The use of the Rényi scalable diversity index to assess diversity trends in comparative and monitoring studies of effects of transgenic crops
Gabor L.LÖVEI,Wan-xue LIU,Jian-ying GUO,Fang-hao WAN
1State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,China;2Department of Agroecology,Aarhus University,Flakkebjerg Research Centre,DK-4200,Slagelse,Denmark
Transgenic crops may have significant environmental impacts (Wolfenbarger & Phifer,2000).Consequently,commercial cultivation of such crops is conditional on an environmental risk assessment,during which the risk to the environment is assessed.The pre-release risk assessment regulations currently vary by country.Different countries have different regulations,and these sometimes contain inconsistencies and insufficiently supported assumptions (National Research Council,2002).An emerging additional regulation,already codified by the European Community (directive 2001/18/EC),is the post-release monitoring of the transgenic plants,but its methodology is not yet fully developed (National Research Council,2002).The evaluation of biodiversity changes is often part of such an assessment (Chen et al.,2011;Liu et al.,2011).
Agriculture crucially depends on ecological services (MEA,2005;Tilman et al.,2002),and more so in developing than developed countries (Mertz et al.,2007).The effect of transgenic crops on ecosystem services was suggested as a conceptual framework to structure and unify the otherwise fragmented concerns about "non-target effects" (Lövei,2001).One of the important biological services is natural pest control(MEA,2005),increasingly used and not necessarily in good condition world-wide (Carpenter et al.,2009).Agricultural habitats can also be significant in supporting biodiversity,especially in heavily cultivated areas (see,for example,Duelli et al.,1999;Mészáros,1984),yet we do not have a general understanding of the level of biodiversity that can be supported by an agricultural landscape (Daily,1999)and which part of this is important in beneficial ecological functions.A diverse array of natural enemies is thought to boost the biological control of pests (Crowder et al.,2010).
Biodiversity is often evaluated in biosafety studies(e.g.Dillon & Sharma,2013;Liu et al.,2011).Our aim was to increase the sophistication of assessing the impacts of transgenic plants on biodiversity.To analyse the possible differences in biodiversity,we suggest that the method of scalable diversity profiles(Rényi,1961;Tóthmérész,1995)can be very useful to detect the impact of different management regimes,including transgenic crops.This method is linked to a generalisation of the one-parameter diversity index families,developed by the Hungarian mathematician Alfred Rényi (Rényi,1961),and allows a more comprehensive evaluation than traditional one-dimensional diversity indices (Lövei,2005;Tóthmérész,1995).In China,this method has been rarely used to compare the above-ground arthropod community structures with different crop management patterns (but see Guo et al.,2007,2009).Even these papers lack a detailed description of the general features of the method.After discussing these details,we use an example from China to illustrate how diversity comparisons can be made using the Rényi-diversity index.
MATERIAL AND METHODS
The Rényi diversity index and its features
The Rényi diversity,HR(a),was first suggested by Hungarian mathematician Alfred Rényi (Rényi,1961),in the form:

where piis the relative abundance of the i-th species,and S is the total number of species in the sample,and a is a scale parameter.The scale parameter,a is a mathematical abstraction and has no direct biological meaning.The equation is interpreted for the range a≥0,a≠1.Four special scale parameter values merit extra consideration:
(ⅰ)when the scale parameter a =0,the value of the Rényi diversity is the logarithm of the number of species of the community;HR(0)=logS.In this case the method is extremely sensitive to the contribution of the rare species to the diversity of the assemblage.
(ⅱ)When the scale parameter,a approaches 1(it cannot take the exact value:a≠1,see above),the Rényi diversity gives the value of the Shannon diversity index.In this case the diversity is sensitive to the rare species,although not so extremely as for a=0.
(ⅲ)at a =2,the Rényi diversity is related to the quadratic or Simpson diversity.In this case the method is more sensitive to the frequent species than to the rare ones.
(ⅳ)When the value of the scale parameter is large (formally a→+∞),the value of the Rényi diversity is closely related to the relative abundance of the most common species.This is the logarithm of the reciprocal value of the so-called Berger-Parker or dominance index (Southwood & Henderson,2000).
Thus it can be seen that the generalised Rényi diversity index is sensitive to the rare species for small values of the scale parameter (close to 0),whereas it is sensitive to the abundant species for larger values of the scale parameter.Diversity profiles can be calculated by several packages,including the DivOrd package(Tóthmérész,1993),the R package vegan (Oksanen et al.,2012),and the BiodiversityR package (Kindt,2011).
