合成ZnO纳米阵列及刺突状CuO/ZnO异质结
李湘奇 范庆飞 李广立 黄瑶翰 高 照范希梅,* 张朝良 周祚万(西南交通大学材料科学与工程学院,材料先进技术教育部重点实验室,成都6003;四川大学华西口腔医院,口腔疾病国家重点实验室,成都6003)
合成ZnO纳米阵列及刺突状CuO/ZnO异质结
李湘奇1范庆飞1李广立1黄瑶翰1高 照1范希梅1,*张朝良2周祚万1
(1西南交通大学材料科学与工程学院,材料先进技术教育部重点实验室,成都610031;2四川大学华西口腔医院,口腔疾病国家重点实验室,成都610031)
采用低温水热法在掺氟SnO2(FTO)导电玻璃表面制备ZnO纳米阵列,研究了前驱体溶液浓度摩尔配比对ZnO纳米阵列形貌、光学性能及其生长机理的影响.研究发现,随着前驱体溶液浓度摩尔配比的增加,ZnO纳米阵列形貌及光学性能也随之变化.ZnO纳米阵列高度逐渐降低,ZnO纳米阵列直径和光学带隙值大体上出现先增大后降低的趋势.而当前驱体溶液(Zn(NO3)2:环六亚甲基四胺(HMT,C6H12N4))浓度摩尔配比为5:5时,其光学禁带值(3.2 eV)接近于理论值.结果显示制备ZnO纳米阵列的最优浓度摩尔配比为5:5.随后选用最优浓度摩尔配比下制备的ZnO纳米阵列为基底,通过一种两步溶液法成功在其表面制备刺突状CuO/ZnO异质结.从场发射扫描电镜(FE-SEM)结果中可以清楚看见,大量的CuO纳米粒子沉积在ZnO纳米阵列表面形成刺突状异质结结构.研究发现该CuO/ZnO纳米异质结相对于纯ZnO纳米阵列在紫外光下光催化性能明显增加.最后,讨论了CuO/ZnO纳米异质结光催化机理.
ZnO纳米阵列;CuO/ZnO异质结;水热法;光学性能;摩尔比
1 Introduction
ZnO is a II-VI semiconductor with a wide direct band gap of 3.37 eV at room temperature and large exciton binding energy(60 meV).As a promising functional material,its nanostructures have attracted great attention.There are lots of literature about ZnO nanostructure,1-4such as nano-film,nano-flower,nano-sphere, nano-array and so on.Because of its high aspect ratio and faster rate of electronic transmission,the ZnO nano-arrays are widely used in light emitting diodes,5field emission devices,6solar cells,7etc.Especially in photocatalysis,there are lots of papers about the photocatalysis of ZnO.8,9Kuo et al.8used the chemical vapor transport method to synthesize ZnO nanowires as recyclable photocatalysts on silicon substrates coated with a very thin gold catalyst film.Zhai et al.9used the sol-gel method to synthesize ZnO nanowires for photocatalytic degradation of 4-chlorophenol on the surface of ZnO nanorod arrays.There are many methods that were used to synthesize ZnO nanorods,such as electrochemical growths,10physical vapor deposition,11magnetron sputtering deposition,12high pressure pulsed laser deposition,13and hydrothermal method14.In more recent years,the hydrothermal method has become a hot spot of research in the growth of ZnO nanorods because of its simple procedure,moderate-temperature, and low cost.For example,Liu and Zeng15reported that ZnO nanorods in the diameter regime of 50 nm were grown on the fluorinated tin oxide(FTO)glass substrate by hydrothermal method.Kumar et al.16reported that ZnO nanorods were successfully grown on glass substrate by a hydrothermal method through controlling the ZnO nanostructure seed layer.
However,one-dimensional(1D)ZnO nano-arrays could not meet the demand for the development of modern advanced materials,due to the wide band-gap and easy recombination of the photo-induced electrons and holes in the photocatalytic reactions.17Hence,the separation process is considered as one of the most important roads for modification of single ZnO nano-arrays. Recently,coupled semiconductors formed between ZnO and other metal oxides or sulfides have been studied,18-20such as In2O3,CdS, CuO and so on.CuO is a p-type semiconductor with a narrow band gap(1.2-1.9 eV),21,22owning unique electrical,magnetic, and catalytic properties,which had been widely used in various applications such as lithium ion electrode materials,heterogeneous catalysts,gas sensors,and solar cells.23-26Therefore,when CuO nano-structure coupled on the surface of ZnO nano-arrays and formed the nano-arrays heterojunction,it may produce more applications.In the past few years,there are a lot of researches about CuO/ZnO nano-arays.27-29However,most of them are used for hydrogen productions,gas sensors,and solar cells,few used for photodegradations.For instance,Kim et al.27used photochemical method to synthesize CuO/ZnO heterostructured nanorods as H2S gas sensor at low temperature.Kargar et al.28synthesized ZnO/CuO heterojunction branched nano-arrays by a thermal oxidation method,applied as photocathodes for photoelectrochemical solar hydrogen production.Jung and Yong29used photochemical method to prepare CuO/ZnO nano-array structure on the mesh substrate,which shows highly efficient photocatalytic applications for dye.
The aim of this study is to optimize the preparation of the 1D ZnO nano-arrays and investigate the role of CuO in enhancing the photocatalytic properties of 1D ZnO nano-arrays.Firstly,we reported a hydrothermal method for the synthesis of 1D ZnO nanoarrays with polyethyleneimine(PEI)as additive,PEI could efficiently increase the length of ZnO nano-arrays.30In this process, different molar ratios of Zn(NO3)2to C6H12N4(HMT)were added into the growth solutions to synthesize ZnO nano-arrays.The effects of OH-ion concentrations on the ZnO nano-arrays,including the structures,growth mechanism,and optical properties, were investigated.In this case,the molar ratios of Zn(NO3)2to HMT not only changed the geometrical shape of ZnO nano-arrays, but also affected optical properties of ZnO nano-arrays.Secondly, the spike-shaped CuO/ZnO heterostructure arrays were prepared by a two-step solution-system method,the structures and morphology were also discussed in details.Further,the samples were used for the photocatalytic degradation of methyl orange(MO) under UV light irradiation.We also discussed the possible photodegradation mechanism.
