Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
Have you heard of NG2 cells or NG2 glia or polydendrocytes? These are new names for the precursor cells that used to be referred to as oligodendrocyte precursor cells (OPCs), which become the oligodendrocytes that myelinate central nervous system (CNS) axons. Evidence suggests, however, that they have other functions, besides differentiating into oligodendrocytes. Most notably, the OPCs/ NG2 cells are uniformly distributed in grey matter as well as in white matter, which matches poorly with the distribution of myelinating oligodendrocytes. Furthermore, not every NG2 cell is fated to become an oligodendrocyte. Hence the term OPC can be fairly applied only when discussing the role of these cells in the oligodendrocyte lineage.
NG2 cells have been identifed by their expression of alpha receptor for platelet-derived growth factor (PDGFRα) and the proteoglycan NG2 (nerve/glia antigen-2), which is one of the major growth-inhibitory CSPGs and is also known as chondroitin sulfate proteoglycan 4 (CSPG4) (Boda and Buffo, 2014; Nishiyama et al., 2014). These cells are abundant, comprising 2—8% of all the cells in the CNS parenchyma, and have morphological and physiological characteristics distinct from astrocytes, oligodendrocytes, microglia and neural stem cells (Nishiyama et al., 2014). NG2 cells emerge between E12.5 and E14.5, proliferate and migrate to fll the entire parenchyma of the CNS, but do not enter the peripheral nervous system (PNS). They remain uniformly distributed in adult CNS, and extend numerous processes that can extend up to 100 μm from the cell body. Their distribution is also tiled;i.e., each NG2 cell and its processes occupy a territory that is not overlapped by neighboring NG2 cells and processes. Thus a large population of tiled and uniformly distributed NG2 cells persists as proliferative precursors throughout grey and white matter. Their potential to differentiate into oligodendrocytes, however, is limited in healthy mature CNS.
Perhaps the most striking feature of NG2 cells is their ability to form anatomical and functional synapses with CNS neurons (Bergles et al., 2010). This attribute contradicts the old dogma that only neurons or muscle cells receive synapses, and is apparently unique among CNS glia. A number of studies have now confirmed the presence of postsynaptic machinery in NG2 cells, including receptors for glutamate and GABA, and described functional features such as rapidly evoked depolarization, spontaneous quantized responses and activity-dependent modifcations. Ultrastructural analyses have also revealed nerve terminal endings exhibiting apparent synaptic specializations formed at discrete sites along the processes of NG2 cells. These neuron-NG2 cell synapses emerge in parallel with neuron-neuron synapses during brain development and persist in adults unless they rapidly become disassembled upon the differentiation of NG2 cells into oligodendrocytes.
Neuron-NG2 cell synapses have been observed in all brain areas and at all ages explored so far. These areas include hippocampus, cerebellar and cerebral cortex, and white matter tracts such as corpus callosum. Although they have not been reported in intact adult spinal cord, to the best of our knowledge, the ability to synapse with neurons seems universal among NG2 cells, suggesting that NG2 cells are overt postsynaptic targets of axon projections. The functional significance of neuron-NG2 cell synapses in intact adult brain remains to be elucidated, although possible roles in homeostatic signaling, stabilization and the modulation of neural function have been suggested (Boda and Buffo, 2014). Likewise, it remains unknown whether neuron-NG2 cell synapses play a role in diseased or injured CNS. Over the past several years, our laboratory has been studying regeneration of primary sensory axons after dorsal root injury using time-lapsein vivoimaging. Our unexpected fndings made us extremely interested in testing whether neuron-NG2 cell synapses play a role in CNS axon regeneration. More specifcally, we are testing the idea that axons fail to regenerate into the adult spinal cord because forming synapses with NG2 cells prematurely terminates their extension before they reach their neuronal targets.