Because the diversity profile is a monotonously decreasing curve,the relationship between two such profiles (i.e.two assemblages or communities,whose diversity is to be compared)can be of three types:
1.An unequivocal ordering of two assemblages occurs if the diversity profiles of the two assemblages to be compared do not cross each other at any point.For the assemblage represented by the upper profile,we can,in common words,claim that this assemblage is "more diverse" than the other one.
2.Due to the mentioned monotony,two profiles can cross each other once or twice.This depends on the rate of decline of the profile.This rate of decline is related to the evenness of the assemblage:a more even assemblage displays a more gradual,less steep decline.It often occurs that one assemblage starts out more diverse than the other,meaning higher diversity for rare species,but at one point,as emphasis gradually shifts towards the more common species,the lines cross.This means that for common species,the other assemblage is more diverse.The situation represents a not unequivocal ordering.
3.The occurrence of two crossings may indicate a stressed,species-poor and low density assemblage,because this assumes that a diversity profile starts low(low species richness)but ends up not so dominated by the most common species (low dominance index).This can occur when the evenness is high — a species-and individual-poor assemblage will,due to statistical constraints,have a high evenness,and thus its diversity profile will decline at low rate.
The diversity profiles of the assemblages to be compared are presented graphically,and analysed verbally.Only in the case of unequivocal ordering (see above)can one assemblage or community be declared to be"more diverse" than another,thus a precise description should usually accompany the graphical presentation of the diversity profiles.
Field example
In order to illustrate the use of the method,we selected a spider survey from China (Liu et al.,2004).We present some experimental detail below —but we stress that the census results serve only an illustrative purpose.
St udy area
The study site was at the Nan-Pi Agricultural Research Station,CAAS Institute of Plant Protection,Hebei Province,north central China (38°00'N,116°70'E).In 1998,this station had a total of 15 ha of experimental fields planted in cotton as well as a range of other cultivated crops and trees.The field census was done on a series of 0.3 ha cotton plots separated by a non-cultivated strip of 10 ~50 m.Four different management regimes were compared:
1.Conventionally managed cotton with pesticide treatments (Conventional plot).This plot was planted with the locally developed cv.Xinxi-82.There were five insecticide treatments per season,on 25 June,3,7,25 July,6 August 1998.For sprayings,different insecticides were used.They were,in the sequence of the treatments:60% methamidophos EC,50% parathion-methyl EC,20% esfenvalerate EC,and 10%cypermethrin EC on the last two occasions.
2.Cotton under an experimental,integrated pest management regime (IPM plot).This field,also planted with cv.Xinxi-82,received only two insecticide treatments,with the same mixture as the conventional field,on 30 June and 27 July 1998.The egg parasitoid Trichogramma chilonis was released twice during the second generation of Helicoverpa armigera,4 times during the third generation,and 3 times during the fourth generation.The parasitoid was released at a density of 180,000 ~210,000 wasps/ha at one time.
3.Bt-transgenic cotton,cv.Monsanto 33B (33B plot).This cv.expresses the Cry1A(c)endotoxin gene from Bacillus thuringiensis and is toxic to the cotton bollworm,H.armigera,and several other species of Lepidoptera (Perlak et al.,1990).There was no pesticide treatment in this plot.
4.Chinese Bt-transgenic cotton,cv.Zhongmian 30 (Zhongmian 30 plot).This is a Chinese-developed line of cotton,containing the Cry1Ab gene,also toxic to Lepidoptera.There was no pesticide treatment in this plot,either.
This design was unreplicated but under the logistical and land constraints,it was decided that smaller plots would be unrealistic as they would be too much influenced by spill-over effects from neighbouring areas(Holt,1985).This is also the reason why we refrained from the statistical evaluation of the patterns.In the neighbouring farming areas,cotton is grown on even smaller plots,the average family land being in the range of 0.5 ha and supporting several crops (G.L.Lövei,personal observation).
Survey method
Starting in early June 1998,a visual inspection of 10 (until 17 July)or 5 (22 July ~end of September)plants at 10 locations per plot (total of 50 ~100 plants)was done every 5 days to the end of September 1998.The locations were regularly distributed within the field,and the plants around them were randomly chosen,and labelled.All censuses were done on the same selected plants.During census,the observer counted and identified all spiders seen on the plants and on the ground.Unidentifiable adult spiders were collected and taken to the laboratory for rearing and identification.The taxonomy followed Zhao (1995)and Platnick (2003).Voucher specimens are deposited in the CAAS Institute of Agro-Environment & Sustainable Development,Beijing,China.