2 Experimental
2.1 Materials
All chemicals were of analytical reagent grade and used without further purification.All the aqueous solutions were prepared using distilled water.Fluorinated tin oxide(10 Ω·cm-2,Xiang Town Technologies Ltd.,China)was used as substrates and cleaned by standard procedures prior to use.
2.2 Preparation of ZnO nano-arrays on FTO coated glass substrates
ZnO nano-arrays were grown by a low temperature hydrothermal route with a two-step method of seed deposition and nanoarray growth.The ZnO nano-arrays were deposited onto FTO coated glass substrates.First,FTO glass substrates were rinsed ultrasonically in acetone,ethanol,and distilled water for 15 min, successively.ZnO seed layer was prepared on FTO glass by dipcoating(10 cm·min-1)in 5-7 mol·L-1ethanolic solutions of zinc acetate(99%,analytical grade),followed by thermal decomposition at 300°C for 15 min.Then,the obtained ZnO seed layer was transferred into a Teflon crucible filled with a growth solutionof Zn(NO3)2(99%,analytical grade),HMT(99%,analytical grade) and PEI(branched,low molecular weight,Chengdu Xiya Chemical Co.Ltd.,China)and kept inside the autoclave at 90°C for 24 h.The concentrations of Zn(NO3)2,HMT,and PEI in the resulting solution are listed in Table 1.The growth solutions were refreshed every 6 h during the reaction period.After reaction,all samples were taken out to rinse with deionized water and ethanol,successively,for several times,and annealed in air at 450°C for 30 min to remove any residual organics before investigating their properties.
2.3 Preparation of CuO hybridized ZnO nano-arrays
CuO/ZnO nano-arrays were also grown by a two-step solutionsystem method of seed deposition and nanoparticle(NP)growth. Firstly,the as-prepared ZnO nano-arrays on FTO substrate(molar ratio of Zn(NO3)2to HMT is 5:5)was immersed in the reaction solutionof 60°Cfor 2h.31The reactionsolutionconsistedof 1mol· L-1copper nitrate((Cu(NO3)2,99%,analytical grade)as copper source,2.6 mol·L-1potassium sodium tartrate tetrahydrate(PSTT, 99%,analytical grade)as a complexing agent,2.7 mol·L-1sodium hydroxide(NaOH,90%,analytical grade)and 6.3 mol·L-1sodium carbonate(Na2CO3,99.8%,analytical grade)as an alkaline medium,and a drop of formaldehyde(HCHO,37%,analytical grade) as a reducing agent,all these chemicals were purchased from Kelong Chemical Co.Ltd.(Chengdu,China).Then,the substrate was rinsed thoroughly with deionized water and immediately annealed in air at 250°C for 1 h to form CuO seeds on the surfaces of the ZnO nano-arrays.After annealing,the oxidized substrate was immersed in above-mentioned solution at 65°C for 7 h to obtain the desired thicknesses of CuO shells.
2.4 Characterization
The morphology of the nano-arrays was characterized using scanning electron microscopy(SEM,Fei Quanta 200,USA).The crystal phase of the nano-arrays was determined by X-ray diffraction(XRD,PanalyticalX'pertPRO,Netherlands),using Cu Kαradiation from 20°to 80°.X-ray photoelectron spectroscopy (XPS)measurement was performed with a PHI 5600 multitechnique system by using a monochromatic Al KαX-ray source. The UV-Vis diffuse reflectance spectra in the wavelength range of 300-800 nm were carried out with a UV-Vis 2550 spectrophotometer(Shimadzu 2550,Japan).
2.5 Photocatalytic test
The photocatalytic activities of the pure ZnO nano-arrays and CuO/ZnO heterostructures were investigated using the photodegradation of MO solution.Firstly,the sample(2 cm×3 cm)was vertically immersed in a beaker filled with 20 mL MO aqueous solution(1 mg·L-1,99%,analytical grade,Chengdu Kelong Chemical Co.Ltd.,China),and then kept for 30 min in the dark to equilibrate.Subsequently,the solution with the samples was irradiated under a 100 W UV-lamp with 254 nm emission wavelength or a 100 W visible light for 5 h,the location of the lamp was on the right above the front side of the samples,the distance between the lamp and the solution surface was 14 cm, and meanwhile stirred continuously at 25°C.The solution was sampled every 1 h during UV irradiation in order to determine the degree of degradation of MO,which was done by measuring the absorbance at 466 nm using a UV-Vis 2550 spectrophotometer.

Table 1 Synthesis conditions for the growth of ZnO nano-arrays
3 Results and discussion
3.1 Structural characterization of ZnOnano-arrays

Fig.1 (a)X-ray diffraction patterns of ZnO nano-arrays under different molar ratios of Zn(NO3)2to HMT on FTO glass substrates;(b)enlarged(002)diffraction peaks about ZnO
The crystallinity of ZnO nano-arrays prepared under the different synthesis conditions was investigated using X-ray diffraction analysis.Fig.1 shows the XRD patterns of ZnO nanoarrays grown for 24 h in the aqueous solutions with different molar ratios of Zn(NO3)2to HMT.As shown in Fig.1,FTO glass substrate had three characteristic diffraction peaks at 2θ=26.6°, 37.8°,51.8°,which corresponded to the(110),(200),(211) crystalline planes of tetragonal rutile structure of SnO2(JCPDS file No.41-1445),respectively.Besides,the diffraction peaks at 2θ=34.4°,36.1°,47.4°,62.7°for all the samples were attributed to the(002),(101),(102),(103)crystalline planes of typical wurtzite structure of ZnO(JCPDS file No.36-1451),respectively.The dominant(002)peak appearance in XRD patterns strongly supported that the ZnO nano-arrays show a high orientation along c-axis which was perpendicular to the FTO substrates.The results could also be inferred from the next SEM observations.Furthermore,enlarged(002)peaks of ZnO nano-arrays grown under different molar ratios of Zn(NO3)2to HMT were inserted in Fig.1b.We can find that all the(002)diffraction peak located on the same positions,and the(002)peak with the molar ratio of 5: 5 has the highest intensity.This result can be attributed to different molar ratios of Zn(NO3)2to HMT.ZnO is formed in the solution of OH−and Zn2+ions,and their stoichiometry is very critical parameter for ZnO.In the reaction process,if OH−concentrationis too low,this may lead to slow-growing and non-full reaction to form incomplete grain structure,while OH−concentration is too high,this may lead to fast-growing and the dissolution of ZnO crystals due to the presence of excess OH−.32All these reasons might result in the decrease of preferential growth trend.