The idea that axons may terminate regeneration by synapsing with non-neuronal cells is not new but was proposed many years ago by Carlstedt. He observed that ventral root axons coapted to a dorsal root stop regenerating at the dorsal root entry zone (DREZ), the transitional zone between the CNS and PNS, by exhibiting ‘synapse-like’ ultrastructural features, such as copious vesicles and mitochondria. He termed these structures ‘synaptoids’ and speculated that they formed between axon tips and astrocytes (Carlstedt, 1985). Subsequently, Liuzzi and Lasek (1987) proposed that astrocytes stop axons by inducing them to form synapses, which acts as a physiological ‘stop signal’. This provocative idea was not further pursued and virtually forgotten for many years. It is now experiencing a resurgence, at least by our group and Jerry Silver’s group, in a modifed version that points to NG2 cells, rather than astrocytes, as the postsynaptic cells that arrest regenerating axons.
After dorsal root injury, axons proximal to the lesion, which remain attached to cell bodies in the dorsal root ganglion (DRG), extend new axons that grow well along the axotomized roots, which are filled with growth-promoting Schwann cells, and then stop or turn around at the DREZ. The molecular and cellular events that repel or arrest axons at the DREZ remain elusive, but this regeneration failure has been generally attributed to the weak growth potential of sensory neurons, in combination with growth-inhibitory or repulsive molecules extrinsic to the neuron (Han et al., 2012). For our own approach to understanding the regeneration failure, we have been applying two new, extremely useful techniques that have not been utilized in earlier studies (Han et al., 2012). One isin vivoimaging with which we have directly observed the behavior of DR axons arriving at the DREZ. The other is a wholemount analysis of the DREZ, which is prepared so as to include a thin slice of dorsal spinal cord with one or multiple dorsal root stumps attached. Axons and glial cells are pre-labeled or immunolabeled later in this sliced wholemount preparation. This techniqueavoids the need to prepare serial sections, and allows simultaneous three-dimensional analysis of many axons in their entirety under high-resolution microscopy. Both approaches were more challenging to develop than we had anticipated but provided unexpected insights which might have been overlooked by earlier studies that principally relied on static analysis of tissue sections.
We were very surprised to observe that many axons regenerating along the roots did not turn around upon arriving at the DREZ, but instead stopped there (Di Maio et al., 2011). Moreover, once axons arrived at the DREZ, they were rapidly immobilized and rarely moved forward or retracted. We also found that most axons that stopped did so in the CNS territory of the DREZ, whereas the small number that turned around did so in the PNS territory. Thus many axons cross the CNS-PNS border and then are immobilized in CNS territory, where they remain completely stationary for at least 4 months. These observations were surprising because we had anticipated that axons entering the DREZ would demonstrate far more dynamic responses, such as a transient growth cessation by inhibitory or repulsive factors, followed by revitalization of growth by Schwann cells, which might induce axons to resume extension into CNS territory or to turn around and grow along an alternative pathway back into PNS.
We were even more surprised to find that, in line with the idea of Carlstedt and Lasek, arrested axons exhibited multiple varicosity-like swellings at their tips and adjacent axon shafts that were intensely immunolabeled with synaptic markers (Di Maio et al., 2011). Ultrastructural analysis specifcally targeting the DREZ confrmed that these swellings were synaptic boutons with the presynaptic features of differentiated nerve terminals. They resembled synapses far more closely than the ‘synaptoids’, and included differential distribution of organelles with abundant synaptic vesicles aggregated selectively at electron-dense active zones. Notably, postsynaptic cells in contact with presynaptic boutons were not astrocytes, which contradicts the proposals of Carlstedt and Lasek, because they lacked the abundant intermediate flaments characteristic of astrocytes. The postsynaptic cells were thinner than axons and contained fine, if any, postsynaptic densities, which excludes the possibility that these profles are axon-axon synapses. Moreover, their presynaptic active zones were thinner than at neuron-neuron synapses, resembling those reported at neuron-NG2 cell synapses!
We have therefore examined in greater detail the possibility that NG2 cells are the postsynaptic cells with which dorsal root axons form synapses as they enter the DREZ. We have found that resident NG2 cells in spinal cord are rapidly activated in response to distant injury of dorsal roots;i.e., they proliferate, migrate and extend more processes to become highly ramifed (Kim et al., unpublished data; Rhodes et al., 2006). These reactive NG2 cells, with their highly branched processes, form a wide and dense ‘cellular net’ or ‘barricade’in the CNS territory of the DREZ exactly where ourin vivoimaging showed axons to stop and become immobilized (Figure 1). Presynaptic boutons of arrested axons identifed by immunolabeling for synaptic markers are co-localized with NG2 cells (Kim et al., unpublished data). We are currently carrying out additional experiments, such as immuno-electron microscopic (immuno-EM) examination and NG2 cell ablation, to obtain stronger evidence that NG2 cells are the postsynaptic targets, albeit inappropriate, of regenerating dorsal root axons.
