Study of polluted soil remediation based on freezing and thawing cycles
DaHu Rui ,BaiYang Song ,Yuzuru Ito ,Li Wang
1.School of Civil Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China
2.Dept.of Civil Engineering,Setsunan University,Neyagawa,Osaka Prefecture 572-0074,Japan
1 Introduction
With rampant industrialization and urbanization,as well as the utilization of Earth resources on a large scale over recent decades,the problem of soil pollution is getting worse.It is generally known that soil pollution poses a terrible hazard to the environment,but the present techniques of contaminated soil remediation cannot control this growing threat.Therefore,the purpose of technology research on cost-effective remediation of contaminated soils is to improve environmental quality;this has become an international multi-disciplinary research hotspot (Zhou and Song,2004;Liet al.,2006;Luo,2009).
Heavy-metal pollution contaminates soil in different forms,such as dissolving in the liquid phase of soils,precipitating with hydrates and carbonates,mixing with minerals and organic matter,and adsorbing in the surfaces of colloids.The current method of remediating polluted soil can only address one or two metal cation pollutants,so it is of great significance to seek a simple way to deal with a variety of pollutants.As society uses more and more chemical products,the pollution resulting from organic chemicals has become increasingly serious.Furthermore,the control and governance of dense nonaqueous phase liquids (DNAPLs) has become the most important problem confronting international environmental and water resources,because DNAPLs can reach the bottom of aquifers through the vadose zone due to their density being less than that of water.This type of pollution can seriously harm our environment and its management is very difficult.
As a solution to this problem,this paper discusses the technique of remediating polluted soil by freezing.The main principles are:
1) Fen-Chonget al.(2006),Gay and Azouni(2007),and Shafiqueet al.(2012) presented a"low-temperature purification method" that utilized a propagated freezing front that led to the rejection of metallic pollutants in the suspension system.Its mechanism was mainly that,because the solubility of most of metal salts was relatively high,the solubility decreased along with the temperature,and accordingly the metal salt precipitated.Heavy-metal ions in contaminated soil were often separated from other particles,and they could be expelled by propagation of the freezing front under a certain temperature gradient(Guillaume and Azouni,2003).The advantage of this method is that a variety of heavy-metal ions can be extracted (Wuet al.,2001;Qiet al.,2008;Xuet al.,2010;Bing and Heng,2011).The soil freezing process(either natural or artificial) causes the water to shift from unfrozen soils to the freezing front,thus enhancing the permeability of the soil.This paper assesses how this effect,combined with traditional pumping and treating methods,can improve pollutant extraction efficiency.The cleansing mode is shown in figure 1.
2) For DNAPLs in soil,suction by freezing is based on the different freezing points of moisture and pollutants (Table 1),in addition to the increase of the permeability coefficient by the freezing-thawing action.Figure 2 shows the simulation graph of this (Itoet al.,2002,2004).The main process is that,first,the freezing and return pipes are buried in the contaminated soil,and then the moisture in the soil is frozen(the pollutant would not be frozen due to its different freezing point).Afterwards,the traditional pumping and treating method would be applied to extract the high concentrations around the pollution sources with the help of the increscent permeability coefficient caused by freezing and thawing action.