3.2 Surface morphology analysis of ZnO nanoarrays
Fig.2 shows the top-down and cross-section SEM images of the ZnO nano-arrays synthesized under the conditions of different molar ratios of the Zn(NO3)2to HMT.From Fig.2,we observed that the majority ZnO nano-arrays were uniform and vertical grown on the FTO substrate with a high aspect ratio.The results were consistent with the XRD analysis.Averages were taken from the measurements for the ZnO nano-arrays on FTO substrate, where the lengths were measured using the cross-sectional SEM images and the diameters were measured using the top-down SEM images.33The mean values of the ZnO nano-array dimension, including the length and diameter estimated from a statistical evaluation of SEM images,are given in Fig.2f.It was obvious that as the molar ratios of Zn(NO3)2to HMT increased from 5:2 to 5: 15,the lengths of ZnO nano-arrays correspondingly decreased from 6.78 to 2.92 μm.The diameters of the nano-arrays increased to 311 nm with increasing the molar ratio of Zn(NO3)2to HMT to 5:6,and when further increasing the molar ratio to 5:15 the diameters of the nano-arrays decreased to 190.7 nm.

Fig.2 Top-down SEM images of ZnO nano-arrays grown under different molar ratios of Zn(NO3)2to HMT of (a)5:2,(b)5:5,(c)5:6,(d)5:10,and(e)5:15;(f)mean diameter and length from a to e
It was reported that ZnO was an amphoteric substance,it could not only act as an acid to form[Zn(OH)4]2−in the presence of OH−, but also act as a base to produce Zn2+in the presence of H+. Therefore,it was deduced that increasing the molar ratios of Zn(NO3)2to HMT could suppress the growth rate.This might result from the dissolution of ZnO crystals due to the presence of excess OH−.32
As all we know,the growth of ZnO nano-arrays in the aqueous solution is based on the crystallization theory of solid phase from the solution,which involves two steps of nucleation and growth.
In our experiments,the ZnO seeds derived by the thermal decomposition of zinc acetate were used as the nuclei,which was propitious to lower activation energy barrier for ZnO nano-arrays. The following reactions are involved in the formation of ZnO seeds:34


Next,ZnO nano-arrays were grown in aqueous solution of Zn(NO3)2and HMT at 90°C.In our experiment,Zn(NO3)2was used as source of zinc,HMT as source of OH−,and PEI as additive.No precipitate generated initially when HMT was added to the mixture solution.With the increase of growth temperature,the HMT began to decompose and form ammonia,which reacted with Zn(NO3)2to generate Zn(OH)2and finally obtained ZnO nanoarrays on the substrate.The involved reactions can be expressed as follows:35
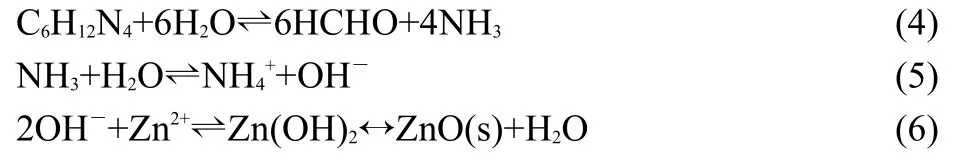
The formation for superior alignment on ZnO seed film is attributed to the polar nature of the ZnO surface.36ZnO,as a polar crystal,its surface is either positively charged or negatively charged.34,37Therefore,the seed surface attracts ions of opposite charges(OH−or Zn2+)to form the new surface,which in turn attracts ions with opposite charges to cover the surface and thereby reacting to form ZnO.It was reported that the optimized(001) surface had roughly a 60%higher cleavage energy than the nonpolar(100)and(110)faces,and ZnO had a dipole moment along(001)direction.These properties suggested that the c-axis was the fastest growth direction and the ZnO(001)had the highest energy of the low-index surface.34,37Hence,ZnO could easily grow along this direction and the resultant ZnO nano-arrays stand mostly perpendicular onto substrates as shown in Fig.2.
3.3 Optical properties of ZnO nano-arrays
The optical band gap is an important parameter for the semiconductor materials.It links to the absorption line in the spectrum for these materials.Fig.3a shows the optical transmittance spectra of ZnO nano-arrays deposited at different growth conditions in the wavelength range of 300-800 nm.The average transmittance of the samples can be calculated by this formula:

where Aois absorbance tested by a UV-Vis 2550 spectrophotometer,T is the average transmittance.It can be found that the ZnO nano-arrays present low transmittance in the wavelength range of 300-800 nm and the average transmittances are 1.57%, 2.01%,0.67%,11.68%,and 3.54%for the molar ratios of Zn(NO3)2to HMT from 5:2 to 5:15,respectively.This indicates a strong adsorption in the wavelength range from 300 to 800 nm.
However,absorption coefficient is related to transmittance. From the transmission spectra of Fig.3a,the values of the absorption coefficient α are calculated using the equation:38

The relationship between absorption coefficientαand the incident photon energy hνcan be described by Tauc formula:

where A is a constant,Egis the optical band gap energy,νis the frequency of the incident photon,h is the Planck's constant,and d is the nano-array length.The optical band gaps for the as-prepared samples are 2.70,3.20,2.90,3.15,and 3.05 eV,respectively, which are given in Fig.3(b-f).With increasing the molar ratios of Zn(NO3)2to HMT from 5:2 to 5:15,the optical band gap increased at first with the molar ratio increasing from 5:2 to 5:5 and then decreased from 5:5 to 5:15,the change trend of optical band gap was generally like as the changing trend of diameter.Wang39and Schmidt40et al.also found the width of the band gap was connected with nano-arrays diameter.Therefore,we can regulate the optical band gap of ZnO nano-arrays through controlling the structure and diameter by changing the molar ratios of precursor solution.
3.4 CuO hybridized ZnO nano-arrays
Although the ZnO nano-arrays have higher specific surface area and excellent electron transmission,the photocatalytic activity is relatively low.The recombination process is considered as one of the most important factors to control photocatalytic activity.Therefore,we try to deposite nano-CuO on the surface of ZnO nano-arrays to form p-n heterojunctions to reduce the recombination of photo-induced electrons and holes.
