Analysis on Bacterial Community Structure in Mushroom(Agaricus bisporus)Compost Using PCR-DGGE
Yaping GUO,Guoqing ZHANG,Qingjun CHEN*,Kai YANG
1.College of Plant Science and Technology,Beijing Key Laboratory for Agricultural Application and New Technique,Beijing University of Agriculture,Beijing 102206,China;
2.College of Biological Sciences and Engineering,Key Laboratory of Urban Agriculture (North) of Ministry of Agriculture,Beijing University of Agriculture,Beijing 102206,China
Responsible editor:Qingqing YIN Responsible proofreader:Xiaoyan WU
Agaricus bisporus is the world’s most widely cultivated edible mushroom.In European countries it is grown mainly with straw and chicken manure in factories.In China,a large agricultural country with rich resources of crop straw,especially rice straw and corn stover,mushroom is mainly cultivated by composting with rice straw,corn stover,wheat straw and animal manure,etc.,which greatly reduces economic investment,makes full use of local agricultural wastes and increases the employment rate and income of farmers[1].
The compositions and processes of compost are the most important factors affecting the yield and quality of mushroom.Composting is a process of microbial succession.Microbial community succession and physiological metabolism determine the quality of compost piles[2-3].Therefore,study on the changes in microbial community during mushroom composting reveals the fermentation mechanism of the compost materials.
There are various methods to study the changes of microbial community in compost piles,such as pure culture,denaturing gradient gel electrophoresis (DGGE) and metagenomics.The compost procedure of mushroom has been well improved,standardized,industrialized in foreign countries,and pure culture is used as the main way to monitor microbial succession[4-6].PCR-DGGE can reveal the genetic information of microorganisms,and the differences in electrophoretic profiles at different periods directly reflect the changes in microbial communities[4-6].In this study,PCR-DGGE was adopted to explore the changes in microbial community structure in mushroom compost piles composed of rice straw and corn stover respectively,with an attempt to provide theoretical basis for better utilization of agriculture wastes and improvement of mushroom compost.
Materials and Methods
Mushroom cultivation
Raw materialsThe primary raw materials of compost rice straw,corn stover and cow dung were collected from the mushroom production cooperative in Taishi Village,Miyun County of Beijing.
Culture mediaFormula A included 70% rice straw,23% cow dung,3%bean flour,2% superphosphate,1%gypsum and 1% lime.Formula B included 51% corn stover,40% cow dung,3% bean flour,4% superphosphate and 2%corn flour.Their physicochemical properties are shown in Table1.
The rice straws were chopped into 15 -30 cm long,and corn stover into about 10 cm lone,and cow dung was crushed and sieved before composting.
CompostingA layer of corn stover(30 cm thick) was paved on bottom,alternately covered by 25 cm thick cow dung and 15 cm thick corn stover to 150 cm high in total.The materials from the third bottom layer were watered,covered by cow dung on the top layer.The temperature of compost piles rose to 70-75 ℃2 or 3 d later.The compost pile of Formula B was turned over for the first time on the 7thd,and then for the second time on the 11thd and for the third time on the 15thd.The compost pile of Formula A was turned over for the first time on the 14thd,and then for the second time on the 21thd and for the third time on the 28thd.Water content of the compost piles was adjusted to 75% and pH to 8.0 during when the piles were turned.Then the compost was transferred onto the racks in greenhouse.After 10-15 h of steam injection,the temperature of compost rose to about 60 ℃.Then,steam was continuously injected for another 10-12 h (pasteurization),before being gradually ventilated.48 h later,the temperature of compost dropped from 50 ℃to about 25 ℃,when the secondary fermentation was completed.
Sampling
The samples of each formula were collected at three different composting periods.Samples No.A-h of Formula A were collected 2 d after composting and samples No.B-h of Formula B were collected 3 d after composting.Samples No.A-I of Formula A (d 27)and samples No.B-I of Formula B(d 14)were collected during fermentation phase I.Samples No.AII of Formula A(d 36)and samples No.B-II of Formula B(d 22)were collected during fermentation phase II,the period from the beginning of pasteurization to mushroom seeding.Every sample was collected at five random directions of compost pile,or from the third layer of racks in three rooms,about 1 000 g in total.200 g of each sample was taken,divided into 10 copies,immediately frozen in liquid nitrogen and finally preserved at-80 ℃.
