Novel anti-androgen receptor signaling agents:Understanding the mechanisms of resistance
Stylini Krnik,Theodoros Krntnos,Jinhu Yin,Likun Li,Timothy C.Thompson,*
Reviews
Novel anti-androgen receptor signaling agents:Understanding the mechanisms of resistance
Styliani Karanikaa,1,Theodoros Karantanosa,1,Jianhua Yina,b,Likun Lia,Timothy C.Thompsona,*
aDepartment of Genitourinary Medical Oncology,The University of Texas MD Anderson Cancer Center,Houston,TX,USA
bDepartment of Epidemiology,Second Military Medical University,Shanghai,China
Received 2 August 2014;received in revised form 27 August 2014;accepted 3 September 2014
Available online 15 September 2014
Prostate cancer;
Castration-resistant
prostate cancer;
Anti-androgen
receptor agents;
Combination
therapies
Prostate cancer remains an intractable threat to the lives of men worldwide.Although deaths from prostate cancer(PCa)in the United States have declined in recent years,in other parts of the world Pca mortality is increasing.The introduction of 2nd generation antiandrogen receptor agents into the therapeutic armamentarium for metastatic castrationresistant prostate cancer(mCRPC)has resulted in modestly increased survival advantages as demonstrated by initial clinical trials.However,analysis of the molecular pathways affected by these agents may lead to new insight into mechanisms of resistance that drive mCRPC,including proliferation and survival signaling pathways that are derepressed by maximum repression of androgen signaling.Combination therapies that involve anti-AR signaling agents together with agents that target these pathways establish a paradigm for the development of more effective treatment of mCRPC.In this review,we briefly summarize the current clinical trial literature with regard to novel anti-AR signaling agents such as abiraterone acetate and enzalutamide.We discuss observational data that point to mechanisms of resistance that emerged from these studies.We further present and discuss recent experimental studies that address the mechanisms of resistance to these treatments.Finally,we discuss novel and rational therapeutic approaches,including combination therapy,for patients with mCRPC.
ª2014 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier(Singapore)Pte Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Prostate cancer(PCa)is the most frequently diagnosed cancer and the second leading cause of death by cancer in men in western countries[1].The critical role of the androgen receptor(AR)in the progression of this disease has been described thoroughly[2],while its role in PCa development is more controversial[3].Thus,for more than 60 years systemic hormonal therapy aimed at decreasing secretion of testosterone by the testes has been the mainstay of therapy for patients with metastatic disease.However,despite an initial response,metastatic disease eventually progresses in the majority of cases upon systemic androgen depletion,a state known as castration-resistant prostate cancer(CRPC)which is characterized by significant morbidity and mortality[4].Notably,a minority of cases of metastatic disease presents with initial resistance to systemic hormonal therapy;for these cases,clinicians need to consider alternative therapeutic approaches,including chemotherapy such as taxanes(Data reported at 2014 ASCO Annual Meeting,Abstract No.:LBA2)and carboplatin[5]or novel agents targeting oncogenic signaling,such as cabozatinib[6].
The findings that AR is amplified[7]and its downstream signaling remains active under systemic androgen deprivation[8]led to introduction of agents targeting either androgen biosynthesis in the tumor microenvironment,such as abiraterone acetate(AA),or AR signaling,such as enzalutamide.It should be noted that,according to recent studies,ARN-509,a new AR inhibitor,provides stronger inhibitory effects than enzalutamide.Numerous clinical trials confirmed the efficacy of these agents in patients with CRPC[9e13],even those who had received no previous chemotherapy[14],indicating that these agents are the optimal therapeutic approach for this state of disease.However,the progression-free survival and overall survival advantages that these agents provide in patients with CRPC are relatively poor and all of these patientseventually develop resistance [15e17].No known therapeutic approach has been shown to be effective for patients whose disease becomes resistant to these novel anti-androgen agents,although cabazitaxel has shown evidence of efficacy for PCa that is resistant to AA[18].This conclusion suggests that a greater understanding of the molecular mechanisms involved in the development of resistance to novel anti-androgen agents is critical for the development of new approaches that will improve the quality of life and survival of patients with CRPC.
The aim of this review was to briefly summarize the current clinical trial literature with regard to novel anti-AR signaling agents such as AA and enzalutamide,and to discuss insights into the mechanisms of resistance that emerged from these studies.We also present recent experimental studies that address the mechanisms of resistance to these treatments.Finally,novel and rational therapeutic approaches,including combination therapy,for patients with CRPC are also discussed.
2.Novel anti-AR signaling agents
2.1.Novel inhibitors of androgen synthesis:AA
Numerous reports support the finding that androgen biosynthesis in adrenal glands and the tumor microenvironment are strongly implicated in the development of CRPC [19].In particular,CYP17A1,a steroidogenic enzyme implicated in the conversion of progestins to adrenal androgens,increasing the subsequent production of testosterone and dihydrotestosterone,is induced in metastatic CRPC(mCRPC)compared to primary PCa[19].These findings led to introduction of AA,which inhibits CYP17A1,leading to significant decrease of testosterone levels in the tumor microenvironment[20].Phase III clinical trials enrolling patients with mCRPC in pre-and post-docetaxel settings demonstrated that AA plus prednisone prolonged overall survival in the post-docetaxel setting and radiologic progression-free survival in the pre-docetaxel setting[11,12].These findings were associated with evidence of efficacy in decreasing serum androgens[21].On the basis of these results,AA was approved for mCRPC in both pre-and post-docetaxel settings during the last year.
In a recent observational study,Efstathiou et al.[20]evaluated the effects of AA in 57 patients with mCRPC.The authors demonstrated a?50%reduction of prostatespecific antigen(PSA)level in 50%of patients and showed that stronger pre-treatment nuclear AR and CYP17A1 cytoplasmic staining were associated with longer treatment duration(>4 months),suggesting better clinical response.These observations suggested that AA-mediated androgen depletion in the tumor microenvironment may be more effective in patients with evidence of active AR and upregulated CYP17A1.Unfortunately,the clinical data also suggested that almost all patients will eventually experience disease progression during AA treatment.According to another study,notably,the TMPRSS:ERG fusion gene,which is considered to be an AR target,is not predictive of the outcome of AA therapy[22].