3.4.1 Structural characterization of CuO/ZnOnanoarrays
The XRD patterns of the pure CuO and the as-synthesized samples with the 5:5 molar ratio of Zn(NO3)2to HMT were shown in Fig.4.As shown in Fig.4,the diffraction peaks at 2θ=34.4°, 36.2°,47.5°,62.8°,72.4°of as-prepared sample were attributed to the typical wurtzite structure of ZnO(JCPDS file No.36-1451). Then,the other peaks at 2θ=26.5°,37.8°,51.6°,65.8°were belong to the(110),(200),(211),(301)crystalline planes of tetragonal rutile structure of SnO2(JCPDS file No.41-1445),respectively. Besides,the two characteristic diffraction peaks at 2θ=61.6°,68.3° were assigned to the(113),(221)crystalline planes of monoclinic structure of CuO(JCPDS file No.80-1917),respectively.This observation indicated that the crystal structure of ZnO was intact during the CuO fabrication process.What's more,no other peaks for possible impurity phases such as Cu2O and metal Cu could be detected.So,it could be concluded that the nanocomposites were only composed of CuO and ZnO.
3.4.2 Composition analysis of CuO/ZnOnano-arrays
It is well known that surface composition and chemical states of materials are very important during photodegradation process since they can strongly affect the photocatalytic activity.The XPS analysis is carried out to investigate the composition information by the characteristic binding energy of different elements and element chemical states on material surfaces.The XPS results are given in Fig.5.The binding energies in the XPS spectra were calibrated by using that of C 1s(284.8 eV).All peaks in the XPS full spectrum of the as-prepared CuO/ZnOnano-arrays shownin Fig.5a could be ascribed to Zn,Cu,O,and C elements and no peaks of other elements are observed.However,the appearance of weak C peak mainly came from gaseous carbon molecules of vacuum treatment before the XPS test.
The high resolution spectra for Zn,O,and Cu species are shown in Fig.5(b-d),respectively.The two strong peaks of Zn 2p at 1021.8 and 1045.0 eV in Fig.5b corresponded to Zn 2p3/2and Zn2p1/2,respectively.The peaks were symmetrical and nearly the same positions as those of pure ZnO.41Thus,it could be confirmed that Zn element existed mainly as the form of Zn2+chemical states on sample surfaces.As shown in Fig.5c,the broad O 1s core-level spectrum could be fitted to two symmetrical peaks by Gaussian rule.The peak at a lower binding energy(529.9 eV)in Fig.5c agreed with lattice oxygen(OL)in mental oxide such as CuO and ZnO,42,43while the other peak at 531.4 eV was attributed to chemisorbed oxygen and/or hydroxide(OH).42,43Fig.5d shows the Cu 2p XPS spectrum,the Cu 2p spectra of the as-synthesized sample show two main 2p3/2and 2p1/2spinorbit lines at 933.8 and 953.7 eV,respectively,which corresponded to the binding energies of Cu 2p3/2and Cu 2p1/2of CuO.44,45Simultaneously,the peak at 941.1, 943.6,and 962.7 eV were considered to be the satellite peaks of CuO,which could also confirm the presence of CuO.46,47So,it was further deduced that CuO was formed on the ZnO.

Fig.3 (a)Optical transmittance of all the ZnO nano-arrays under different molar ratios of Zn(NO3)2to HMT on FTO glass substrates;(b-f)optical band gap calculations of ZnO nano-arrays grown under different molar ratios of Zn(NO3)2to HMT

Fig.4 XRD patterns of the bare CuO and the as-prepared CuO/ZnO nano-arrays
3.4.3 Surface morphology analysis of CuO/ZnO nanoarrays
SEM tests were subsequently carried out to try to observe the location and morphology of CuO.Fig.6(a-c)shows the typical FE-SEM images of CuO NPs prepared on the surface of ZnO nano-arrays which were obtained under the conditions of 5:5 molar ratio of Zn(NO3)2to HMT,which was shown in Fig.2b.As can be seen from Fig.6(a-c),lots of CuO NPs uniformly enwrapped on the surface of ZnO nano-arrays and the structure of the CuO hybridized ZnO still presented the spike shape.In addition,no isolated CuO particles were found in the mixture, meaning that CuO particles were strongly anchored to the ZnO surface.The average size of CuO NPs was about 48.37 nm by using image analysis software(Image Pro Plus 6.0).48Therefore, combination with the results of XRD and XPS tests,we can be sure that this sample was CuO/ZnO heterostructure.