DNA extraction and purification
Total DNA was extracted and purified from compost using MOBIO Power Soil®DNA Isolation Kit[7].
PCR-DGGE
The genomic DNA extracted from compost was served as the template to amplify the V3 domain of 16S rDNA using the universal primers in the V3 domain of E.coli 16S rDNA:357F(5’-CCT ACG GGA GGC AGC AG-3’)and 518R (5’-ATT ACC GCG GCT GCT GG-3’),with a 40-bp GC-clamp added to the 5’-end of 357F[8].PCR system(50 μl)contained 4 μl of dNTPs(2.5 mmol/L),5 μl of 10×Buffer,4 μl of MgCl2(25 mmol/L),1 μl of 357F-GC(10 μmol/L),1 μl of 518R (10μmol/L),30-50 ng of template DNA and 0.3 U of Taq polymerase (TaKaRa).The PCR was started with pre-denaturing at 94 ℃for 5 min,followed by 35 cycles of denaturing at 94 ℃for 30 s,annealing at 57 ℃for 30 s,and extension at 72 ℃for 1 min;the amplification was completed by holding the reaction mixture at 72 ℃for 10 min.The PCR products were separated through electrophoresis on 1.0%(m/V)agarose gel.
DGGE was performed according to Wang et al[9].After electrophoresis the gel was stained with SYBR GreenⅠand visualized under gel imaging system (Bio-Rad,USA).The target DNA bands were recovered,and amplified again using primers 357F(without GC-clamp) and 518R.The PCR products were ligated into pEASY-T3 vector (purchased from Beijing Transgen Biotech Co.,Ltd.),and sequenced by Beijing Genomics Institute.
Analysis on DGGE profiles and establishment of phylogenetic tree
Quantity one software was adopted for analyzing the DGGE profiles above,and Shannon-Weaver index was used to measure the diversity of microbial communities in compost piles.Canoco 4.5 software was used to conduct principal component analysis(PCA) and plot.The sequences of target DNA fragments were aligned with related sequences in GenBank database online using Blast (http://www.ncbi.nlm.nih.gov),and finally the phylogenetic tree was constructed using MEGA 5.1 software.
Results and Analysis
DGGE profiles and diversity index of microbial communities
Bacterial 16S rDNA V3 domain was amplified from the samples collected at different composting stages.The DGGE profiles as shown in Fig.1Arevealed the changes of bacterial communities in Formula A and Formula B compost piles.Then,the schematic diagram of relative band intensities in the DGGE profiles was obtained using Quantity one software (Fig.1B).The compost piles of both Formula A and Formula B had abundant microbial diversity,and the dominant bacteria varied at different composting stages.The DNA bands of the DGGE profiles of Formula A and Formula B had significant differences in number and size at the beginning of composting,but became similar at the end of fermentation phase II.The DNA bands of Formula B were slightly different at the end of fermentation phase I and fermentation phase II,indicating that at the end of fermentation phase I the microbial community had become stable and the fermentation was almost finished.
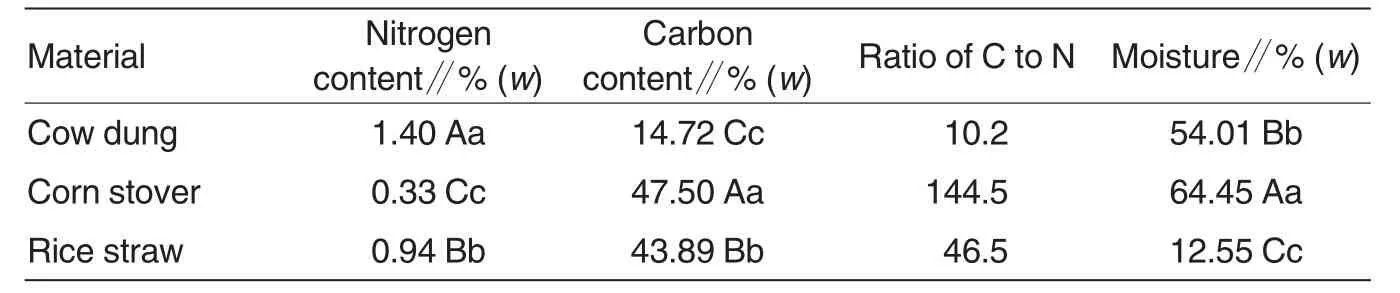
Table1 Basic properties of compost materials
According to the number and brightness of DNA bands in DGGE profiles,Shannon-Weaver index was calculated to measure the microbial diversity in compost.As shown in Fig.2,the Shannon-Weaver index of Formula A and Formula B was 3.73 and 3.63 on average.The Shannon-Weaver index of Formula B was the smallest at the beginning of composting,only 3.50,and the Shannon-Weaver index of Formula A was the largest at the end of fermentation phase II,up to 3.80.The microbial diversity index of Formula A compost kept increasing throughout the composting period,while that of Formula B compost increased before decreasing.The reason may be that the two compost piles were composed of different materials at different proportions.The microbial diversity index of Formula A compost continued to increase at the end of fermentation phase II,indicating that the fermentation had not finished.The microbial diversity index of Formula B compost showed a bell-shaped curve,indicating that the compost was already well decomposed.