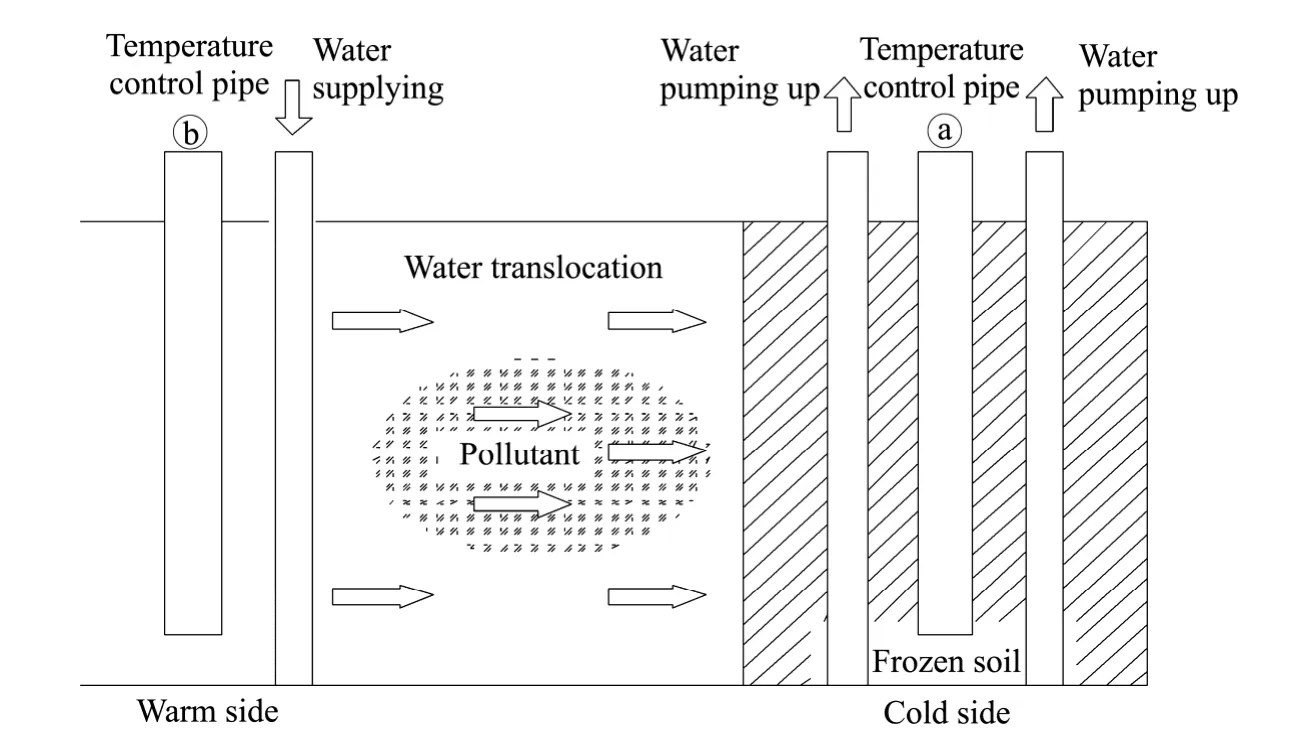
Figure 1 Freezing and thawing method for polluted soil remediation

Table 1 Properties of some DNAPLs
2 Remediation of contaminated soil by freezing and thawing
2.1 Experimental methods
The physical parameters of the clay we used are shown in table 2.The sample preparation process was:a 2% NaCl solution,the amount of which was nearly 1.5 times the liquid limit,was injected into the soil specimen (2 g NaCl per 100 g distilled water),and then the water content ratio was adjusted to 70%.Next the sample was put in a soil tank with a 60-cm diameter and 80-cm height and the sample was mixed for 4 hours after 12 hours of being static.Finally,preconsolidation was achieved by an applied pressure of 30 kPa.
A high-level cistern was employed for moisturizing through a central water supply pipe (Figure 3).The head difference was 80 cm.Drain holes (2-mm diameter) were arranged from bottom to top along its 40-cm length,and three layers of twisted geotechnical cloth were installed to prevent the pipe from being clogged by soil particles.The upper applied load in the test was 20 kPa.

Figure 2 Removal technique of DNAPLs:Suction by freezing

Figure 3 Freezing and thawing test system

Table 2 Properties of the studied soil
The test conditions are shown in table 3.F/T-1 was the freezing and thawing test process,wherein the freezing process was induced from the side to the center by the freezing plate fixed on the side,in which the coolant circulated.The movement of the freezing front was verified by thermocouples installed at the bottom and center of the soil tank.The freezing process would be shut off if the temperature of the center reduced to-1.45 °C,and then warm water (40 °C) was injected into the freezing plate to force thawing.This process,as well as the vacuum attraction,was like freezing and the water supply was in an open system (that is,the water supplement was from the high-level cistern through the central water supply pipe).Moisture was extracted by a plastic drainage belt inside the soil tank.For comparison,F/T-2 was the same experiment without freezing.