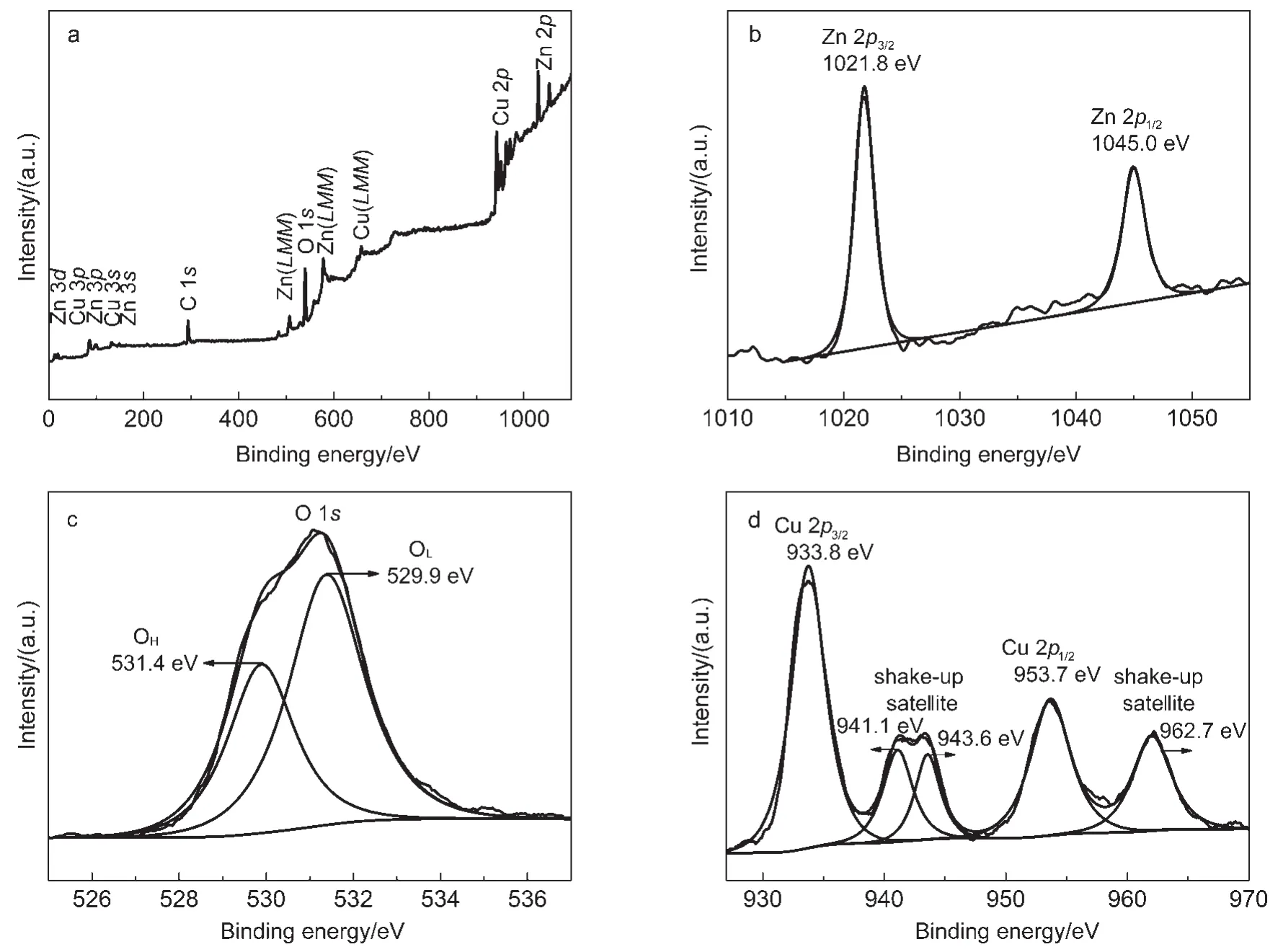
Fig.5 XPS spectra of the as-prepared CuO/ZnO nano-arrays
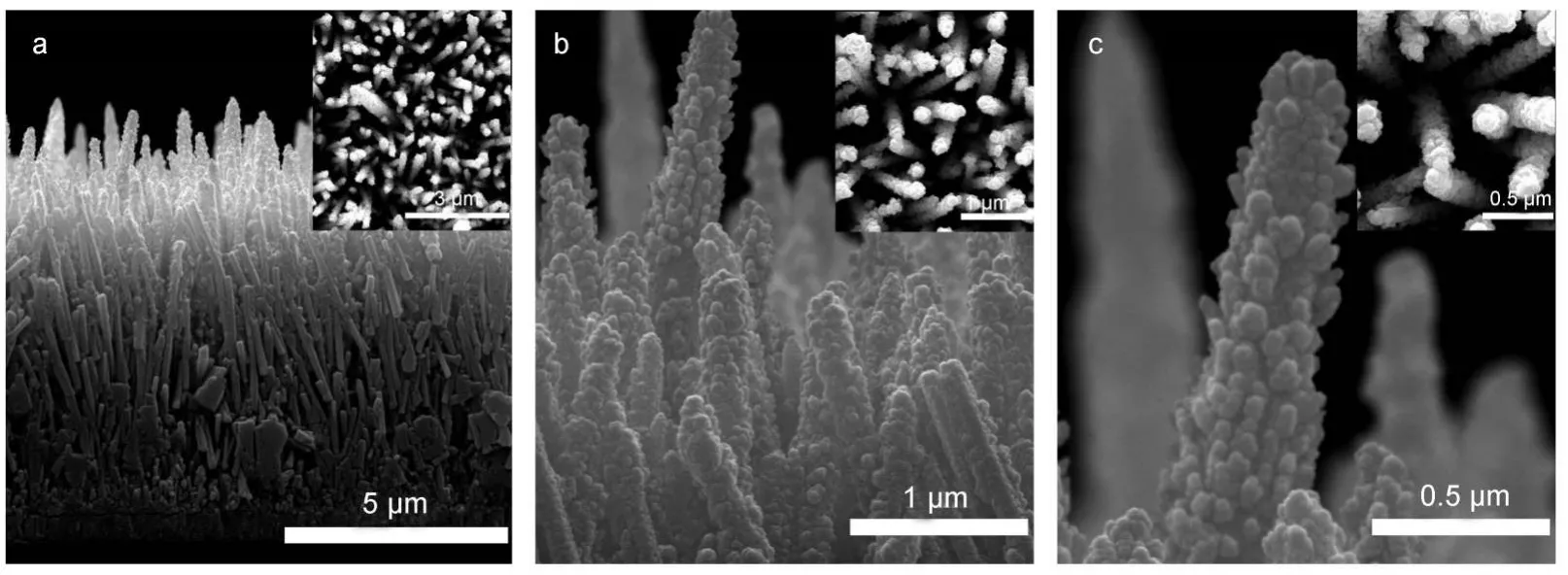
Fig.6 Cross-sectional FE-SEM images of ZnO and CuO/ZnO heterostructure nano-arrays
3.4.4 Photocatalytic performance of CuO/ZnOnanoarrays
The photocatalytic activities of the CuO/ZnO photocatalysts were evaluated with respect to the degradation of MO dye aqueous solution(1 mg·L-1)under UV light irradiation,and the results are shown in Fig.7a.The rate of photodegradation was tested by measuring the absorbance at 466 nm for MO.The degradation efficiency is calculated by the following equation:

where D is the degradation rate of dye solution,A0and Atare theinitial maximum absorbance and the maximum absorbance of dye solution after irradiation for t hours,respectively.