Principal component analysis
The principal component analysis on the DGGE profiles revealed that the principal component 1(PC1)and principal component (PC2) contributed 34.2% and 20.3%,respectively.According to PCA,all the samples were classified into two groups.One group included A-h,B-h and A-I,and the other group include B-I,A-II and B-II.A-h and B-h were distantly clustered,indicating that the microbial communities of Formula A and Formula B were significantly different at the beginning of composting.The samples of Formula A at three different stages (Ah,A-I and A-II) were also distantly clustered,indicating that the microbial communities significantly changed during the composting period.Samples B-I and B-II were clustered together,indicating that the fermentation of Formula B compost had finished at phase I.As shown in Fig.3,the PC1 factors of both formulas presented similar order,from left to right they were samples collected at the beginning of composting,fermentation phase I and fermentation phase II,indicating that the microbial succession of Formula A and Formula B was similar during composting period.
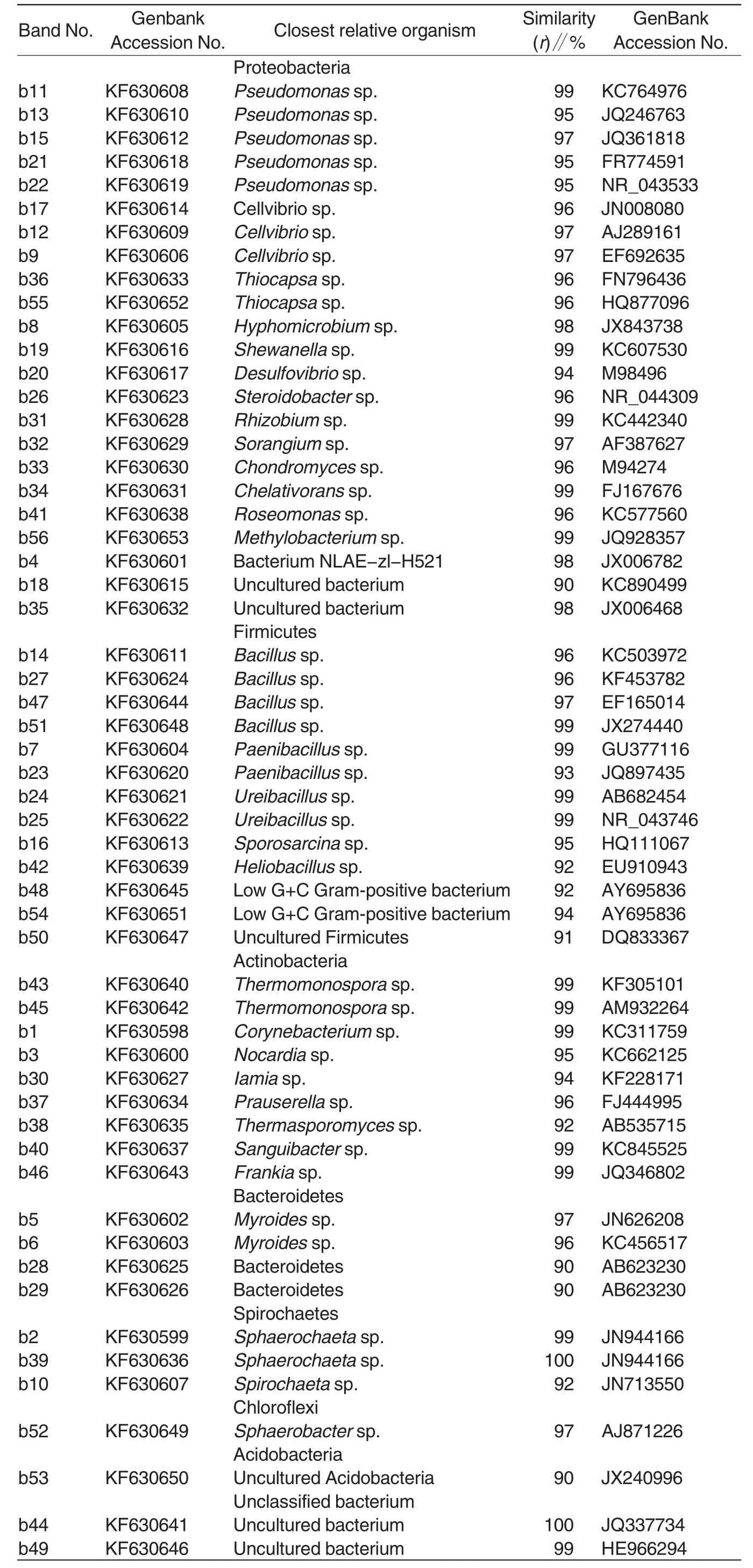
Table2 Nucleotide sequence identity of analyzed DGGE fragments
Sequence analysis and phylogenetic relationship of specific DNA bands
The dominant DNA bands in DGGE profiles were recovered,cloned and sequenced,and as a result,56 16S rDNAsequences were obtained and submitted to GenBank under accession number from KF630598 to KF630653.Sequence alignment using Blast in GenBank database was shown in Table2.Phylogenetic tree showed that the 56 specific sequences were classified into seven phyla and 42 genera,including 13 genera of phylum Proteobacteria,six genera of phylum Firmicutes,8 genera of phylum Actinobacteria,several genera of Spirochaetes,Bacteroidetes,Chloroflexi and Acidobacteria.Pseudomonas and Cellvibrio,the dominant genera of Proteobacteria mainly grew at the beginning of composting of