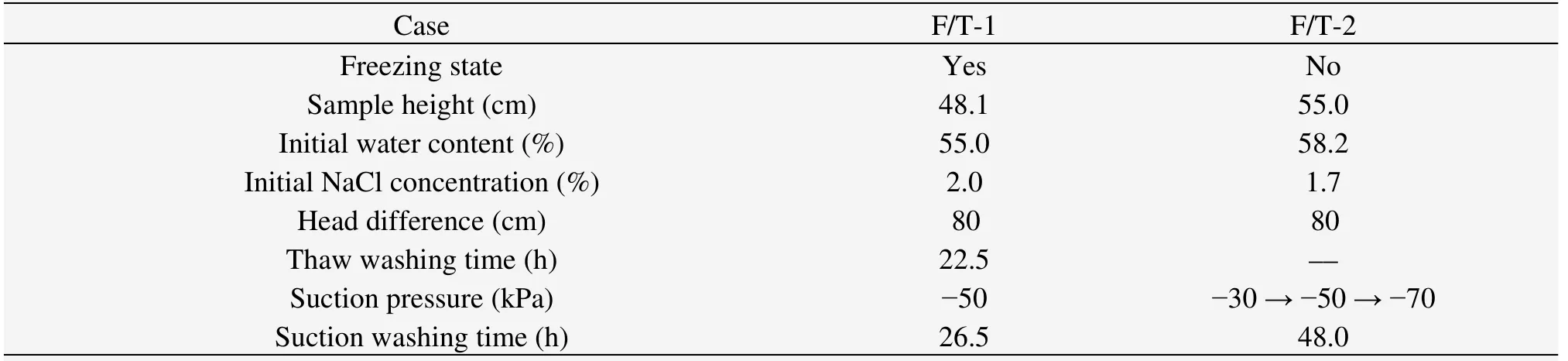
Table 3 Experimental conditions
2.2 Experimental results
2.2.1 Pumping test without freeze-thaw process (F/T-2)
1) Concentration of NaCl in discharge water
The experimental results are shown in table 4,and figure 4 shows the relationship between the water outflow by vacuum suction and the NaCl concentration.Water outflow increased by a certain percentage and the final water outflow was 8.3 L during the pumping test without a freeze-thaw process.By calculating the water outflow,the permeability coefficients of times 16-25 hours and 39-48 hours were both 1×10-6–2×10-6.The concentration of NaCl in the discharge water was equal to that of the pore water(1.7%);from suction to vacuum,the negative pressure reached to-50 kPa.When the pressure changed from-50 kPa to-70 kPa,the NaCl concentration reduced to 1.2% as the water outflow was increasing.
2) Distribution of remaining NaCl in the specimens
The distribution of remaining NaCl in the specimens is shown in figure 5.The NaCl removal rate was about 9.3% overall,although around the center it was reduced 50% from its initial value.
2.2.2 Pumping test without freeze-thaw process (F/T-1)
1) Concentration of NaCl in discharge water
The relationship between water discharge and concentration of NaCl during the freeze-thaw extraction process is shown in figure 6.The concentration of NaCl in the drainage water was similar to that at the beginning of pumping.The concentration reduced to 1.1% after 15 hours.The concentration was restored to 1.5% before vacuum suction and the removed NaCl concentration was higher.This indicates that the pore water in the specimen was evenly substituted from the supply water in the high-level cistern.
Negative pressure occurred after 22.5 hours hours with thawing.It’s worth noting that the water supplement must be expedited.The water discharge increased sharply at the beginning of extraction,while the NaCl concentration reduced and finally reached 0.1%.The reason for this was that the structure of the soil was changed into a fixed the seepage path by the action of extraction,and the path affected the remediation of NaCl.It increases NaCl removal.
From figure 4 it can be seen that the NaCl concentration was 2% and the water content ratio was 55%at the beginning of freezing;the calculated NaCl content was therefore 1,478.2 g.By calculating the water discharge and the NaCl concentration,the removed NaCl was 609.6 g during the thawing,and the removed NaCl was 323.7 g during the vacuum negative pressure suction.Ultimately,about 63% of the NaCl was removed.
By the water discharge,the permeability coefficient was calculated to be 8×10-5–9×10-5after 14–21.5 hours,and it was 5×10-6–6×10-6after 40–48.8 hours.This difference illustrates that the permeability coefficient changed during the suction process.Compared with the F/T-2 test (pumping without a freeze-thaw process),the variation of the permeability coefficient increased 5–60 times by a factor of the changed of structure of the soil after the freeze-thaw process.
2) Distribution of water content in the specimens
The distribution of water content in the specimens at the end of the freeze-thaw test is shown in figure 7.Compared with the initial water ratio (55%),the water ratio in the specimens was reduced after the test.The water discharge was 9.2 L,and this result indicates the drainage consolidation caused by freezing during the moisture transfer process.