Fig.7 (a)Photoactivity of CuO/ZnO nano-arrays in the MO aqueous solution under UV irradiation;(b)photocatalytic degradation of MO in recycling tests of CuO/ZnO nano-arrays
The blank test with only UV irradiation exhibited a decomposition rate of only 2.2%after 5 h irradiation.Pure CuO NPs show slightly progressed degradation for MO solution with 5.43%.And it could be noticed that the degradation rate of pure ZnO nano-arrays was 56.7%which was superior to that of pure CuO NPs,which might be due to the fact that ZnO nano-arrays had higher aspect ratio and higher specific surface area than pure CuO NPs.Further,the degradation rate of MO experienced another considerable increase and reached to 92.8%when CuO/ZnO nano-arrays were applied.As a contrast,we used commercial titania(P25,Degussa)as a reference catalyst,and the degradation rate of MO for P25 under UV irradiation for 5 h was 81.1%. Therefore,the photocatalytic activities were in the order of CuO/ ZnO>P25>pure ZnO>pure CuO>the blank test under UV light irradiation.The phenomenon is attributed to the formation of heterojunctions between the interface of CuO and ZnO nanoarrays,which favors the separation of photo-generated electrons and holes.20,49
Photocatalytic recycling experiments were used for photocatalytic stability test.Fig.7b shows the results for photocatalytic recycling degradation of MO under UV irradiation.The photocatalytic activity decreased slightly after the third cycle;the MO degradation rate was 92.8%at the end of the first cycle and 84.6% after the third cycle,showing that the photocatalyst had excellent stability.
The enhanced activities of the CuO/ZnO heterostructure could be explained based on the schematic diagram of excitation and separation of electrons and holes for CuO/ZnO heterojunction under UV irradiation,shown in Fig.8.It was reported that interparticle transfer of charge carriers contributes to the enhanced photocatalytic efficiency of coupled semiconductors when the energies of valence band(VB)and conduction band(CB)were properly matched.50As shown in Fig.8,when the CuO/ZnO heterostructure was irradiated by UV light,the valence band electrons of CuO and ZnO could be excited to their conduction band, respectively.According to the thermodynamic theory,51the photogenerated electrons transfer from CB of CuO to that of ZnO, while the photogenerated holes immigrate from VB of ZnO to that of CuO.Consequently,the photogenerated electrons and holes are effectively separated,the electrons accumulated in CB of ZnO and holes accumulated in VB of CuO can be consumed for degradation of pollutants.The possible photo-degradation mechanism of CuO/ZnO nano-arrays was as follows:29,52

From the above reactions,it had been confirmed that the photodegradation of dyes was mainly governed by the direct oxidation of photo-induced holes53,54which could reduce H2O molecules to OH·,and OH·was strong oxidizing agent for organic pollutant and electrophilic reagent.46
The UV-Vis absorption abilities of the one-dimensional ZnO nano-arrays and the CuO/ZnO nano-arrays are shown in Fig.9a. The absorption edge was about 380 nm for bare ZnO nano-arrays, while the absorption edge of the CuO/ZnO nano-arrays was about 550 nm.Obviously,the CuO/ZnO nano-arrays had the better light utilization rate than one-dimensional ZnO nano-arrays.The enhanced absorption of visible light for CuO/ZnO nano-arrays might be also very useful in the photo-degradation of dyes applications.

Fig.8 Schematic diagram of excitation and separation of electrons and holes for CuO/ZnO heterojunction under UV irradiation

Fig.9 (a)Diffuse reflectance UV-Vis spectra of the bare ZnO and CuO/ZnO nano-arrays;(b)photoactivity of CuO/ZnO nanoarrays in the MO aqueous solution under visible light irradiation
The photocatalytic activities for the degradation of MO dye aqueous solution(1 mg·L-1)of the one-dimensional ZnO nanoarrays and the CuO/ZnO nano-arrays under 100 W visible light irradiation are shown in Fig.9b.The results show that the degradations of MO dye aqueous solution of the one-dimensional ZnO nano-arrays,P25,and CuO/ZnO nano-arrays were very lower from 10.4%to 14.9%under visible light than the results under the UV irradiation,these results might be attributed to the optical absorption.The UV light can stimulate CuO and ZnO to generate photoproduction electron-holes at the same time,and be more effective to separate the photogenerated electrons and holes, while the visible light can only stimulate CuO.Therefore,the photodegradation of MO under UV irradiation is higher than that under the visible light.
4 Conclusions
In summary,the spike-shaped CuO/ZnO nano-arrays were successfully fabricated using a two-step solution-system method. Firstly,the characters of the ZnO nano-arrays,such as the structures,growth mechanism,and optical properties could be controlled by the molar ratios of Zn(NO3)2to HMT.As the molar ratios increased from 5:2 to 5:15,the lengths of ZnO nano-arrays correspondingly decreased from 6.78 to 2.92 μm,and the diameter firstly increased to 311.0 nm and then decreased to 190.7 nm.By and large,the change of optical band gap also had the same trend as the change of diameter,when the molar ratio is 5:5,the(002) peak had the highest intensity and the optical gap was 3.20 eV. Afterwards,the CuO NPs were deposited on the surface of ZnO nano-arrays which were obtained under the 5:5 malar ratio of Zn(NO3)2to HMT.The prepared sample presented spike shape, excellent photocatalytic properties for MO under UV irradiation, and also showed advanced light absorption ability in the UV-Vis region.Next,we expect that this unique morphology will provide more promising applications,such as solar energy conversion and gas sensor devices.