RESULTS
The empirical example:diversity comparison of four spider assemblages
Assemblage composition— A total of 5,605 individuals,belonging to 16 identified and 13 unidentified species,were observed during the sampling season.The most species-rich was the Zhongmian 30 Bt-cotton plot,followed by the Monsanto 33B,the IPM plot and finally,the conventionally managed plot (Table 1 ).The 33B Bt-cotton plot had the highest total number of spiders observed,followed by the other Bt-cotton cultivar,Zhongmian 30.In the conventional plot,only about one-fourth of the numbers found in the Bt-cotton plots were present (Table 1 ).
Diversity & diversity ordering— The rank-abundance curve indicated that the two Bt-cotton plots had about equal diversity (Fig.1 )and they were the two most diverse assemblages.The conventional plot and the IPM plot curves indicated lower diversity,the latter having a "longer tail",indicating more species present(Fig.1 ).
The diversity ordering showed a more complete but more complex picture.The spider assemblage in the cv.Zhongmian 30 was unequivocally the most diverse as its respective diversity profile did not cross any of the other profiles at any value of the scale parameter (Fig.2 ).The other three assemblages could not be unequivocally ordered.At small values of the scale parameter,the IPM and conventional plots supported a less diverse assemblage than the Monsanto Bt-cotton cv.33B but the situation changed and the 33B curve descended to remain the lowest when the scale parameter value was a >1.6.The profile of the pesticide-treated,conventional field crossed the other two curves at about a =0.4 ~0.6 and remained the highest until ca.a=4.1 (Fig.2 ).
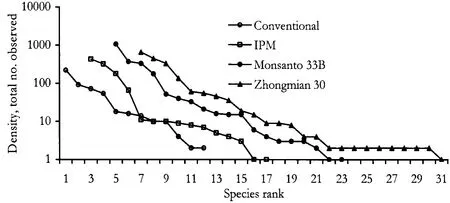
Fig.1 Rank-abundance curves of the four spider assemblages in transgenic Bt-and non-transgenic cotton fields at Nan-Pi Research Station,Hebei Province,north central China,in 1998
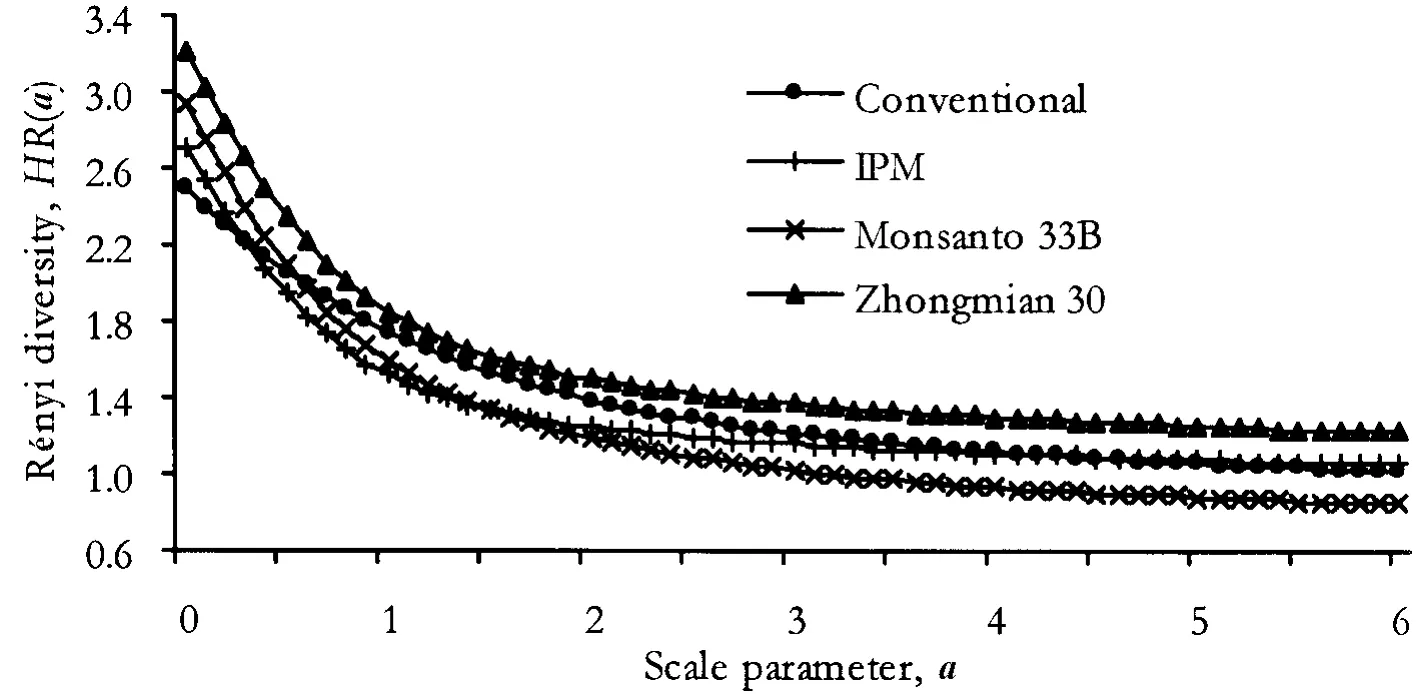
Fig.2 The Rényi diversity profiles of the four spider assemblages studied at Nan-Pi Research station,Hebei Province,north central China,in 1998
DISCUSSION
The spider fauna encountered at Nan-Pi was not particularly species-rich.A survey of cotton plants in South Africa (van den Berg et al.,1990)detected 76 spider species.The known fauna of cotton fields in Arkansas,USA is 189 species (Heiss et al.,1988)although this cannot be directly compared to our survey because that list was compiled using different methods and a wider spatial and temporal scale.In Australia,the species richness is close to ours (25 species,Bishop,1980).An extensive survey in China(Qu et al.,1986)found 61 species,but only 30 species were found in cotton fields in Wuhan Province,Southern China (Li & Zhao,1993).