Interestingly,VCaP xenografts in which the disease relapsed during AA therapy were found to have induced CYP17A1,while human CRPCs treated with ketoconazole,which also inhibits CYP17A1,showed higher levels of CYP17A1 than untreated CRPC[16].Moreover,Mostaghel et al.[17]showed that AA decreased tumor androgens but alsoinducedCYP17A1expressionin LuCaP23CR and LuCaP35CR human xenografts.These findings suggest that PCa cells expressing CYP17A1 may develop particular dependency on these enzymatic pathways,while the development of resistance to AA may be related to upregulation of CYP17A1.Moreover,CYP11A1 mediates progesterone synthesis from cholesterol,leading to accumulation of this metabolite during AA treatment,while a mutant form of AR(T877A)expressed in LNCaP and LNCaP C4-2 PCa cells has been found to respond to progesterone[23],further suggesting that upregulation of androgen biosynthesis pathways in PCa cells is implicated in the development of resistance to AA.
An interesting and critical consideration is whether treatment with AA may have an impact on the efficacy of subsequent chemotherapy,particularly taxanes.With regard to this question,taxanes have been shown to inhibit AR transcriptional activity[24],mainly through inhibition of microtubule-mediated nuclear translocation of AR.These findings were further supported by the results of a recent study that showed that AR splice variants are implicated in the development of resistance to taxanes[25].In a retrospective analysis of CRPC patients who were either treated or not treated with AA prior to docetaxel,Schweizer et al.[26]found that men treated with AA prior to docetaxel therapywere more likelyto progresson docetaxel compared to those who did not receive prior AA.Similarly,docetaxel was shown to decrease PSA by>50%in only 26%of patients with AA-resistant disease,compared to 45%of AA-naïve patients,while all cases in which PSA did not respond on AA were also resistant to docetaxel[27].On the other hand,it was recently reported that cabazitaxel,another newer taxane,provides significant efficacy in terms of PSA response and overall survival in patients whose tumor developed resistance to docetaxel and AA,suggesting that the activity of cabazitaxel is not mainly mediated by inhibition of AR signaling[18].These critical clinical conclusions suggest that mechanisms related to the development of resistance to AA are complicated,clearly related to AR signaling in a subset of patients but also associated with AR-independent pathways.
2.2.Novel anti-AR agents:enzalutamide and ARN-509
Based on the above presented findings,AR remains active in CRPC[28],suggesting that AR signaling is a reasonable target for novel therapies in patients with this state of PCa.Enzalutamide is a novel antagonist of AR that inhibits its nuclear translocation and chromatin binding and decreases the interactions between AR and its co-regulators[29].Phase I/II studies showed that enzalutamide had significant activity in men with CRPC[9],which led to the assessment of this agent in phase III trials showing prolongation of the survival of patients with chemotherapy-resistant disease by 4.8 months[30].However,median treatment duration was short(8.3 months).This agent was also evaluated in chemotherapy-naïve patients with mCRPC in the phase III PREVAIL trial[31].After a median follow-up duration of 22 months,overall survival of a total of 1717 patients was significantly longer(by 2.2 months)with enzalutamide than with placebo;risk of radiographic progression was signif icantly lower with enzalutamide than with placebo,and there was a statistically significant benefit from enzalutamide for all the secondary endpoints,including time to initiation of chemotherapy and time to PSA progression.
Interestingly,Schrader et al.[32]presented a study in which patients with docetaxel-and abiraterone-resistant CRPC were treated with enzalutamide;in three of the nine patients,the disease did not progress[32].This finding suggests that resistance to AA can be partially inhibited by administration of a novel AR inhibitor such as enzalutamide.For example,AR gain-of-function mutants that can be activated by non-androgenic steroids that do not require CYP17A1 for synthesis may contribute to the development of resistance to AA,but enzalutamide can inhibit AR mutants such as the progesterone-sensitive AR mutant T877A[16,29].
ARN-509 is a next-generation AR antagonist found to inhibit AR nuclear translocation and DNA binding,downregulate AR transcriptional activity and provide greater efficacy than any other AR antagonist to which it was compared[33].In a recent phase I clinical trial,ARN-509 was notably safe and well-tolerated by patients while offering significant antitumor efficacy;PET imaging demonstrated robust AR blockade after 4 weeks of treatment[13].The results of a phase II clinical trial evaluating ARN-509 efficacy in 100 men with CRPC are pending.
The efficacy of two other novel AR antagonists,ODM-201 and ezn-4176,has also been evaluated in clinical trials.ODM-201 inhibits AR nuclear translocation without agonistic activity in the context of AR overexpression(Data reported in 2012 ESMO Congress,Abstract No.:LBA25_PR).The results of the first multicentered phase I/II dose-escalation trial in progressive mCRPC(NCT013117641)were recently presented.A PSA decline of?50%was obtained in 13 of 15 patients(87%)at 12 weeks,including the ones previously treated with docetaxel(Data reported in 2012 ESMO Congress,Abstract No.:LBA25_PR).Ezn-4176 is a nucleic acid-based antisense oligonucleotide targeting AR mRNA.When administered as a single agent,it specifically inhibited AR mRNA and decreased AR protein levels,inhibiting growth of androgen-sensitive and CRPC tumors in vitro and in vivo[34].This agent is currently being examined in a phase I clinical trial(NCT01337518)[35].
Other anti-androgens are in preclinical development.Compound-30 is a novel anti-androgen developed by optimizing AR ligand-binding efficacy.Guo et al.[36]have shownthatcompound-30inhibited AR activitymore potently than bicalutamide and had a significant antitumor effect in an in vivo CRPC model.Exploring the capabilities of this new drug,Kuruma et al.[37]suggested that compound-30 is a viable therapeutic approach for patients with CRPC that develop resistance to enzalutamide and other AR inhibitors.The authors concluded that the new drug is more potent than enzalutamide in inhibiting AR transcriptional activity and PCa cell proliferation,decreasing cell growth and AR transcriptional activity even in enzalutamide-resistant cells[37].