Table 4 Experiment results
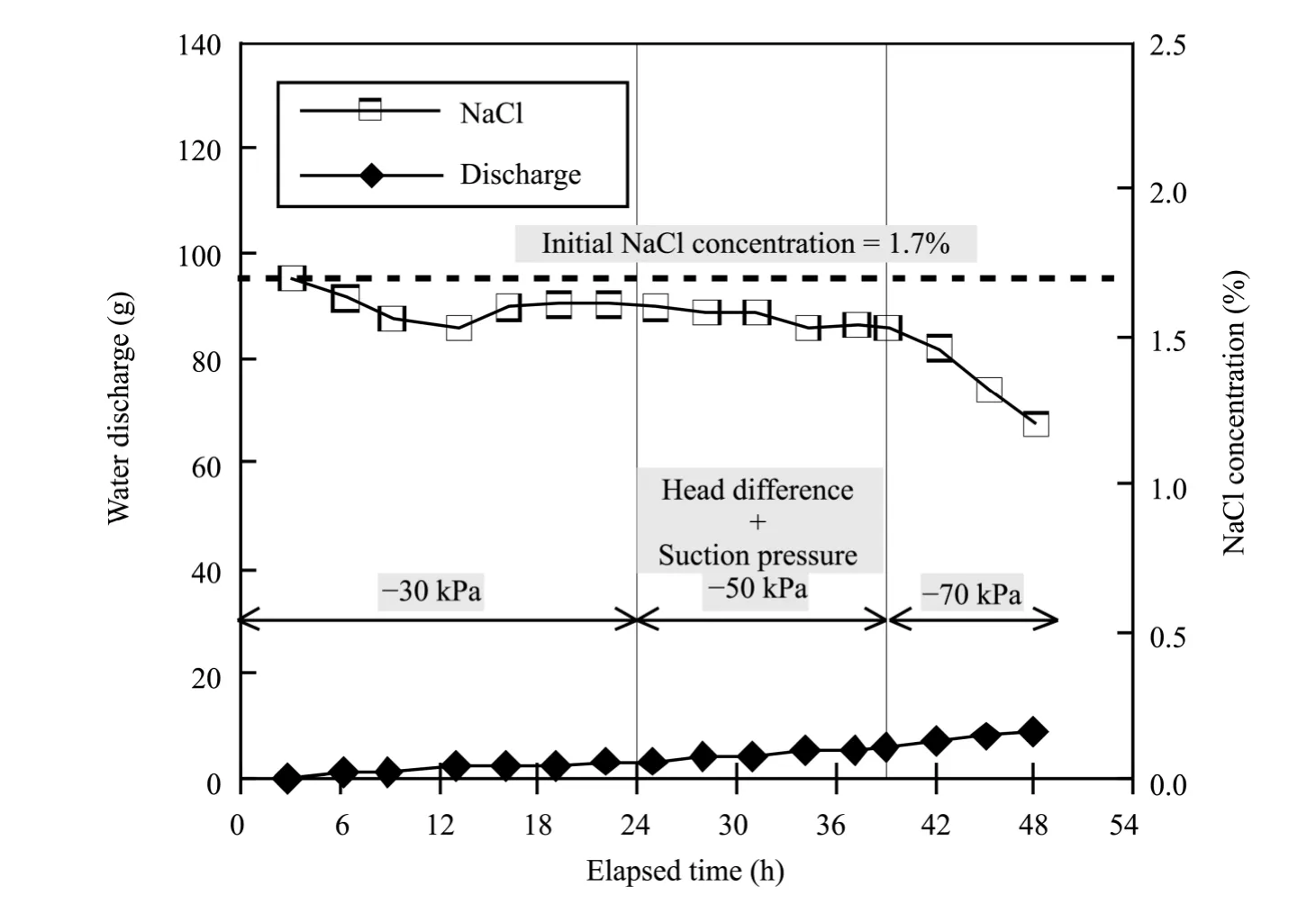
Figure 4 Relationship between water discharge and removed NaCl
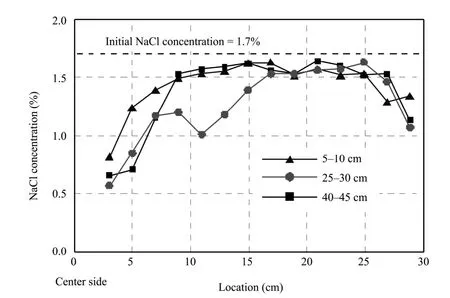
Figure 5 NaCl distribution in the specimens without freeze-thaw

Figure 6 Relationship between water discharge and removed NaCl

Figure 7 Distribution of water content in the specimens
3) Distribution of remaining NaCl in the specimens
The distribution of the remaining NaCl in the specimens after the experiment is shown in figure 8.The sampling areas were the upper part of each specimen (40 cm from the bottom),the middle(25-30 cm from the bottom),and the lower part(5-10 cm from the bottom).The NaCl in the soil was gradually removed from the center of the soil bin to the side during the process of thawing and pumping.The NaCl concentration declined obviously with respect to the initial state in the lower and middle areas of each sample.In particular,the concentration within 9 cm from the middle of each sample reached to <0.1%.
