developmental stages to gain a better understanding of the interactions between glial cells and neurons that are responsible for myelination and myelin sheath maintenance.
In the postnatal period, Schwann cells experience several important stages, including the proliferating stage (postnatal day 0 to postnatal day 2), the premyelinating stage (postnatal day 2 to postnatal day 5) and the myelinating stage (postnatal day 5 to postnatal day 10; Martinez et al., 2004; Reinhard et al., 2009). Thus, in the present study, we chose postnatal days 1, 3 and 7 as the time point which represented the proliferating stage, the premyelinating stage and the myelinating stage, respectively. In addition, postnatal day 14 to postnatal day 56 was chosen to represent the postmyelinating stage. Our results showed that neuregulin-1 type III had an increasing tendency from postnatal day 1 to postnatal day 14, which supported the view that neuregulin-1 type III synthesized by dorsal root ganglia neurons contributes to the signal that promotes the proliferation of Schwann cells from postnatal day 0 to postnatal day 2, and axonal myelination by Schwann cells from postnatal day 5 to postnatal day 15 (Birchmeier and Nave, 2008; Reinhard et al., 2009).
Recent studies have provided compelling evidence that the level of axon-derived neuregulin-1 type III provides a key instructive signal for Schwann cell committing to a myelinating fate and regulating myelin sheath thickness (Michailov et al., 2004; Taveggia et al., 2005). However, neuregulin-1 type III exerts no effect on the maintenance of the myelin sheath in adult animals (Atanasoski et al., 2006; Fricker et al., 2011). To our surprise, the expression of neuregulin-1 type III was not decreased in the postmyelinating stage from postnatal day 14 to postnatal day 56, a relative high level of neuregulin-1 type III mRNA was also observed. Interestingly, neuregulin-1 type III protein increased gradually from postnatal day 14 to postnatal day 28 and reached a peak at postnatal day 28 which was confrmed using both western blot assay and immunofuorescent staining. The high expression of neuregulin-1 type III can account for the idea that axonally presented neuregulin-1 type III inhibits the expression of neuregulin-1 type I in Schwann cells (Stassart et al., 2013). However, the exact role of neuregulin-1 type III in the mature peripheral nervous system has not been identifed. This is an important question for further study.
The expression of neuregulin-1 type III mRNA reached a peak at postnatal day 3 and then stabilized at a relatively high expression level from postnatal day 3 to postnatal day 56. However, the expression of neuregulin-1 type III increased gradually from postnatal day 1 to postnatal day 28, reached a peak at postnatal day 28, and then decreased at postnatal day 56. Thus, there were no clear correlations between neuregulin-1 type III protein expression patterns and the mRNA levels at these developmental time points, indicating the importance of the translational control of neuregulin-1 type III expression.
In conclusion, this study has identified the expression pattern of neuregulin-1 type III in the peripheral nervous system during development. This study also confirmed a persistent expression of neuregulin-1 type III from postnatal day 14 to postnatal day 56, indicating a continued need for neuregulin-1 type III signaling in the mature nervous system. Further studies of neuregulin-1 type III in the postmyelinating stage would contribute to understanding the role of neuregulin-1 type III in physical and pathological conditions in matured nervous systems and help fnd new therapeutic approaches for nervous system diseases.
Author contributions:LLH was responsible for animal surgery, quantitative real-time polymerase chain reaction, western blot assay and immunofluorescent staining. ZYL participated in histomorphometric measurements and statistical analysis. JHH and ZJL were responsible for study design, study supervision, and manuscript preparation. All authors approved the final version of the paper.
Conficts of interest:None declared.
Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U (2006) ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci 26:2124-2131.
Birchmeier C, Nave KA (2008) Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56:1491-1497.
Buonanno A, Fischbach GD (2001) Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11:287-296.
Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL (1996) GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron 17:229-243.
Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G (2003) Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci 6:1186-1193.
Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G (2006) Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci 26:3079-3086.
Falls DL (2003a) Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol 32:619-647.
Falls DL (2003b) Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284:14-30.
Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DL (2011) Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci 31:3225-3233.
Fricker FR, Zhu N, Tsantoulas C, Abrahamsen B, Nassar MA, Thakur M, Garratt AN, Birchmeier C, McMahon SB, Wood JN, Bennett DL (2009) Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not for long-term axon maintenance. J Neurosci 29:7667-7678.
Garratt AN, Britsch S, Birchmeier C (2000a) Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays 22:987-996.
Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C (2000b) A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol 148:1035-1046.
Heermann S, Schmucker J, Hinz U, Rickmann M, Unterbarnscheidt T, Schwab MH, Krieglstein K (2011) Neuregulin 1 type III/ErbB signaling is crucial for Schwann cell colonization of sympathetic axons. PLoS One 6:e28692.
Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R (2006) Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci 9:1520-1525.
Limpert AS, Carter BD (2010) Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. J Biol Chem 285:16614-16622.
Lyons, DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS (2005) erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafsh. Curr Biol 15:513-524.
Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M (1993) Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 362:312-318.
Martinez JC, Malave C, Bosch I, Castillo C, Nunez J, Villegas, GM, Villegas R (2004) A real-time quantitative PCR comparative study between rat optic and sciatic nerves: determination of neuregulin-1 mRNA levels. Brain Res Mol Brain Res 130:49-60.
Meyer D, Birchmeier C (1995) Multiple essential functions of neuregulin in development. Nature 378:386-390.
Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA (2004) Axonal neuregulin-1 regulates myelin sheath thickness. Science 304:700-703.
Nave KA, Salzer JL (2006) Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol 16:492-500.
Newbern J, Birchmeier C (2010) Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol 21:922-928.
Reinhard S, Vela E, Bombara N, Devries GH, Raabe TD (2009) Developmental regulation of Neuregulin1 isoforms and erbB receptor expression in intact rat dorsal root ganglia. Neurochem Res 34:17-22.
Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA (2013) A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci 16:48-54.
Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW, Jiang CC (2013) miR-10b promotes migration and invasion in nasopharyngeal carcinoma cells. Asian Pac J Cancer Prev 14:5533-5537.
Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA (2010) Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci 30:6122-6131.
Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL (2005) Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47:681-694.
Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, Et A (1992) Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell 69:559-572.
Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW (2000) Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron 25:79-91.
Copyedited by Apricò K, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to: Jing-hui Huang, M.D., Ph. D., huangjh@fmmu.edu.cn. Zhuojing Luo, M.D., Ph.D., zjluo@fmmu.edu.cn, zhuojingl@163.com.
10.4103/1673-5374.150708
http://www.nrronline.org/
Accepted: 2014-12-14
- 中国神经再生研究(英文版)的其它文章
- Letter from the Editors-in-Chief
- Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
- Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury
- “Bad regenerators” die after spinal cord injury: insights from lampreys
- Can cinnamon bring aroma in Parkinson’s disease treatment?
- Acupuncture: a potent therapeutic tool for inducing adult neurogenesis