Finally,a novel approach to the development of AR antagonists is to target the amino-terminus domain of the AR.The small molecule AR antagonist EPI-001 inhibits proteineprotein interactions necessary for AR transcriptional activity[38].This type of anti-AR activity may have distinct advantages compared to androgen synthesis inhibitors or AR antagonists that target ligand-AR interactions,since it does not require interference with ligand-mediated AR activation.In addition,in preclinical studies EPI-001-based analogs were shown to inhibit constitutively active AR splice variants that contribute to CRPC and resistance to AR inhibitors[39].
Despite this progress,clinical data indicate that mCRPC tumors only partially respond to novel inhibitors of androgen synthesis,including AA and AR inhibitors such as enzalutamide,while the disease eventually relapses.Evaluation of the molecular mechanisms of resistance to these agents is anecessary and critical step in substantially improving on these results and is currently a field of intense research.
3.Mechanisms of resistance to novel anti-AR signaling agents
3.1.Alterations of AR as mechanisms of resistance to AR inhibitors
Various studies have reported numerous molecular mechanisms related to AR signaling implicated in the development of resistance and cancer progression upon androgen depletion.In particular,increased expression of AR[19],induction of alternative splicing of AR creating AR splice variants that lack the ligand-binding domain(LBD)and remain active under low androgen levels[40],selection for AR-mutated forms that are sensitive to low levels of androgens[41],and post-transcriptional modifications of AR that result in its activation even in the absence of androgens have been found to contribute to the development of CRPC.Although the mechanism of action for AA is different from that of standard luteinizing hormone releasing hormone agonist-based ADT,and the effects of AA are largely manifested in the tumor microenvironment,we could hypothesize that similar mechanisms may underlie resistance to standard ADT and AA,on the basis of a common downstream therapeutic action,i.e.,androgen depletion.
AR mutations have been found to occur more frequently in CRPC than in hormone-naïve tumors[42];in fact,recent data show that AR is one of the most frequently mutated genes in mCRPC[43].Earlier studies have highlighted the role of AR mutations,including the T877A mutant,which increases the sensitivity of AR to steroids[44]and converts anti-androgens to strong agonists[45],in the development of resistance to AR antagonists such as flutamide[46].According to a recent report by Korpal et al.[47],LNCaP clones resistant to enzalutamide do not present significant differences in terms of AR expression and nuclear translocation compared to baseline celllines.However,sequencing analysis revealed that all of the strongly resistant clones express the F876L mutation in AR,which switches the inhibitory effects of enzalutamide to agonistic activity[47].Similarly,clones resistant to ARN-509 express the F867L mutation,while circulating cancer cells from cases in which resistance to ARN-509 developed expressed this particular mutation at a higher frequency than cases in which this resistance did not develop[48].These findings suggest that treatment with AR inhibitors targeting the LBD,such as enzalutamide and ARN-509,may select for mutations that alter the sensitivity of AR to steroids and convert the antagonistic effects of these agentsto agonistic.Interestingly,Noonan et al.[49]and Loriot et al.[50]demonstrated that PCa patients that progress on enzalutamide therapy may respond to AA,further suggesting that there are distinct mechanisms of resistance to these two agents.
AR amplification has been reported in relapsed PCa following androgen depletion,but its incidence is not considered high enough to fully explain the development of CRPC[8,51,52].Interestingly,it has been reported that increased expression of AR related to its amplification converts the anti-androgenic effects of an old AR inhibitor,bicalutamide,to induced activity[53].On the other hand,AR amplification was detected in a man with mCRPC whose disease subsequently responded to enzalutamide,with loss of AR amplification and gain of MYC and c-METamplification noted at progression(Data reported on 2014 Genitourinary Cancers Symposium,Abstract No.:65).This fi nding suggests that the mechanisms of resistance to novel anti-androgens are not limited to AR-related signaling but may also be associated with derepression of alternative oncogenic signaling(discussed later in this review).
3.2.Expression of AR splice variants as a mechanism of resistance to anti-AR signaling agents Alternative splicing of AR leads to formation of AR species lacking the LBD that are constitutively active despite androgen depletion and is believed to be implicated in the development of resistance to hormonal therapy[54].Thus,the formation of AR variants as a mechanism of resistance to novel anti-androgen agents is now a subject of intense research.
Mostaghel et al.[17]demonstrated that AA is associated with rapid formation of C-terminaletruncated constitutively active AR-Vs lacking the LBD,leading to development of resistance.Interestingly,according to a recent study by Antonarakis et al.[55]that evaluated mRNA levels of AR-V7 in circulating cancer cells from patients receiving AA,none of the AR-V7-positive patients achieved PSA response?50%,whereas 68%of the AR-V7-negative patients did.Thus,AR-V7-positive patients were more than 16 times more likely to experience both PSA and clinical progression concluding that expression of AR-V7,one of the most frequently detected AR splice variants,is associated with poor response to AA in patients with mCRPC.This finding suggests that common mechanisms may underlie the development of CRPC and resistance to AA.On the other hand,another recent study by Small et al.showed that mCRPC resistant to AA expresses lower levels of AR than AA-naïve mCRPC,suggesting that AA selects for tumors with lower levels of AR,representing disease less dependent on AR(Data reported at also 2014 ASCO Annual Meeting,Abstract No.:5020).Antonarakis et al.[55]also reported that expression of AR-V7 predicts for poor response to enzalutamide,suggesting that AR variant formation may be a common mechanism of resistance to novel anti-androgen agents.Specifically,they found that patients who had AR-V7 in their circulating tumor cells had worse response to enzalutamide than those without detectable AR-V7 in a total sample of 31 patients.PSA levels did not decline in any of the patients with detectable AR-V7;in fact,no patient with detectable AR-V7 achieved a PSA response,while PSA levels dropped by at least 50%in 10 of the 19 AR-V7-negative patients after enzalutamide treatment[55].Consistent with these clinical data was the finding that the expression of AR variants driven by AR gene rearrangements is sufficient for resistance to enzalutamide in CWR22Rv1 cells,inducing a transcriptional program that is similar to that of the full-length AR[15].Hu et al.[56]demonstrated that enzalutamide treatment promotes expression of the AR-V7 variant similarly to AR siRNA treatment,furthersupporting the critical role of this variant in the development of resistance to enzalutamide.Moreover,Nadiminty et al.[57]found that upregulation of AR variants by NF-kB2/p52 induces resistance to enzalutamide in LNCaP C4-2B and CWR22Rv1 PCa cells,while knockdown of either fulllength AR or the AR-V7 variant increased the efficacy of enzalutamide in these two cell lines.Given the evidence presented here that resistance to enzalutamide and ARN-509 is associated with alterations or deletions through alternative splicing of the LBD,small molecules such as EPI-001 that target the N-terminal AR domain[39]are considered to be promising novel therapeutic candidates for patients with CRPC.