As we continue our investigation using a dorsal root injury model, Jerry Silver’s group has been pursuing similar studies using a spinal cord injury model. Lesioned axon tips retract from the site of dorsal column injury and become stabilized in close association with cells that are highly immunolabled with NG2 antibody (McTigue et al., 2006; Busch et al., 2010). These cells have been referred as NG2+cells because of some uncertainty about their identity;i.e., multiple cell types present at the lesion express high levels of NG2, among them NG2+OPC cells, pericytes, denervated Schwann cells and possibly some astrocytes and macrophages. Silver and his colleagues found that these NG2+cells form synapse-like contacts with the dystrophic tips of severed axons, which are immunolabeled by synaptic markers (Filous et al., 2014). They also purifed NG2+cells from adult spinal cord and cocultured them with DRG neurons. Intriguingly, imaging of vesicle endocytosis and exocytosis with FM dye revealed that these axons released and recycled neurotransmitters at their contacts with NG2+cells. The contacts were also labeled by synaptic markers and ultrastructurally looked like ‘synaptoids’. They also demonstrated that conditions unfavorable for stable and sustained contacts between axons and NG2+cells, such as removal of CSPGs or NG2 (NG2 knockout mice) or a conditioning lesion, resulted in formation of fewer synaptoids or synaptic contacts than usual and in dieback of lesioned axon tips to a greater than normal distance. Additionally, some axons with enhanced growth potential due to a conditioning lesion extended further in NG2 proteoglycan knockout mice than in wild type. These data suggest that damaged axons undergoing retraction at the lesion site become stabilized upon synapsing with NG2+cells, and thus, paradoxically, that there may be some beneficial aspects to the formation of neuron-NG2 cell synapses. Nevertheless, the observation that regeneration of conditioned axons is increased when synaptic contacts with NG2+cells are reduced implies that neuron-NG2 cell synapses have a net negative effect on CNS axon regeneration.
In summary, recent data obtained from spinal cords with injured dorsal roots or dorsal column axons revitalize a virtually forgotten idea proposed by Carlstedt and Liuzzi that synapse formation between axons and glial cells may play a role in CNS regeneration failure. Thus far the evidence is only suggestive of a causal relationship, and the anatomical, physiological and ultrastructural data obtained from the axotomized DREZ and spinal cord are inconclusive. Moreover, region- and age-specifc differences in the proliferation and differentiation of NG2 cells have been noted (Nishiyama et al., 2014). It is therefore critically important to obtain more direct and defnitive evidence that resident NG2 cells in adult spinal cord are capable of forming synapses, and that this blocks regeneration.
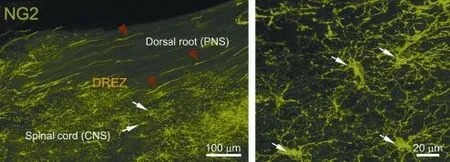
Figure 1 Confocal views of NG2 cells at intact dorsal root entry zone (dREZ) under low (left panel) and high magnifcation (right panel).
Several features of NG2 cells must be carefully taken into account in the further identification and evaluation of the cells themselves and of neuron-NG2 cell synapses. First, the NG2 can be highly expressed by other cell types, as discussed above, and large quantities of NG2 proteoglycans can be proteolytically shed from cell surfaces, forming plaques of diffuse NG2 labeling. Second, the lineage plasticity of NG2 cells may be regionally specific and may be further diversified after injury. Third, given that neuron-NG2 cell synapses are widespread in adult CNS, it is important to show defnitively that any such synapses observed after injury are formed between regenerating axons and NG2 cells, rather than pre-existing ones that survived injury. In this context it is also important to note that neurons in grey matter often extend profuse dendritic arbors that establish neuron-neuron synapses with axons deep in white matter (Abbadie et al., 1999). It is therefore essential to use additional markers and multiple criteria for careful and unequivocal identifcation of NG2 cells bothin vitroandin vivo.