(1) Zhang,C.H.;Wang,G.F.;Liu,M.;Feng,Y.H.;Zhang,Z.D.; Fang,B.Electrochim.Acta 2010,55(8),2835.doi:10.1016/j. electacta.2009.12.068
(2) Jiang,C.Y.;Sun,X.W.;Lo,G.Q.;Kwong,D.L.Appl.Phys. Lett.2007,90(26),263501.doi:10.1063/1.2751588
(3) Zhang,Y.Z.;Liu,Y.P.;Wu,L.H.;Li,H.;Han,L.Z.;Wang,B. C.;Xie,E.Q.Appl.Surf.Sci.2009,255(9),4801.doi:10.1016/ j.apsusc.2008.11.091
(4) Yang,P.D.;Yan,H.Q.;Mao,S.;Russo,R.;Johnson,J.; Saykally,R.;Morris,N.;Pham,J.;He,R.H.;Choi,H.J.Adv. Funct.Mater.2002,12(5),323.doi:10.1002/1616-3028 (20020517)12:5<323::AID-ADFM323>3.0.CO;2-G
(5) Liu,C.H.;Zapien,J.A.;Yao,Y.;Meng,X.M.;Lee,C.S.;Fan, S.S.;Lifshitz,Y.;Lee,S.T.Adv.Mater.2003,15(10),838.doi: 10.1002/adma.200304430
(6) Lee,C.J.;Lee,T.J.;Lyu,S.C.;Zhang,Y.;Ruh,H.;Lee,H.J. Appl.Phys.Lett.2002,81(19),3648.doi:10.1063/1.1518810
(7) Zhu,S.B.;Chen,X.N.;Zuo,F.B.;Jiang,M.;Zhou,Z.W. J.Solid State Chem.2013,197,69.doi:10.1016/j.jssc. 2012.09.001
(8) Kuo,T.J.;Lin,C.N.;Kuo,C.L.;Huang,M.H.Chem.Mater. 2007,19(21),5143.doi:10.1021/cm071568a
(9) Zhai,X.H.;Long,H.J.;Dong,J.Z.;Cao,Y.A.Acta Phys.-Chim.Sin.2010,26(3),663.[翟晓辉,龙绘锦,董江舟,曹亚安.物理化学学报,2010,26(3),663.]doi:10.3866/ PKU.WHXB20100317
(10) Elias,J.;Lévy-Clément,C.;Bechelany,M.;Michler,J.;Wang, G.;Wang,Z.;Philippe,L.Adv.Mater.2010,22(14),1607.doi: 10.1002/adma.200903098
(11) Lyu,S.C.;Zhang,Y.;Lee,C.J.;Ruh,H.;Lee,H.J.Chemistry of Materials 2003,15(17),3294.doi:10.1021/cm020465j
(12) Kang,S.W.;Mohanta,S.K.;Kim,Y.Y.;Cho,H.K.Crystal Growth and Design 2008,8(5),1458.doi:10.1021/cg701216f
(13) Sun,Y.;Fuge,G.M.;Ashfold,M.N.R.Chemical Physics Letters 2004,396(1),21.
(14) Gao,Y.F.;Nagai,M.;Chang,T.C.;Shyue,J.J.Crystal Growth and Design 2007,7(12),2467.doi:10.1021/cg060934k
(15) Liu,B.;Zeng,H C.Journal of the American Chemical Society 2003,125(15),4430.doi:10.1021/ja0299452
(16) Kumar,P.S.;Raj,A.D.;Mangalaraj,D.;Nataraj,D.Applied Surface Science 2008,255(5),2382.doi:10.1016/j.apsusc.2008.07.136
(17) Liu,Z.Y.;Bai,H.W.;Sun,D.D.Int.J.Photoenergy 2011,2012.
(18) Yan,W.P.;Wang,D.J.;Chen,L.P.;Lu,Y.C.;Xie,T.F.;Lin,Y. H.Acta Phys.-Chim.Sin.2013,29(5),1021.[闫伟平,王德军,陈礼平,卢永春,谢腾峰,林艳红.物理化学学报,2013,29 (5),1021.]doi:10.3866/PKU.WHXB201303043
(19) Zhang,Q.B.;Feng,Z.F.;Han,N.N.;Lin,L.L.;Zhou,J.Z.; Lin,Z.H.Acta Phys.-Chim.Sin.2010,26(11),2927.[张桥保,冯增芳,韩楠楠,林玲玲,周剑章,林仲华.物理化学学报, 2010,26(11),2927.]doi:10.3866/PKU.WHXB20101113
(20) Wang,J.;Fan,X.M.;Wu,D.Z.;Dai,J.;Liu,H.R.;Zhou,Z.W. Appl.Surf.Sci.2011,258(5),1797.doi:10.1016/j. apsusc.2011.10.048
(21) Koffyberg,F.P.;Benko,F.A.J.Appl.Phys.1982,53(2),1173. doi:10.1063/1.330567
(22) Wang,L.;Han,K.;Song,G.;Yang,X.;Tao,M.Characterization of Electro-Deposited CuO as a Low-Cost Material for High-Efficiency Solar Cells.In Photovoltaic Energy Conversion;the 2006 IEEE 4th World Conference,Singapore,2006;IEEE, 2006,1,130-133.
(23) Rai,A.K.;Anh,L.T.;Gim,J.;Mathew,V.;Kang,J.;Paul,B.J.; Singh,N.K.;Song,J.;Kim,J.J.Power Sources 2013,244, 435.doi:10.1016/j.jpowsour.2012.11.112
(24) Nezamzadeh-Ejhieh,A.;Karimi-Shamsabadi,M.Chem.Eng.J. 2013,228,631.doi:10.1016/j.cej.2013.05.035
(25) Steinhauer,S.;Brunet,E.;Maier,T.;Mutinati,G.C.;Kock,A.; Freudenberg,O.;Gspan,C.;Grogger,W.;Neuhold,A.;Resel, R.Sensor Actuat.B-Chem.2013,187,50.doi:10.1016/j. snb.2012.09.034
(26) Anandan,S.;Wen,X.G.;Yang,S.H.Mater.Chem.Phys.2005, 93(1),35.doi:10.1016/j.matchemphys.2005.02.002
(27) Kim,J.;Kim,W.;Yong,K.J.Phys.Chem.C 2012,116(29), 15682.doi:10.1021/jp302129j
(28) Kargar,A.;Jing,Y.;Kim,S.J.;Riley,C.T.;Pan,X.Q.;Wang, D.L.ACS Nano 2013,7(12),11112.doi:10.1021/nn404838n
(29) Jung,S.;Yong,K.Chem.Commun.2011,47(9),2643.doi: 10.1039/c0cc04985a
(30) Law,M.;Greene,L.E.;Johnson,J.C.;Saykally,R.;Yang,P.D. Nat.Mater.2005,4(6),455.doi:10.1038/nmat1387
(31) Goldie,W.Plating 1964,51(11),1069.
(32) Jung,J.;Myoung,J.;Lim,S.Thin Solid Films 2012,520(17), 5779.doi:10.1016/j.tsf.2012.04.052
(33) Zhu,K.X.;Wang,W.J.;Chen,X.L.;Liu,J.;Song,B.;Jiang,L. B.;Guo,J.G.;Cheng,J.Y.J.Alloy.Compd.2011,509(24), 6942.doi:10.1016/j.jallcom.2011.04.007
(34) Chen,Z.T.;Gao,L.J.Cryst.Growth 2006,293(2),522.doi: 10.1016/j.jcrysgro.2006.05.082
(35) Lee,Y.L.;Zhang,Y.;Ng,S.L.G.;Kartawidja,F.C.;Wang,J. J.Am.Ceram.Soc.2009,92(9),1940.doi:10.1111/ jace.2009.92.issue-9
(36) Wang,Z.L.Mater.Today 2004,7(6),26.doi:10.1016/S1369-7021(04)00286-X
(37) Vayssieres,L.;Keis,K.;Lindquist,S.E.;Hagfeldt,A.J.Phys. Chem.B 2001,105(17),3350.doi:10.1021/jp010026s
(38) Pankove,J.I.Optical Process in Semiconductor;Dover Publications:New York,2012.