The development of AA to decrease androgen biosynthesis in the tumor microenvironment and enzalutamide,ARN-509 and other novel anti-androgens to inhibit AR signaling,as single agents or in combination,represent novel approaches to maximal AR signaling inhibition.With regard to combination approaches,a phase III trial is underway to test the concept that two separate anti-AR signaling agents,i.e.,AA and enzalutamide that act at different levels,will be more effective than single-agent inhibitors that act at one level(NCT01949337).Given that numerous studies have highlighted the role of alternative oncogenic signaling such as c-myc and Akt in the development of PCa[58,59],it can be hypothesized that the majority of patients whose disease progresses under maximal AR inhibition will have a cancer less dependent on AR and more dependent on these alternative oncogenic signaling pathways(Fig.1).
4.Alternative signaling and resistance to maximal androgen depletion
The maximal AR inhibition that probably can be achieved by combining androgen depletion in the tumor microenvironment(AA)and AR inhibition by novel agents(enzalutamide or ARN-509)is expected to select for cancer cells with minimal or no dependency on AR signaling.Maximal AR inhibition can be a strong selective pressure for induction and/or manifestation of alternative pathways promoting survival and growth of PCa cells independently of AR[60].This would partly explain the clinical observation that a subset of patients experience very rapid progression upon systemic androgen depletion or AR inhibition by novel antiandrogen agents.It can be hypothesized that alternative oncogenic signaling may be already activated in thesetumors prior to initiation of the hormonal or novel antiandrogen therapy,which would further induce these pathways through the mechanism of selection.The necessity for predictive biomarkers for these novel anti-AR agents is evident from these initial clinical studies.
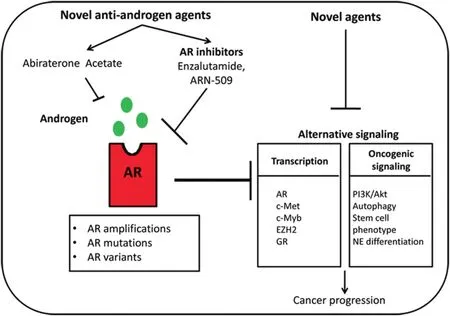
Figure 1 Resistance mechanisms associated with novel agents that target AR signaling by decreasing androgen biosynthesis(abiraterone acetate)or inhibiting AR(enzalutamide and ARN-509).Induced androgen biosynthesis and alterations of AR,such as amplifications,mutations,and AR variants,are implicated in the development of resistance to these novel agents,while combination of abiraterone acetate and an AR inhibitor leads to maximal AR inhibition.This downregulation of AR signaling can lead to derepression of alternative oncogenic signaling at the transcriptional level and through induction of various oncogenic pathways.These signaling activities set the stage for the introduction of novel therapeutic approaches targeting these alternative oncogenic signaling pathways.Specifically,induction of the expression of c-Met and EZH2 has led to the experimental trial of novel agents such as cabozatinib and DZNep,respectively,while the induction of oncogenic signaling pathways including PI3K/Akt,autophagy and development of a stem cell phenotype has resulted in testing novel agents such as BEZ235,early and late stage autophagy inhibitors,and IL-6 inhibitors,respectively.The final therapeutic goal is combination pharmaceutical targeting of both AR(novel anti-AR signaling agents)and alternative signaling(novel agents)early during PCa progression.AR,androgen receptor;NE,neuroendocrine;GR,glucocorticoid receptor.
According to this general concept,AR may repress oncogenic signaling in PCa cells and AR inhibition may derepress these pathways,rendering the PCa progression more dependent on them.Given that the majority of patients with CRPC are now treated with novel anti-androgen agents such as enzalutamide and AA,it is really critical to consider the hypothesis that maximal AR inhibition by these agents may lead to derepression of alternative oncogenic signaling in CRPC,promoting survival and growth of cancer cells.Based on this idea,numerous studies have revealed that enzalutamide can induce several pathways with oncogenic potential in PCa cells.
To explore and develop new insight into the concept of derepression of oncogenic pathways by novel anti-AR signaling agents,we will describe particular examples of this phenomenon,focusing on(1)specific examples of gene derepression at the transcriptional level and(2)promotion of oncogenic signaling by anti-AR-signaling.
4.1.AR inhibition-mediated derepression of oncogene transcription
The concept that androgen deprivation and subsequent AR inhibition may derepress particular AR-suppressed genes was supported by the recent findings of Cai et al.[61].The authors showed that and rogen-liganded AR decreases the expression of AR gene by repressing the activity of ARBS2 as a transcriptional enhancer[61].Moreover,the authors demonstrated that numerous genes related to DNA synthesis and repair,DNA metabolism and cell cycle are also repressed by androgen-liganded AR and are induced under androgen depletion,while genes related to cellular metabolism were found to be induced by androgen-liganded AR.Interestingly, many of these androgen-liganded AR-repressed genes were shown to be upregulated in CRPC VCaP xenografts together with some androgen-liganded AR-induced genes implicated in cellular metabolism.According to the authors,the intratumoral synthesis of androgens in the state of CRPC leads to partial restoration of AR transcriptional activity,while these levels are not sufficient to downregulate the expression of androgen-liganded AR-repressed genes related to cell cycle and DNA metabolism[61].Thus,the results of this study highlight that inhibition of androgen biosynthesis combined with AR inhibition through combinational approaches may lead to derepression of alternative oncogenic signaling,favoring the survival of PCa cells and disease progression.