The diversity comparison of the four treatments indicated that only one of the assemblages,the one living in the Chinese Bt-cotton cv.Zhongmian 30,can be considered unequivocally more diverse than the others.The diversity profile for the Bt-cotton cv.33B,surprisingly,indicated the least diverse spider assemblage for most of the range.This was probably caused by unidentified conditions that made this crop a very favourable habitat for one theridiid species,A.tepidariorum.This was the most common species overall,and nearly half of all specimens observed were found in this cotton cultivar.The diversity under scale parameters sensitive to medium-rare species showed that the conventional,pesticide-treated field had the second most diverse assemblage.Evaluating diversity based on only the frequently used Shannon-diversity(near scale parameter a =1,see Material and Methods section)would have given a similar result that would not be representative of the total impact on diversity.The diversity in this region of the scale parameter is influenced by the combined effect of species number and their relative density.In the conventional plot,the very low densities of even the common species increased the evenness of the assemblage,thus inflating its diversity.The impact of restricted pesticide use on spider diversity in relation to the Bt-cotton cultivars is better reflected in the run of the IPM profile over a wider interval of the scale parameter values.This also indicates that spraying indeed was harmful for the diversity of the spider assemblage (Fig.2 ).
The density differences for spiders active on plants,however,probably reflect real differences as they are directly comparable.The effect of frequent pesticide spraying in the conventional plot was evident on the density and diversity of spider assemblage in conventionally managed cotton.This was the only treatment where P.astrigera,a wolf spider,was the most common species.Plant-living spider densities were drastically reduced.Pesticide sprayings,especially early in the season,disrupt the beneficial arthropod assemblage,and they fail to recover during the season (Hagerty et al.,2000).In our experiments,the Bt-plot had no insecticide treatments.This,however,is not the usual practice.The number of insecticide sprayings on Bt-cotton in China is much reduced,especially early in the season,but not completely stopped(Huang et al.,2002).Therefore we expect that the composition and dynamics of the spider assemblage in Bt-cotton could be closer to the "IPM assemblage" than in our experiments reported here.The large differences among plots were most likely due to the degree of pesticide application and less likely due to the cotton genotype.A similar trend was reported from cotton fields in the U.S.A.(Hagerty et al.,2000).
There were no noticeable differences in the surrounding crops around the different plots,nor a clear gradient in slope or soil type.If the differences encountered were caused only by differences in the pesticide treatments,the two Bt-crops,having no pesticide treatments,should show only minor differences.This was clearly not so,and thus the causes that generated differences in the spider assemblages cannot entirely be apportioned to differences in pesticide treatments and agronomy.