Formula A compost and fermentation phase I of Formula B compost,and disappeared at the end of fermentation phase II.Bacillus,Paenibacillus and Ureibacillus,the dominant genera of Firmicutes and Gram-positive bacteria with low G + C content,grew in the whole composting period.The microflora of Actinobacteria mainly appeared at the fermentation phases I and II in both formulas.Among them,Thermomonospora,Thermasporomyces and Iamia,the dominant genera in Formula B mainly appeared at the fermentation phases I and II.Frankia,the dominant genus of Formula A mainly appeared at fermentation phase II.In both Formula A and Formula B compost,genus Myroides of Bacteroidetes and genus Spirochaeta of Spirochaetes were detected at the beginning of composting and fermentation phase I,and disappeared at fermentation phase II.The microflora of Chloroflexi and Actinobacteria were detected at fermentation phase I and fermentation phase II of both formulas.Moreover,DNA band b44 was also a dominant species at fermentation phase I and fermentation phase II,but which genus it belonged to was not determined.
Discussion and Conclusions
The quality of compost is directly related to the yield and quality of mushroom.The succession of microbial community during composting has been studied a lot using traditional methods,like separation culture and enzyme activity determination.Sharman et al.[3]determined the fugal community via pure culture during traditional and industrial composting procedures,and found that the fungi in the two compost piles were different in number and species.The study of Raquel et al.[10]proved that the quality of mushroom compost could be determined according to dehydrogenase activity.However,95%of the microbial population in compost cannot be detected by pure culture,and the dynamics in microbial community cannot be directly reflected using enzyme activity,so DGGE and metagenomics become important techniques in research of environmental microbiology.