However,the NaCl concentration around the upper part of each specimen changed little,as did that in the middle of the soil tank (9-25 cm).The reason was that,on the one hand,the permeability did not change distinctly because the freezing was not sufficient around the upper part of each sample,and,on the other hand,the drain hole that was only sated in the range of 40 cm from the bottom of the soil sample reduced the effect of the suction.Also,some water reflowed at the wall of soil tank during the suction,resulting in the sharp reduction of the NaCl concentration.
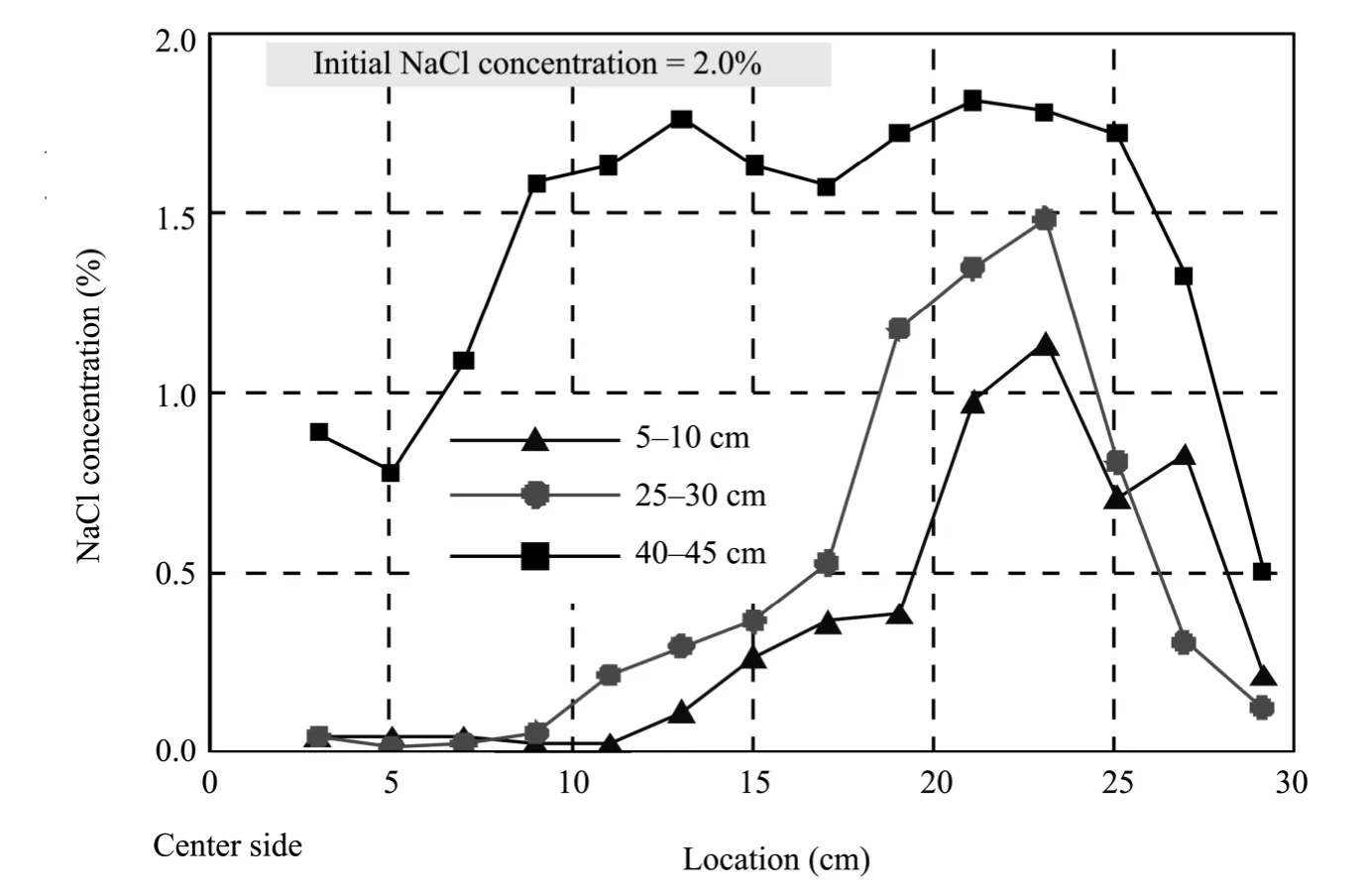
Figure 8 Distribution of NaCl in the specimens after freeze-thaw process
3 Extraction of DNAPL pollutants by the freezing method
3.1 Experimental methods
The physical parameters of the soil used in this experiment are shown in table 5.We chose PF-5080(produced by 3M Company,St.Paul,MN and abbreviated here as P solvent) to simulate the DNAPL pollutants.Its freezing point was-43 °C and its specific gravity was 1.76.This material is environmentally sound and has high safety and performance characteristics.