Due to the census technique used,the presence of wolf spiders that are mostly active on the ground was very probably underestimated.Only one species,P.astrigera,was identified,although that was a very common one.A better understanding of the ground-active fauna would require soil sampling or fenced pitfall trapping (Lövei & Sunderland,1996)or D-vac+hand count (Greenstone,2001;Sunderland & Topping,1992)that can provide real density data.
Overall,the use of the Rényi diversity profiles allows the evaluation of diversity in a more articulated way than the use of the various diversity indices in isolation.The use of the individual indices nearly always gives conflicting results,which are difficult to reconcile (Tóthmérész,1995).The Rényi diversity family index and the graphical evaluation method offers a possibility to understand the validity of the indication by single indices,and can be reconciled by specifying the range over which one particular relationship holds,and allows a biological interpretation of diversity by placing various weights on the different abudance catgegories (rare vs.medium-rare vs.common species).We would recommend the more extended use of this method,as it is eminently suitable for a complex assessment on diversity of any management regime,especially in agriculture and conservation biology.
ACKNOWLEDGEMENTS
This work,done under a Sino-Danish scientific cooperation project studying the environmental impact of transgenic cotton,was supported by the Chinese Ministry of Science&Technology(project no.s G2000016209 and 2002BA516A01),the European Community (ICA4-CT-2001-10069),and The Danish Research Agency.We thank Dr.Jun Chen for help in identifying spiders,Drs.M.Greenstone,T.Magura,D.Mayntz,K.Sunderland,S.Toft,B.Tóthmérész,and three anonymous reviewers for helpful comments on an earlier draft.We thank Prof.M.S.You for taking over the editorial duties for this manuscript.
Bishop A L.1980.The composition and abundance of the spider fauna in South-East Queensland cotton.Australian Journal of Zoology,28:699-708.
Carpenter S R,Mooney H A,Agard J,Capistrano D,DeFries R S,Díaz S,Dietz T,Duraiappah A K,Oteng-Yeboah A,Pereira H M,Perrings C,Reid W V,Sarukhan J,Scholes R J and Whyte E.2009.Science for managing ecosystem services:Beyond the Millennium Ecosystem Assessment.Proceedings of the National Academy of Sciences USA,106:1305-1312.
Chen X W,Lin S,You M S,Yang G and Wang F.2011.Effects of transgenic rice on the structure and function of soil microbial communities.Journal of Biosafety,20:151-159.(In Chinese).
Crowder D W,Northfield,T D,Strand M R and Snyder W E.2010.Organic agriculture promotes evenness and natural pest control.Nature,466:109-112.
Daily G R.1999.Developing a scientific basis for managing earth's life support systems.Conservation Ecology,3(2):14.[online]URL:http:∥www.consecol.org/vol3/iss2/art14.Accessed 20 April 2012.
Dillon M K and Sharma H C.2013.Comparative studies on the effects of Bt-transgenic and non-transgenic cotton on arthropod diversity,seedcotton yield and bollworms control.Journal of Environmental Management,34:67-73.
Duelli P,Obrist M K and Schmatz D R.1999.Biodiversity evaluation in agricultural landscapes:above-ground insects.Agriculture,Ecosystems and Environment,74:33-64.
Greenstone M H.2001.Spiders in wheat:First quantitative data for North America.Biocontrol,46:439-454.
Guo J Y,Wan F H,Hu Y H and Yan Y.2007.Effects of crop arrangement patterns on arthropod community structure in transgenic bollworm-resistant cotton fields.Chinese Journal of Applied Ecology,18:2061-2068.(In Chinese).
Guo J Y,Wan F H and Wu M.2009.Effect of transgenic Bt cotton on soil invertebrate community structure.Chinese Journal of Eco-Agriculture,17:1221-1228.(In Chinese).
Hagerty A M,Turnipseed S G,Sullivan M J and Richter D.2000.Impact of beneficial arthropod conservation in B.t.and conventional cotton∥Dugger P.Proceedings of the Beltwide Cotton Conference.San Antonio,USA,976-978.
Heiss J S,Harris V E and Phillips J R.1988.An illustrated and annotated key to the cotton spiders of Arkansas.Journal of Entomological Science,23:1-35.
Holt R D.1985.Population dynamics in two patchy environments:some anomalous consequences of an optimal habitat distribution.Theoretical Population Biology,28:181-208.