He et al.[11]studied the bacterial community in industrial mushroom compost at late fermentation period using PCR-DGGE and found that the bacteria in industrial compost were much more diverse than that in traditional compost.Szekely et al.[12]studied the dynamics of microbial community in industrial mushroom compost with DGGE and T-RFLP and found that
Pseudoxanthomonas,Thermobifida and Thermomonospora were the dominant microflorae and they were all cellulose-degrading bacteria.Traditional composting is widely adopted in current mushroom cultivation in China.However,the application of PCRDGGE in the study of microbial community succession has rarely reported.In addition,this technique can also be used to optimize compost compositions and proportions and composting procedures.
In this study,PCR-DGGE was conducted to analyze the structural characteristics of bacterial communities in mushroom compost composed mainly of rice straw and corn stover,aimed to provide theoretical basis for improvement of traditional composting procedures and efficient use of agricultural resources.As a result,56 specific DNAsequences were obtained,classified into 42 genera of 7 phyla,among which Proteobacteria,Firmicutes and Actinobacteria were the dominant microflorae.Proteobacteria was the dominant microflora at the beginning of composting,and the other two phyla were the dominant microflorae at the ends of fermentation phase I and fermentation phase II.Bacillus,Paenibacillus,Ureibacillus and Gram-positive bacteria with low G + C content dominant microflorae most widely distributed in both formulas,and they were detected throughout the whole composting period,for they are adaptive to wide ranges of temperature and pH,and produce extracellular hydrolytic enzymes which can hydrolyze macromolecular compounds like cellulose and hemicellulose.Szekely et al.[12]detected the microbial community in mushroom compost composed of wheat straw and chicken manure,and found that the dominant microflorae were γ-Proteobacteria at the beginning of composting and the end of fermentation phase II,Firmicutes and Thermus at the end of fermentation phase I.Jia[13]found that Proteobacteria,Firmicutes and Actinobacteria were dominant microflorae in compost composed mainly of rice straw and corn stover,and most of actinomycetes appeared at the ends of fermentation phase I and fermentation phase II.By amplifying the V3 domain of bacterial 16S rDNA through DGGE,He et al.[11]found that Chloroflexi was most abundant in industrial mushroom compost.But only one band (b52) of genus Sphaerobacter was obtained at the end of composting in our study.The study of Xiao et al.[14]revealed that genus Bacillus existed throughout the composting of food waste,and played an important role.
In this study,we found that Pseudomonas and Cellvibrio existed at the beginning of composting and fermentation phase I,more in corn stover compost than in rice straw compost,because these bacteria are able to decompose carbohydrates where existed more in corn stover.In the study of Agnolucci et al.[15],microbial population in industrial compost of olive processing waste was greatly increased by supplementing Bacillus and Pseudomonas.We also found that at the ends of fermentation phase I and fermentation phase II a large amount of actinomycetes was detected,among which,Thermomonospora and Thermasporomyces were dominant microflorae.Thermophilic actinomycetes are able to decompose hemicellulose,and alter the molecular structure of lignin to some extent,and finally decompose lignin[16].Keiko et al.[17]found that in the compost of white plastic and food waste,actinomycetes,the dominant microflorae at middle and late composting,began to appear at middle composting,accounting for 23% of the total amount of microorganisms,and bloomed at late composting,accounting for 35%of the total amount of microorganisms.
Diversity analysis showed that during the whole composting period,the diversity index of rice straw compost was greater than that of corn stover compost,and the bacterial diversity of rice straw compost kept increasing while that of corn stover compost showed a bell-shaped curve.Rice straw compost was not fully decomposed after two fermentation phases (36 d),so fermentation period of rice straw compost should be prolonged.Microbial growth and reproduction showed no significant increase at the end of fermentation phase II,indicating the microbial community succession met the requirements of mushroom compost,and the compost had been well decompose[15,18-19].
The principal component analysis(PCA) revealed that the sample No.A-I of rice straw compost was clustered together with A-h and B-h,indicating that rice straw compost was not fully fermented at phase I,thus affecting the final maturity; the samples No.B-I and B-Ⅱof corn stover compost were clustered together,indicating that corn stover compost entered into maturity in advance.The reason may be that compared with corn stover,rice straw contains more cellulose and hemicellulose,and thus requiring a longer time for complete degradation;on the other hand,corn stover compost was composed of a higher proportion of cow dung than rice straw compost,and the humic acid in cow dung promoted the acidification during microbial decomposition.As both rice straw and corn stover have certain advantages during composting,they can be mixed at a certain ratio in compost,supplemented with more cow dung and other excipients,to promote the microbial growth at early period and rice straw fermentation during composting.