c-Met is another example of AR inhibition-mediated derepression of an oncogene.This molecule,which functions as an hepatocyte growth factor receptor in PCa cells,promoting PCa proliferation and invasion[62],was found to be induced by castration in LNCaP cells[63].Particularly,it was demonstrated that AR represses c-Met expression through binding in its promoter[63],providing a clear rationale behind the derepression phenomenon.Notably,c-Metistargetable through novelinhibitorssuch as cabozantinib,which is currently being evaluated in two phase III clinical trials in comparison with prednisone and mitoxantrone (COMET-1 [NCT01605227]and COMET-2[NCT01522443]).Thus,it would be important to examine the efficacy of the combination of cabozantinib with enzalutamide and abiraterone in this disease setting.Indeed,a phase II randomized clinical trial evaluating the combination of cabozantinib and abiraterone in chemotherapy-naïve mCRPC is currently ongoing(NCT01995058).
Recently,enzalutamide was found to increase the expression of c-Myb[64],a transcriptional factor reported to be induced in aggressive breast,head and neck and prostate cancers[65].It was demonstrated that c-Myb increases growth and metastatic potential of both AR-positive and-negative PCa cells[64].AR and c-Myb were also found to share a signature of DNA damage response(DDR)-related genesstrongly associated with cancer recurrence,castration resistance,and metastatic disease[64].These results point to a c-Myb-mediated mechanism of resistance to enzalutamide related to DDR.This pathway was correlated with resistance to the novel PARP inhibitor olaparib,showing that inhibition of this pathway combined with olaparib can significantly increase PCa cell toxicity.On the basis of these findings,we can hypothesize that targeting c-Myb in combination with enzalutamide or maximal AR inhibition could be particularly effective in patients with CRPC.The finding of Li et al.[64]that c-Myb regulates DDR through Topbp1,the ataxia-telangiectasia and Rad3-related protein(ATR)and Chk1 protein,which regulate DDR checkpoints,supports that combination of enzalutamide with inhibitors of these c-Myb downstream targets may be effective in PCa.
According to a recent study,activated AR leads to downregulation of enhancer of zeste homolog 2(EZH2)[66].EZH2,which is induced in various malignancies such as PCa,functions as an epigenetic gene silencer of genes such as E-cadherin,which inhibits epithelial cell migration[67].Notably,it has been shown that treatment with androgens decreases the migration of LNCaP cells,but downregulation of E-cadherin abrogated this effect[66].Finally,Xu et al.[68]demonstrated that castration-resistant LNCaP abl cells are more sensitive to EZH2 downregulation than castrationsensitive LNCaP cells,further highlighting the critical role of EZH2 in the development of CRPC.Interestingly,3-deazaneplanocin A(DZNep),a potent S-adenosylhomocysteine hydrolase inhibitor,was found to effectively deplete potentially oncogenic PRC2 components EZH2,SUZ12 and H3K27me3,resulting in reactivation of EZH2-repressed target genes[69].Further studies are needed to clarify the effect of enzalutamide and other novel anti-androgen agents on EZH2 expression and downstream signaling in PCa.Finally,combinations of novel anti-androgen agents with EZH2-targeting agents such as DZNep may have benefits for patients with aggressive metastatic PCa.
According to a report by Sahu et al.[70],AR and glucocorticoid receptor(GR)have overlapping sets of gene targets,supporting that induction of GR can be a potential mechanism related to development of CRPC.It was recently demonstrated that GR and common target genes of AR and GR are upregulated in LNCaP xenografts resistant to enzalutamide[71].The authors also found that GR inhibition in resistant cells can potentiate their sensitivity toenzalutamide,while GR expression was significantly higher in tumors that had a poor response to enzalutamide at 8 weeks of treatment compared to baseline and to those that had a good response at the same time point[71].These results suggested that AR directly represses GR expression at the transcriptional level[71].It could be hypothesized that,in a subset of prostate tumors,AR can repress GR expression while enzalutamide treatment leads to induction of GR,which becomes critical for the survival of cancer cells.These results also support the suggestion that combination of maximal AR inhibition with GR inhibition may be a reasonable therapeutic approach for CRPC and improve the survival and quality of life of patients with this disease.Finally,the discovery of reliable markers downstream of GR for the identification of patients with induced GR pathway is critical for the development of this approach.
4.2.Promotion of oncogenic signaling by AR inhibition
Numerous recent studies have focused more on the derepression of oncogenic signaling by novel anti-AR signaling agents,mainly enzalutamide,at the post-transcriptional level,such as protein interactions and phosphorylation.In particular,Carver et al.[72]found that enzalutamide treatment increased the phosphorylation of Akt in PTEN-deleted LNCaP cells and LAPC4 cells,while the combination of enzalutamide and BEZ235,a PI3K inhibitor,caused marked regression of PCa in animal models.These effects of enzalutamide were attributed to induction of the Akt phosphatase PHLPP by AR inhibition and are consistent with the findings of another study,by Mulholand et al.[73],who demonstrated that AR inhibition,either by castration or AR loss,released PHLPP-mediated suppression of Akt activity and induced Akt-dependent but AR-independent PCa cell proliferation.
It was recently discovered that enzalutamide induces autophagy in both androgen-responsive and CRPC cells,mainly through AMP-dependent protein kinase(AMPK)activation[74].Autophagy is an intracellular degradation system that delivers cytoplasmic constituents to the lysosomes to regenerate energy when the cells are confronted with stressful conditions such as growth factor deprivation or toxic drugs[74,75].It is known that exaggerated autophagy can result in autophagic cell death,also known as type II programmed cell death,and thus has a role in tumor suppression,whereas more generalized deregulation has been linked to non-apoptotic cell death.On the other hand,autophagy was shown to promote resistance to chemotherapy in numerous malignancies[76].Recently this intracellular pathway was reported to be a survival mechanism mediating resistance to AR inhibitors in CRPC cells[77].Consistent with these results was the observation by Nguyen et al.[74]that AR inhibition by enzalutamide caused significant induction of autophagy through activation of the AMPK pathway and suppression of mTOR downstream signaling via phosphorylation of Raptor.According to their preclinical data,when resistance to enzalutamide develops,pharmacological blocking of autophagy bypasses the resistance and achieves better therapeutic results.These results could also be an example of the benefit gained by combination therapy that includes a novel antiandrogen and inhibition of a survival pathway.However,these results point to the need for further studies to clarify whether the mechanistic complexity of autophagy can be exploited in CRPC.