Until now there has been widespread agreement that limited axon regeneration in adult CNS is due to a combination of the weak growth potential of mature neurons and the presence of growth-inhibitory factors primarily associated with astrocytes and oligodendrocytes. However, despite some encouragingin vitroandin vivostudies, therapies targeting growth inhibitory factors or boosting intrinsic growth potential have not produced robust regeneration. It is therefore particularly interesting to note the widespread presence of synapse-forming NG2 cells in adult CNS, including optic nerves, because, by serving as inappropriate postsynaptic targets, they may act in a unique way to pose an additional barrier to regeneration elsewhere in the CNS. If regenerating axons indeed cease growth prematurely due to extensive formation of aberrant synapses with NG2 cells, this will substantially revise the prevailing view in the feld, and may contribute to the development of more effective therapy. The same mechanisms may also limit spontaneous recovery by actively restricting sprouting-based, anatomical plasticity in the injured CNS. It is also notable that NG2 cells make intimate contacts with astrocytes, microglia, pericytes and myelin (Boda and Buffo, 2014), which would allow them to actively sense and integrate information from diverse but relevant cell types and possibly to play a central role in the pathogenesis of various CNS diseases and injuries. Future efforts to understand the roles of NG2 cells in damaged CNS may turn out to be far more fruitful than we originally anticipated.
Our research on NG2 cells and dorsal root regeneration was supported by NIH NS079631, Shriners Hospitals for Children and Craig H. Neilsen Foundation. I also thank members of the Son laboratory who have worked on the projects, including Hyukmin Kim, Tim Himes, Alessandro Di Maio, Andy Skuba, SeungBaek Han and Jinbin Zhai. Special thanks to AlanTessler for advice about science and life over the years.
Young-Jin Son*
Shriners Hospitals Pediatric Research Center and Department of Anatomy and Cell Biology, Temple University School of Medicine, Philadelphia, PA 19140, USA
*Correspondence to: Young-Jin Son, Ph.D., yson@temple.edu.
Accepted:2015-02-11
Abbadie C, Skinner K, Mitrovic I, Basbaum AI (1999) Neurons in the dorsal column white matter of the spinal cord: complex neuropil in an unexpected location. Proc Natl Acad Sci U S A 96:260-265.
Bergles DE, Jabs R, Steinhauser C (2010) Neuron-glia synapses in the brain. Brain Res Rev 63:130-137.
Boda E, Buffo A (2014) Beyond cell replacement: unresolved roles of NG2-expressing progenitors. Front Neurosci 8:122.
Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J (2010) Adult NG2+cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci 30:255-265.
Carlstedt T (1985) Regenerating axons form nerve terminals at astrocytes. Brain Res 347:188-191.
Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, Bishop D, Son YJ (2011) In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci 31:4569-4582.
Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, Kang SH, Bergles DE, Lee SI, Levine JM, Silver J (2014) Entrapment via synaptic-like connections between NG2 proteoglycan+cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci 34:16369-16384.
Han S, Kim H, Skuba A, Tessler A, Ferguson TA, Son YJ (2012) Sensory axon regeneration: A review from an in vivo imaging perspective. Exp Neurobiol 21:83-93.
Liuzzi FJ, Lasek RJ (1987) Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237:642-645.
McTigue DM, Tripathi R, Wei P (2006) NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol 65:406-420.
Nishiyama A, Suzuki R, Zhu X (2014) NG2 cells (polydendrocytes) in brain physiology and repair. Front Neurosci 8:133.
Rhodes KE, Raivich G, Fawcett JW (2006) The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and infammation-associated cytokines. Neuroscience 140:87-100.
10.4103/1673-5374.153672 http∶//www.nrronline.org/
Son YJ (2015) Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration? Neural Regen Res 10(3)∶346-348.
- 中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters
- Empowering sonic hedgehog to rescue brain cells after ischemic stroke