The experimental apparatus is shown in figure 9.To contain the soil specimen we used an acrylic tank which had a 10-cm height,10-cm inner diameter,and a 0.2-cm-thick aluminum base.A suction tube with a diameter of 0.5 cm was installed in the middle;it had two lines of punched holes (1-mm diameter) along the tube extending 80 mm from the top to the bottom.Three layers of geotextile were convolved in the tube to prevent clogging by soil particles.A heating rod encased in a tube ensured unobstructed in the pipe.
The sample was divided into five layers to fill the container,and was compacted by a hammer.Then 100 mL of the P solvent was injected into the soil and water was infused to submerge the sample after the P solvent got to the bottom.
The experimental process was that the receptacle filled with soil was put into antifreeze fluid (-15 °C) to cool;we could verify that the soil was entirely frozen by the thermocouple inserted into soil.The ice crystals melted due to the heating rod before suction using an aspirator,and then we measured the fluid (liquid or DNAPL) in the trap.To calculate the partial vaporization of the DNAPL during the suction process,the integral container was measured before and after suction and the residual DNAPL was calculated through the volumetric water content [the ratio of liquid (including the P solvent) and soil particles].
Table 6 details the experiment conditions.We conducted the freezing and non-freezing experiments on silica sand and clay,and analyzed the amount of extraction.Of these,samples C1 and C2 utilized silica sand while samples C3 and C4 utilized clay.

Table 5 Properties of soil used in DNAPL removal tests (freezing method)
3.2 Experimental results
3.2.1 Experiment with silica sand
The results of experimental suction on the silica sand are shown in figure 10.The C1 experiment recycled 78.5 g of liquid P solvent (accounting for about 43% of the total),and 25% of gaseous P solvent as calculated through the sample weight before and after the experiment.Thus,68% of the solute was removed.However,in the C2 sample,we extracted 62% liquid P solvent together with 22% gaseous P solvent,so a total of 84% was removed.
We thus conclude that the efficiency with non-freezing was 22% higher than that with freezing.However,a large amount of moisture was also extracted in the form of P solvent.Due to the closed condition of the experiment and the limited water extracted,however,there would be a large number of groundwater supplement,this means that a large amount of drainage would be purified in the actual situation.
3.2.2 Experiment with clay
As shown in figure 10,we did not obtain the liquid P solvent in sample C3,and only recycled 57 g(32%) of the gaseous P solvent.Further,we only obtained 4.1 g of gaseous P solvent in sample C4.Unlike the experiment with silica sand,the clay experiments did not have much water discharge.We suspect that the negative pressure caused compression consolidation of the clay around the water supply pipe,which caused displacement collapse of moisture and P solvent.
We therefore conclude that the DNAPL could be extracted selectively because of the difference of solidification points in the area of soil polluted by the DNAPL.