The development of resistance to enzalutamide is associated with the stem-cell phenotype in PCa[78,79].In particular,Bishop et al.[79]having studied patients undergoing long-term androgen withdrawal therapy,indicated that all cancer stem cell markers were expressed at a higher frequency in enzalutamide-resistant cells than in CRPC controls,while neuroendocrine markers were also highly upregulated in these cells.In addition,the majority of the neuroendocrine-like enzalutamide resistant cells expressed PD-L1 and PD-L2 that are known to downregulate T cell-effector responses and engage signaling molecules that have been evaluated as immunotherapy targets.Thus,the emergence of neuroendocrine-like cells through the development of resistance to enzalutamide may be associated with suppression of antitumor T cell responses leading to increased aggressiveness of the disease.At the same time,these data suggest that immunotherapy approaches such as targeting PD-L:PD-1 pathway in combination with enzalutamide may be a reasonable approach for this subset of patients.Finally,Schroeder et al.[78]showed that inhibition of the IL-6-STAT3 pathway reduces the prostate stem-like cancer cell population and prostate tumor growth supporting the approach of combining inhibitors of IL-6 and novel anti-androgen agents.
Svensson et al.[80]using chromatin immunoprecipitation data and location analysis by confocal microscopy and proximity ligation assays,found that AR and the repressor element-1 silencing transcription factor(REST)coexist in close proximity in PCa cells,suggesting functional crosstalk between them.The authors demonstrated that androgen deprivation,AR knockdown,orenzalutamide led to decreased REST protein levels and induction of genes associated with neuroendocrine differentiation,such as chromogranin A[80].The expression of REST was evaluated in tissue microarray sections from prostatectomies and this analysis showed that REST was strongly associated with AR expression while low REST nuclear abundance in these specimens strongly predicted disease recurrence within 3 years after surgery[80].These data suggest that AR signaling regulates the expression of the transcriptional silencer REST,while ADT and enzalutamide can downregulate this factor,leading to upregulation of genes implicated in neuroendocrine differentiation of PCa cells.Thus,treatment with enzalutamide may induce neuroendocrine differentiation in CRPC,which has been associated with rapid disease progression and poor prognosis[81].
The data presented here suggest that AR inhibition with enzalutamide promotes the derepression of numerous oncogenic pathways,potentially through survival selection,setting the stage for development of AR-independent aggressive PCa.Two critical points arise from this conclusion:(1)although an attractive therapeutic approach for CRPC,the combination of AA and enzalutamidefor achieving maximal AR inhibition will require identification of those patients whose tumors will respond to this treatment by induction of specific oncogenic pathways and will rapidly develop resistance.The understanding of thesebiological processes is critical for the development of novel predictive biomarkers,which may improve the progressionfree survival and overall survival benefits of these agents;(2)the combination of maximal AR inhibition with inhibition of alternative derepressed oncogenic signaling is a reasonable therapeutic approach for CRPC patients given thefindings from numerous recent preclinical studies.Predictive biomarkers are critical to get the maximum benefit from novel therapies targeting these pathways.
A clinical trial presented as an abstract at the 2014 Annual Meeting of the American Society for Clinical Oncology evaluated the combination of systemic androgen deprivation with docetaxel in patients with hormone-naïve metastatic PCa.The investigators showed that the addition of docetaxel to hormonal therapy significantly prolonged overall survival(57.6 vs.44.0 months)and that the survival benefit was more impressive for patients with high-extent disease(49.2 vs.32.2 months).Moreover,a greater proportion of patients assigned to docetaxel demonstrated a PSA 2 ng/mL after 1 year(22.7%vs.11.7%)and delayed median time to clinical progression(32.7 vs.19.8 months).These impressive results support the idea that combinational approaches may be very effective when they are used early during disease progression.This concept may lead to a paradigm shift for patients with mCRPC who are receiving a novel anti-androgen agent such as AA or enzalutamide,as the data suggest that introduction of a novel agent in combination with AR inhibition early during disease progression can provide significant advantages for patients with aggressive PCa.
5.Conclusion
Recent results from clinical trials presented in this review indicate that novel anti-androgen agents,such as AA and enzalutamide,provide certain/limited progression-free survival and overall survival benefits in patients with CRPC.Numerous mechanisms are implicated in the development of resistance to these agents.AR alteration and the derepression of alternative oncogenic signaling by AR inhibition seem to make an important contribution to progression of the disease during treatment with these novel agents.Clinical data suggest that the combination of these two agents leads to maximal AR inhibition, and this approach is currently being evaluated in the treatment of CRPC.However,preclinical studies presented in this review indicate that AR inhibition by enzalutamide leads to promotion of oncogenic pathways such as GR,c-Myb,autophagy and development of a stem cell-phenotype in PCa cells.These findings suggest that PCa cells with activated alternative signaling will become rapidly resistant to AR inhibition, whereas their survival will become more dependent on these pathways,rendering early treatment with a combination of maximal AR inhibition and agents that target these pathways a promising novel approach for patients with CRPC.Further preclinical studies and clinical trials evaluating the efficacy of these combination treatments are needed.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
We thank Kathyrn L.Hale,MS,MLIS,for her expert editorial assistance.
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant,5 P30 CA16672,and the Department of Epidemiology,Second Military Medical University,Shanghai,China.
[1]Siegel R,Naishadham D,Jemal A.Cancer statistics.CA Cancer J Clin 2013;2013(63):11e30.
[2]Debes JD,Tindall DJ.The role of androgens and the androgen receptor in prostate cancer.Cancer Lett 2002;187(1e2):1e7.
[3]Kahn BCJ,Kyprianou N.Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer.Int J Biol Sci 2014;10:8.