Huang J K,Rozelle S,Pray C and Wang Q F.2002.Plant biotechnology in China.Science,295:674-677.
Kindt R.2011.BiodiversityR.Version 1.6.http:∥cran.r-project.org/web/packages/BiodiversityR.Accessed on 20 April 2012.
Li D Q and Zhao J Z.1993.The spider community and its diversity in cotton fields.Acta Ecologica Sinica,13:205-213.(In Chinese).
Liu W X,Wan F H,Guo J Y and Lövei G L.2004.Spiders and their seasonal dynamics in transgenic Bt-vs.conventionally managed cotton fields in north-central China ∥Samu F and Szinetár C.European Arachnology 2002.Proceedings of the 20th European Colloquium of Arachnology.Budapest,Hungary:Plant Protection Institute & Berzsenyi College,337-342.
Liu Z C,Chen Y,Tian J C,Lu Z B,Shu Q Y,Hu C,Peng Y F and Ye G Y.2011.Impact of transgenic cry1Ab rice on the arthropod community of rice paddies in China.Journal of Biosafety,20:69-76.(In Chinese).
Lövei G L.2001.Ecological risks and benefits of transgenic plants.New Zealand Plant Protection,54:93-100.
Lövei G L.2005.Generalised entropy indices have a long history in ecology — A comment.Community Ecology,6:245-247.
Lövei G L and Sunderland K D.1996.The ecology and behaviour of ground beetles.Annual Review of Entomology,41:231-256.
MEA.2005.Millenium Ecosystem Assessment.Ecosystems and Human Well-Being:Synthesis.Washington,DC,USA:Island Press.
Mertz O,Ravnborg H M,Lövei G L,Nielsen I and Konijnendijk C C.2007.Ecosystem services and biodiversity in developing countries.Biodiversity and Conservation,16:2729-2737.
Mészáros Z.1984.Results of faunistical and floristical studies in Hungarian apple orchards.Acta Phytopathologica Academiae Scientiarium Hungariae,19:91-176.
National Research Council.2002.Environmental Effects of Transgenic Plants.Washington,D.C.:National Academy Press.
Oksanen J,Blanchet F G,Kindt R,Legendre P,Minchin P R,O'Hara R B,Simpson G L,Solymos P,Stevens M H H and Wagner H.2012.vegan.Community Ecology Package,version 2.0-4.http:∥cran.r-project.org,http:∥vegan.rforge.r-project.org/.Accessed 20 April 2012.
Perlak F J,Deaton R V,Armstrong T A,Fuchs R L,Sims S R,Greenplate J T and Fischoff D A.1990.Insect resistant cotton plants.Biotechnology,8:839-843.
Platnick N I.2003.The world spider catalogue,version 3.10.American Museum of Natural History.http:∥research.amnh.org/iz/spiders/catalog/.Accessed 2 August 2012.
Qu H Z,Huang Y L and Wu R X.1986.Population dynamics of spiders in cotton fields and their protection and utilization.Natural Enemies of Insects,8:141-145.(In Chinese).
Rényi A.1961.On measures of entropy and information∥Neyman J.4th Berkeley Symposium on Mathematical Statistics and Probability.Berkeley,CA.,547-561.
Southwood T R E and Henderson P A.2000.Ecological Methods.3rd ed.Oxford,U.K.:Blackwell.
Sunderland K D and Topping C J.1992.Limitations to the use of pitfall traps in ecological-studies exemplified by a study of spiders in a field of winter-wheat.Journal of Applied Ecology,29:485-491.
Tilman D,Cassman K G,Matson P A,Naylor R and Polasky S.2002.Agricultural sustainability and intensive production practices.Nature,418:671-677.
Tóthmérész B.1993.DivOrd 1.50:A program for diversity ordering.Tiscia,27:33-44.
Tóthmérész B.1995.Comparison of different methods of diversity ordering.Journal of Vegetation Science,6:283-290.
van den Berg A M,Dippenaar-Schoeman A S and Schoonbee J S.1990.The effect of two pesticides on spiders in South African cotton fields.Phytophylactica,22:435-441.
Wolfenbarger L L and Phifer R L.2000.The ecological risks and benefits of genetically engineered plants.Science,290:2088-2093.
Zhao J Z.1995.Natural Enemies of Cotton Pests in China.Wuhan,China:Wuhan Publishing House,762-1148.(In Chinese).