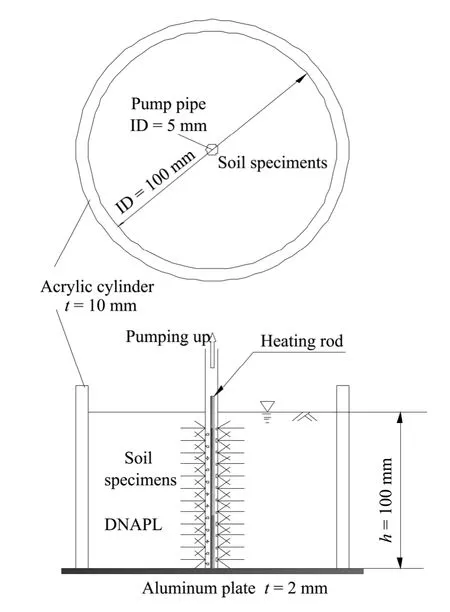
Figure 9 Freezing experiment vessel

Table 6 Experimental conditions,DNAPL extraction
4 Conclusions
This study examines the effect of freezing and thawing cycles on the structure of polluted soil,and discusses the applicability of the freezing method for remediation of such soil.We determined that:
·After a freeze-thaw process,the permeability coefficient of clay increased about 5–60 times by a factor of the changed of structure of the soil;and
·The removal rate of NaCl was about 9% in pumping tests without a freezing-thawing process,but reached 63% with a freezing-thawing process.This indicates that the freeze-thaw action can greatly improve the efficiency of pollutant extraction.
Our study on extracting DNAPLs by the freezing method was based on the difference in freezing points between soil water and DNAPLs,and we found that the permeability coefficients increased due to freeze-thaw action.We therefore conclude:
·DNAPLs can be extracted effectively through the freezing process;and
·DNAPL extraction in silica sand was remarkably effective,while the effectiveness was slightly weaker in clayey soil.
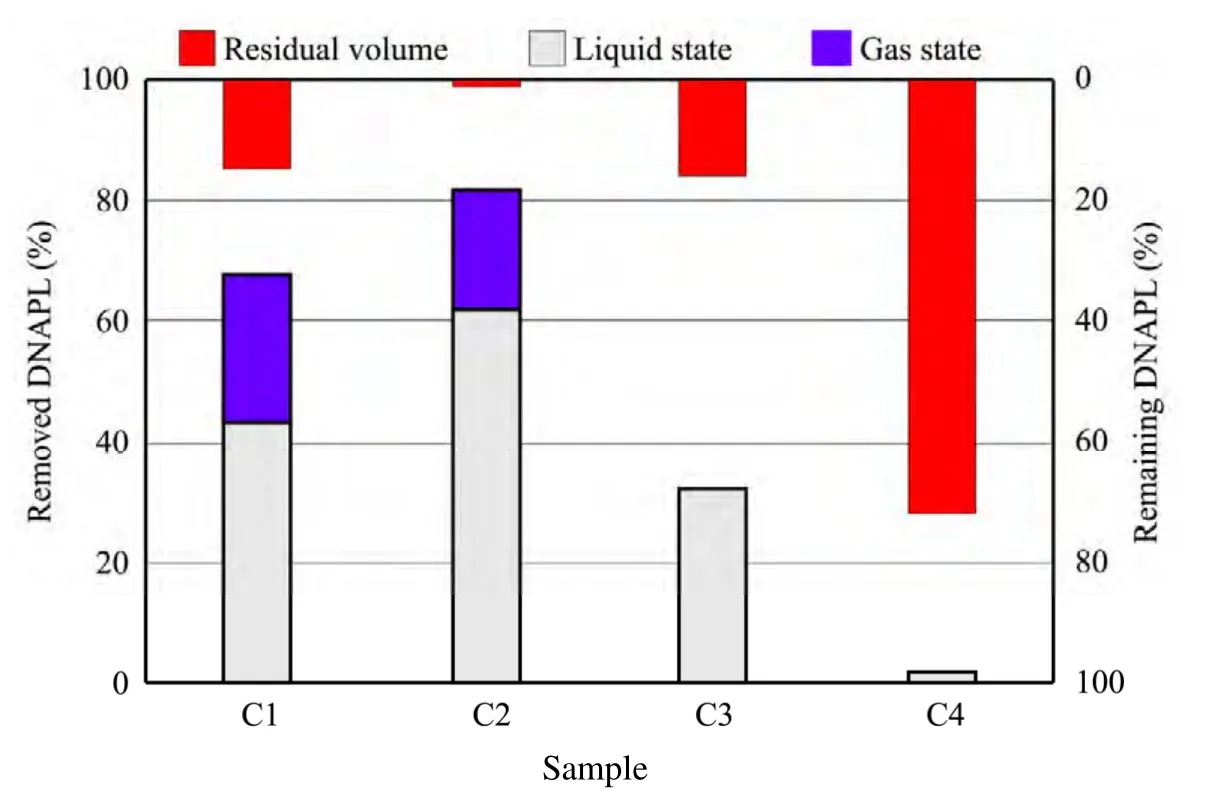
Figure 10 Removal rate and residual ratio of DNAPL
Thus,the freeze-thaw process can effectively remediate certain contaminated soils.This technique not only disturbs the surroundings very little,but also prevents the secondary spread of pollutants during the disposal.The artificial freezing method is feasible to repair contaminated soil but has high maintenance and operation costs due to long-term energy consumption.Because seasonally frozen ground accounts for more than 50% of the Chinese territory,the remediation of contaminated soil would be greatly enhanced if natural freezing and thawing could be combined with artificial methods.In other words,the soil freezing process in which the water shifts from unfrozen soils to the freezing front,and the permeability of the soil,are both enhanced under certain temperature gradients and water conditions.Traditional pumping and treating combined with this artificial freeze-thaw method can significantly improve the extraction efficiency.