[4]Harris WP,Mostaghel EA,Nelson PS,Montgomery B.Androgen deprivation therapy:progress in understanding mechanisms of resistance and optimizing androgen depletion.Nat Clin Pract Urol 2009;6:76e85.
[5]Nakabayashi M,Sartor O,Jacobus S,Regan MM,McKearn D,Ross RW,et al.Response to docetaxel/carboplatin-based chemotherapy as first-and second-line therapy in patients with metastatic hormone-refractory prostate cancer.BJU Int 2008;101:308e12.
[6]Smith DC,Smith MR,Sweeney C,Elfiky AA,Logothetis C,Corn PG,et al.Cabozantinib in patients with advanced prostate cancer:results of a phase II randomized discontinuation trial.J Clin Oncol 2013;31:412e9.
[7]Linja MJ,Savinainen KJ,Saramaki OR,Tammela TLJ,Vessella RL,Visakorpi T.Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer.Cancer Res 2001;61:3550e5.
[8]Visakorpi T,Hyytinen E,Koivisto P,Tanner M,Keinänen R,Palmberg C,et al.In vivo amplification of the androgen receptor gene and progression of human prostate-cancer.Nat Genet 1995;9:401e6.
[9]Scher HI,Beer TM,Higano CS,Anand A,Taplin ME,Efstathiou E,et al.Antitumour activity of MDV3100 in castration-resistant prostate cancer:a phase 1-2 study.Lancet 2010;375:1437e46.
[10]De Bono JS,Logothetis CJ,Molina A,Fizazi K,North S,Chu L,et al.Abiraterone and increased survival in metastatic prostate cancer.N Engl J Med 2011;364:1995e2005.
[11]Fizazi K,Scher HI,Molina A,Logothetis CJ,Chi KN,Jones RJ,et al.Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer:final overall survival analysis of the COU-AA-301 randomised,double-blind,placebo-controlled phase 3 study.Lancet Oncol 2012;13:983e92.
[12]Danila DC,Morris MJ,de Bono JS,Ryan CJ,Denmeade SR,Smith MR,et al.Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxeltreated castration-resistant prostate cancer.J Clin Oncol 2010;28:1496e501.
[13]Rathkopf DE,Morris MJ,Fox JJ,Danila DC,Slovin SF,Hager JH,et al.Phase I study of ARN-509,a novel antiandrogen,in the treatment of castration-resistant prostate cancer.J Clin Oncol 2013;31:3525e30.
[14]Tombal B,Borre M,Rathenborg P,Werbrouck P,Van Poppel H,Heidenreich A,et al.Enzalutamide monotherapy in hormone-naive prostate cancer:primary analysis of an open-label,single-arm,phase 2 study.Lancet Oncol 2014;15:592e600.
[15]Li YM,Chan SC,Brand LJ,Hwang TH,Silverstein KAT,Dehm SM.Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines.Cancer Res 2013;73:483e9.
[16]Cai C,Chen S,Ng P,Bubley GJ,Nelson PS,Mostaghel EA,et al.Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors.Cancer Res 2011;71:6503e13.
[17]Mostaghel EA,Marck BT,Plymate SR,Vessella RL,Balk S,Matsumoto AM,et al.Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer:induction of steroidogenesis and androgen receptor splice variants.Clin Cancer Res 2011;17:5913e25.
[18]Al Nakouzi N,Le Moulec S,Albigès L,Wang C,Beuzeboc P,Gross-Goupil M,et al.Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies.Eur Urol 2014 May 2.http://dx.doi.org/10.1016/j.eururo.2014.04.015.pii:S0302-2838(14)00396-0,[Epub ahead of print].
[19]Montgomery RB,Mostaghel EA,Vessella R,Hess DL,Kalhorn TF,Higano CS,et al.Maintenance of intratumoral androgens in metastatic prostate cancer:a mechanism for castration-resistant tumor growth.Cancer Res 2008;68:4447e54.
[20]Efstathiou E,Titus M,Tsavachidou D,Tzelepi V,Wen S,Hoang A,et al.Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone.J Clin Oncol 2012;30:637e43.
[21]Ryan CJ,Smith MR,Fong L,Rosenberg JE,Kantoff P,Raynaud F,et al.Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy.J Clin Oncol 2010;28:1481e8.
[22]Danila D,Ananda A,Sungc C,Hellerd G,Leversha MA,Cao L,et al.TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate.Eur Urol 2011;60:897e904.
[23]Culig Z,Hobisch A,Cronauer MV,Cato AC,Hittmair A,Radmayr C,et al.Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgensandprogesterone.MolEndocrinol1993;7:1541e50.
[24]Zhu ML,Horbinski CM,Garzotto M,Qian DZ,Beer TM,Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer.Cancer Res 2010;70:7992e8002.
[25]Thadani-Mulero M,Portella L,Sun SH,Sung M,Matov A,Vessella RL,et al.Androgen receptor splice variants determine taxane sensitivity in prostate cancer.Cancer Res 2014;74:2270e82.
[26]Schweizer M,Zhou X,Wang H,Bassi S,Carducci M,Eisenberger MA,et al.The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer.Eur Urol 2014;66:646e52.
[27]Mezynski J,Pezaro C,Bianchini D,Zivi A,Sandhu S,Thompson E,et al.Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone:clinical evidence for cross-resistance?Ann Oncol 2012;23:2943e7.
[28]Shaf iAA,Yen AE,Weigel NL.Androgen receptors in hormonedependent and castration-resistant prostate cancer.Pharmacol Ther 2013;140:223e38.
[29]Tran C,Ouk S,Clegg NJ,Chen Y,Watson PA,Arora V,et al.Development of a second-generation antiandrogen for treatment of advanced prostate cancer.Science 2009;324:787e90.
[30]Scher HI,Fizazi K,Saad F,Taplin ME,Sternberg CN,Miller K,et al.Increased survival with enzalutamide in prostate cancer after chemotherapy.N Engl J Med 2012;367:1187e97.
[31]Beer TM,Armstrong AJ,Rathkopf DE,Loriot Y,Sternberg CN,Higano CS,et al.Enzalutamide in metastatic prostate cancer before chemotherapy.N Engl J Med 2014;371:424e33.