(39) Wang,B.L.;Zhao,J.J.;Jia,J.M.;Shi,D.N.;Wan,J.G.;Wang, G.H.Appl.Phys.Lett.2008,93(2),021918.doi:10.1063/ 1.2951617
(40) Schmidt,T.M.;Miwa,R.H.Nanotechnology 2009,20(21), 215202.doi:10.1088/0957-4484/20/21/215202
(41) Zheng,J.;Jiang,Z.Y.;Kuang,Q.;Xie,Z.X.;Huang,R.B.; Zheng,L.S.J.Solid State Chem.2009,182(1),115.doi: 10.1016/j.jssc.2008.10.009
(42) Ai,Z.H.;Zhang,L.Z.;Lee,S.C.;Ho,W.K.J.Phys.Chem.C 2009,113(49),20896.doi:10.1021/jp9083647
(43) Borgohain,K.;Murase,N.;Mahamuni,S.J.Appl.Phys.2002, 92(3),1292.doi:10.1063/1.1491020
(44) Li,B.X.;Wang,Y.F.Superlattice Microst.2010,47(5),615. doi:10.1016/j.spmi.2010.02.005
(45) Sakai,Y.;Ninomiya,S.;Hiraoka,K.Surf.Int.Anal.2012,44 (8),938.doi:10.1002/sia.4843
(46) Capece,F.M.;Castro,V.D.;Furlani,C.;Mattogno,G. J.Electron.Spectrosc.1982,27(2),119.doi:10.1016/0368-2048(82)85058-5
(47) Wan,Y.;Zhang,Y.D.;Wang,X.L.;Wang,Q.Electrochem. Commun.2013,36,99.doi:10.1016/j.elecom.2013.09.026
(48) Xiang,F.M.;Wu,J.;Liu,L.;Huang,T.;Wang,Y.;Chen,C.; Peng,Y.;Jiang,C.X.;Zhou,Z.W.Polym.Adv.Technol.2011, 22(12),2533.doi:10.1002/pat.v22.12
(49) Saravanan,R.;Karthikeyan,S.;Gupta,V.K.;Sekaran,G.; Narayanan,V.;Stephen,A.Mater.Sci.Eng.C 2013,33(1),91. doi:10.1016/j.msec.2012.08.011
(50) Serpone,N.;Maruthamuthu,P.;Pichat,P.;Pelizzetti,E.; Hidaka,H.J.Photochem.Photobiol.A 1995,85(3),247.doi: 10.1016/1010-6030(94)03906-B
(51) Wei,S.Q.;Chen,Y.Y.;Ma,Y.Y.;Shao,Z.C.J.Mol.Catal.AChem.2010,331(1),112.
(52) Li,J.;Wang,J.;Huang,L.;Lu,G.D.Photochem.Photobiol. Sci.2010,9(1),39.doi:10.1039/b9pp00084d
(53) Chandrinou,C.;Boukos,N.;Stogios,C.;Travlos,A. Microelectron.J.2009,40(2),296.doi:10.1016/j. mejo.2008.07.024
(54) Greene,L.E.;Law,M.;Goldberger,J.;Kim,F.;Johnson,J.C.; Zhang,Y.F.;Saykally,R.J.;Yang,P.D.Angew.Chem.Int.Edit. 2003,42(26),3031.doi:10.1002/anie.200351461
Syntheses of ZnO Nano-Arrays and Spike-Shaped CuO/ZnO Heterostructure
LI Xiang-Qi1FAN Qing-Fei1LI Guang-Li1HUANG Yao-Han1GAO Zhao1FAN Xi-Mei1,*ZHANG Chao-Liang2ZHOU Zuo-Wan1
(1Key Laboratory of Advanced Technologies of Materials,Ministry of Education,School of Materials Science and Engineering, Southwest Jiaotong University,Chengdu 610031,P.R.China;2State Key Laboratory of Oral Diseases, West China Hospital of Stomatology,Sichuan University,Chengdu 610031,P.R.China)
Alow-temperature hydrothermal route was applied to fabricate ZnO nano-arrays on fluorinated tin oxide(FTO)-coated glass substrates.The effects of the molar ratios of the precursor concentrations on the ZnO nano-arrays were studied with respect to morphology,optical properties,and growth mechanism.The results show that the length reduced with the increased molar ratios of precursor concentrations,and the diameter first increased then decreased.In general,the change of optical band gap followed the same trend as that for the change in diameter.When the molar ratio of precursor concentrations is 5:5,the optical band gap is 3.2 eV, which is similar to the theoretical value at room temperature.We propose that the optimal molar ratio of zinc nitrate(Zn(NO3)2)to hexamethylenetetramine(HMT,C6H12N4)is 5:5 for the preparation of ZnO nano-arrays. Spike-shaped CuO/ZnO nano-arrays were also successfully synthesized using a two-step solution-system method.Field emission scanning electron microscope(FE-SEM)results show that there were a large number of copper oxide(CuO)nano-particles(NPs)deposited onto the ZnO nano-array surfaces to form spike-shaped structures.The covered CuO NPs exhibited improved photocatalytic properties over pure ZnO nano-arraysunder UV irradiation,and the possible photocatalytic mechanism of the CuO/ZnO nano-heterojunction was discussed in detail.©Editorial office ofActa Physico-Chimica Sinica
ZnO nano-array;CuO/ZnO heterostructure;Hydrothermal method;Optical property; Molar ratio
O645;O611
10.3866/PKU.WHXB201502062www.whxb.pku.edu.cn
Received:December 15,2014;Revised:February 4,2015;Published on Web:February 6,2015.
∗Corresponding author.Email:xmfan@home.swjtu.edu.cn;Tel:+86-28-87602714;Fax:+86-28-87600454.
The project was supported by the High-Tech Research and Development Program of China(2009AA03Z427).
国家高技术研究发展计划项目(2009AA03Z427)资助