Numerous studies have shown that bacteria,actinomycetes and fungi bloom in succession during composting[20].Some biological agents or surfactants have been made according to the characteristics of various microorganisms in compost,to speed up the composting process,and improve mushroom production[21-26].In our study,a number of dominant thermophilic bacteria like Pseudomonas and Cellvibrio of Proteobacteria,Thermomonospora and Thermasporomyces of Actinobacteria were detected in mushroom compost.If they may be obtained through pure culture or from microbiological culture collection centers and made into biological agents or composite agents for compost production needs to be studied in future work.
[1]WANG DZ(王德芝),LIU RF(刘瑞芳),MA L (马兰),et al.Modern mushroom production technology(现代食用菌生产技术)[M].Wuhan:Huazhong University of Science and Technology Press(武汉:华中科技大学出版社),2012.
[2]XU XH(许修宏),LI HT(李洪涛),ZHANG D (张迪).Compost microbiology principles and Agaricus bisporus cultivation(堆肥微生物学原理及双孢蘑菇栽培)[M].Beijing:Science Press (北京:科学出版社),2009.
[3]SHARMA SS,YONS GL,CHAMBERS J.Comparison of the changes in mushroom(Agaricus bisporus) compost during windrow and bunker stages of phase I and Ⅱ [J].Assoc Appl Biol,2000,136:59-68.
[4]WAGNER M,AMANN R,LEMMER H,et al.Probing activated sludge with oligonucleotides specific for Proteobacteria:inadequacy of culture-dependent methods for describing microbial community structure[J].Appl Environ Microbiol,1993,59:1520-1525.
[5]GERARD M,KORNELIA S.Application of denaturing gradient gel electrophoresis(DGGE) and temperature gradient gel electrophoresis(TGGE) in microbial ecology [J].Anton Leeuw,1998,73:127-141.
[6]NOVINSCAK A,NADINE JD,CELINE S,et al.Characterization of bacterial and fungal communities in composted biosolids over a 2 year period using denaturing gradient gel electrophoresis[J].Can J Microbiol,2009,55:375-387.
[7]WU L,LI F,DENG CY,et al.A method for obtaining DNA from compost [J].Appl Microbiol Biotechnol,2009,84:389-395.
[8]ZENG GM,YU Z,CHEN YN,et al.Response of compost maturity and microbial community composition to pentachlorophenol(PCP)-contaminated soil during composting [J].Bioresource Technol,2011,102:5905-5911.
[9]WANG XF (王小芬),WANG WD (王伟东),GAO LJ(高丽娟),CUI ZJ(崔宗均).Protocols of applications of denaturing gradient gel electrophoresis (DGGE) in studies of environmental microorganism(变性梯度凝胶电泳在环境微生物研究中的应用详解) [J].J China Agric Univ(中国农业大学学报),2006,11(5):1-7.
[10]RAQUEL B,FELICITAS V,ANTONI S.Dehydrogenase activity as a method for monitoring the composting process[J].Bioresource Technol,2008,99:905-908.
[11]HE LH (何丽鸿),CHEN MJ (陈明杰),PAN YJ (潘迎捷).Bacterial communities in the phase Ⅱof Agaricus bisporus compost by denaturing gradient gel electrophoresis(采用变性梯度凝胶电泳研究双孢蘑菇培养料后发酵过程中的细菌群落结构)[J].Acta Microbiol Sin (微生物学报),2009,49 (2):227-232.
[12]SZEKELY AJ,SIPOS R,BERTA B,et al.DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequenceaided T-RFLP profile of mature compost[J].Microbial Ecol,2009,57:522-533.
[13]JIA YY (贾洋洋).Microbial diversity analysis in composting environment by metagenomic method(利用宏基因组方法分析堆肥生境中微生物区系的变化)[D].Jinan:Shandong University (济南:山东大学),2012.
[14]XIAO Y,ZENG GM,YANG ZH,et al.Effects of continuous thermophilic composting(CTC)on bacterial community in the active composting process[J].Environ Microbiol,2011,62:599-608.