The authors are very thankful to reviewers for proposing good suggestions.This work was supported by the National Natural Science Foundation of China (No.41371092),the Scientific Research Foundation for Returned Overseas Students,the Education Department of Henan Province Science and Technology Research projects (No.14B170007),and the doctoral foundation of Henan Polytechnic University (No.648349).
Bing H,Heng P,2011.Experimental study of water and salt redistributions of saline soil with different freezing modes.Rock and Soil Mechanics,32(8):2307–2312.
Fen-Chong T,Fabbri A,Azouni A,2006.Transient freezing thawing phenomena in water-filled cohesive porous materials.Cold Regions Science and Technology,46:12–26.
Gay G,Azouni A,2007.Concentration of soluble and non-soluble zinc-based impurities by unidirectional freezing:Basis for a method of sludges treatment.Environmental Science and Technology,41(15):5466–5470.
Guillaume G,Azouni MA,2003.Experimental study of the redistribution of heavy metals contaminants in coarse-grained soils by unidirectional freezing.Cold Regions Science and Technology,37(2):151–157.
Ito Y,Kamon M,Hato H,2002.A laboratory experiment to investigation the applicability of freezing and thawing method for remediation of contaminated ground.Journal of the Society of Materials Science Japan,51(1):42–45.
Ito Y,Nii K,Kamon M,et al.,2004.The onsite washing of contaminated fine-grained soils using freezing and thawing effect.The Conference of 39th Japan Geotechnical Society,pp.2243–2244.
Li PJ,Liu W,Sun TH,et al.,2006.Remediation of contaminated soil:Its present research situation and prospect.Chinese Journal of Ecology,25(12):1544–1548.
Luo YM,2009.Current research and development in soil remediation technologies.Progress in Chemistry,21(2P3):558–564.
Qi JL,Ma W,Song CX,2008.Influence of freeze-thaw on engineering properties of a silty soil.Cold Regions Science and Technology,53(3):397–404.
Shafique U,Anwar J,uz-Zaman W,et al.,2012.Forced migration of soluble and suspended materials by freezing front in aqueous systems.Journal of Hydro-Environment Research,6(3):221–226.
Wu QB,Sun T,Tao ZX,et al.,2001.Experimental studies on the salt expansion of coarse grain saline soils under constant temperature.Journal of Glaciology and Geocryology,23(3):1238–1243.
Xu XZ,Wang JC,Zhang LX,2010.Geocryology Physics.Science Press,Beijing,China.
Zhou QX,Song YF,2004.Remediation of Contaminated Soils:Principles and Methods.Science and Technology Press,Beijing,China,pp.330–408.
 Sciences in Cold and Arid Regions2014年4期
Sciences in Cold and Arid Regions2014年4期
- Sciences in Cold and Arid Regions的其它文章
- Thermal conductivity of reinforced soils:A literature review
- In-situ testing study on convection and temperature characteristics of a new crushed-rock slope embankment design in a permafrost region
- Advances in studies on concrete durability and countermeasures against freezing-thawing effects
- Cooling effect of convection-intensifying composite embankment with air doors on permafrost
- Case studies:Frozen ground design and construction in Kotzebue,Alaska
- Thermal state of ice-rich soils on the Tommot-Yakutsk Railroad right-of-way