[15]AGNOLUCCI M,CRISTANI C,BATTINI F,et al.Microbially-enhanced composting of olive mill solid waste(wet husk):bacterial and fungal community dynamics at industrial pilot and farm level [J].Bioresource Technol,2013,134:10-16.
[16]XI BD (席北斗),LIU HL (刘鸿亮),BAI QZ (白庆中),et al.Study on current status of lignin and cellulose biodegradation in composting process (堆肥中纤维素和木质素的生物降解研究现状)[J].Techniq Equip Environ Pollut Control(环境污染治理技术与设备),2002,3(3):19-23.
[17]KEIKO W,NORIO N,TATSUKI T,et al.The dominant bacteria shifted from the order"Lactobacillales"to Bacillales and Actinomycetales during a start-up period of large-scale,completelymixed composting reactor using plastic bottle flakes as bulking agent [J].World J Microbiol Biotechnol,2009,25:803-811.
[18]WANG HL (王鸿磊),WANG HY (王红艳),SONG JF(宋俊芬),et al.Variation of microorganisms and physicochemical properties of Agaricus bisporus compost (双孢菇培养料工厂化发酵过程中微生物及物质变化研究)[J].J Anhui Agric (安徽农业科学),2011,39(1):94-96.
[19]LIU J(刘佳),LI W(李婉),XU XH(许修宏),et al.Effect of cellulose-decomposing strain on microbial community of cow manure compost(接种纤维素降解菌对牛粪堆肥微生物群落的影响)[J].Environ Sci (环境科学),2011,32(10):3073-3081.
[20]JIANG C (姜成),WANG ZS (王泽生).The development and functions of microorganisms in the composting process for Agaricus bisporus cultivation(蘑菇培养料堆制过程中微生物的演替及作用)[J].J Microbiol(微生物学杂志),2003,23(1):56-58.
[21]GERBEN S,TINEKE W,OLIJNSM A,et al.Inoculation of Scytalidium thermophilum in button mushroom compost and its effect on yield [J].Appl Environ Microbial,1994,60 (9):3049-3054.
[22]WIEGANT WM.Growth characteristics of the thermophilic fungus Scytalidium thermophilum in relation to production of mushroom compost [J].Appl Environ Microbial,1992,58:1301-1307.
[23]ZARENEJAD F,YAKHCHALI B,RASOOLI I.Evaluation of indigenous potent mushroom growth promoting bacteria(MGPB) on Agaricus bisporus production [J].World J Microbiol Biotechnol,2012,28(1):99-104.
[24]WU XJ (吴小建).Isolation and identification of thermophilic fungi and cultivation of Agaricus bisporus on substrates pre-colonized by Scytaidum thermophilum(嗜热真菌的分离鉴定及其在双孢蘑菇栽培中的应用研究)[D].Nanning:Guangxi University (南宁:广西大学),2012.
[25]XI BD (席北斗),DANG QL (党秋玲),WEI ZM (魏自民),et al.Effects of microbial inoculants on actinomycetes communities diversity during municipal solid waste composting(生活垃圾化堆肥对放线菌群落的影响)[J].Trans CSAE(农业工程学报),2011,27:223-228.
[26]WANG J (王晶),XU XH (许修宏).Effects of adding to complex bacteria on microbial community of compost assessed by PCR-DGGE techniques(利用PCR-DGGE 方法研究添加复合菌剂对堆肥微生物群落的影响)[J].J Agro-Environ Sci (农业环境科学学报),2011,30(12):2602-2607.
 Agricultural Science & Technology2015年8期
Agricultural Science & Technology2015年8期
- Agricultural Science & Technology的其它文章
- On Genetic Parameter Estimation of Xinjiang Brown Cattle’s Main Economic Characters
- Effect of Flooding and Air-drying on Nutrition Content of Soil in Embankment WLFZ of Chaohu Lake
- Influences of Nitrogen-phosphorus Ratio on the Growth and Competition of Chlorella vulga and Anabaena sp.strain PCC
- Fatty Acid Composition and Seed Quality Traits of the Transgenic Rapeseed W-4(Brassica napus L.)with Down-regulated Expression of fad2 Gene
- Research on the Construction of Remote Plant and Animal Hospital in Omnimedia Era
- Study on Physical Properties and Related Spectral Characteristics of Composited Soil with Different Ratio of Feldspathic Sandstone and Sand