[32]Schrader AJ,Boegemann M,Ohlmann CH,Schnoeller TJ,Krabbe LM,Hajili T,et al.Enzalutamide in castrationresistant prostate cancer patients progressing after docetaxel and abiraterone.Eur Urol 2014;65:30e6.
[33]Clegg NJ,Wongvipat J,Joseph JD,Tran C,Ouk S,Dilhas A,et al.ARN-509:a novel antiandrogen for prostate cancer treatment.Cancer Res 2012;72:1494e503.
[34]Zhang YX,Castaneda S,Dumble M,Wang M,Mileski M,Qu Z,et al.Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer.Mol Cancer Ther 2011;10:2309e19.
[35]Bianchini D,Omlin A,Pezaro C,Ferraldeschi R,Mukherji D,Crespo M,et al.First-in-human phase I study of EZN-4176,a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castrationresistant prostate cancer.Br J Cancer 2013;109:2579e86.
[36]Guo C,Linton A,Kephart S,Ornelas M,Pairish M,Gonzalez J,et al.Discovery of aryloxy tetramethylcyclobutanes as novel androgen receptor antagonists.JMedChem 2011;54:7693e704.
[37]Kuruma H,Matsumoto H,Shiota M,Bishop J,Lamoureux F,Thomas C,et al.A novel antiandrogen,compound 30,suppresses castration-resistant and MDV3100-resistant prostate cancer growth in vitro and in vivo.Mol Cancer Ther 2013;12:567e76.
[38]Myung JK,Banuelos CA,Fernandez JG,Mawji NR,Wang J,Tien AH,et al.An androgen receptor N-terminal domain antagonist for treating prostate cancer.J Clin Invest 2013;123:2948e60.
[39]Sadar MD.Small molecule inhibitors targeting the “achilles’heel”of androgen receptor activity.Cancer Res 2011;71:1208e13.
[40]Yu Z,Chen S,Sowalsky AG,Voznesensky OS,Mostaghel EA,Nelson PS,et al.Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer.Clin Cancer Res 2014;20:1590e600.
[41]Wilson EM.Androgen receptor molecular biology and potential targets in prostate cancer.Ther Adv Urol 2010;2:105e17.
[42]Taplin ME,Rajeshkumar B,Halabi S,Werner CP,Woda BA,Picus J,et al.Androgen receptor mutations in androgenindependent prostate cancer:cancer and leukemia group B study 9663.J Clin Oncol 2003;21:2673e8.
[43]Grasso CS,Wu YM,Robinson DR,Cao X,Dhanasekaran SM,Khan AP,et al.The mutationallandscape oflethal castration-resistant prostate cancer. Nature 2012;487:239e43.
[44]Steketee K,Timmerman L,Ziel-van der Made AC,Doesburg P,Brinkmann AO,Trapman J.Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer.Int J Cancer 2002;100:309e17.
[45]Newmark JR,Hardy DO,Tonb DC,Carter BS,Epstein JI,Isaacs WB,et al.Androgen receptor gene mutations in human prostate cancer.Proc Natl Acad Sci U S A 1992;89:6319e23.
[46]Taplin ME,Bubley GJ,Ko YJ,SmallEJ,Upton M,Rajeshkumar B,et al.Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist.Cancer Res 1999;59:2511e5.
[47]Korpal M,Korn JM,Gao X,Rakiec DP,Ruddy DA,Doshi S,et al.An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100(enzalutamide).Cancer Discov 2013;3:1030e43.
[48]Joseph JD,Lu N,Qian J,Sensintaffar J,Shao G,Brigham D,et al.A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509.Cancer Discov 2013;3:1020e9.
[49]Noonan KL,North S,Bitting RL,Armstrong AJ,Ellard SL,Chi KN.Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide.Ann Oncol 2013;24:1802e7.
[50]Loriot Y,Bianchini D,Ileana E,Sandhu S,Patrikidou A,Pezaro C,et al.Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide(MDV3100).Ann Oncol 2013;24:1807e12.
[51]Bubendorf L,Kononen J,Koivisto P,Schraml P,Moch H,Gasser TC,et al.Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays.Cancer Res 1999;59:803e6.
[52]Edwards J,Krishna NS,Grigor KM,Bartlett JM.Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer.Br J Cancer 2003;89:552e6.
[53]Chen CD,Welsbie DS,Tran C,Baek SH,Chen R,Vessella R,et al.Molecular determinants of resistance to antiandrogen therapy.Nat Med 2004;10:33e9.
[54]Hu R,Denmeade SR,Luo J.Molecular processes leading to aberrant androgen receptor signaling and castration resistance in prostate cancer.Expert Rev Endocrinol Metab 2010;5:753e64.
[55]Antonarakis ES,Lu C,Wang H,Luber B,Nakazawa M,Roeser JC,et al.AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer.N Engl J Med 2014;371:1028e38.
[56]Hu R,Lu C,Mostaghel EA,Yegnasubramanian S,Gurel M,Tannahill C,et al.Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer.Cancer Res 2012;72:3457e62.
[57]Nadiminty N,Tummala R,Liu C,Yang J,Lou W,Evans CP,et al.NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer:role of androgen receptor and its variants.Mol Cancer Ther 2013;12:1629e37.
[58]Sarker D,Reid AHM,Yap TA,de Bono JS.Targeting the PI3K/AKT pathway for the treatment of prostate cancer.Clin Cancer Res 2009;15:4799e805.
[59]Buttyan R,Sawczuk IS,Benson MC,Siegal JD,Olsson CA.Enhanced expression of the c-myc protooncogene in highgrade human-prostate cancers.Prostate 1987;11:327e37.
[60]Karantanos T,Corn PG,Thompson TC.Prostate cancer progression after androgen deprivation therapy:mechanisms of castrate resistance and novel therapeuticapproaches.Oncogene 2013;32:5501e11.
[61]Cai CM,He HH,Chen S,Coleman I,Wang H,Fang Z,et al.Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1.Cancer Cell 2011;20:457e71.
[62]Knudsen BS,Edlund M.Prostate cancer and the met hepatocyte growth factor receptor.Adv Cancer Res 2004;91:31e67.
[63]Verras M,Lee J,Xue H,Li TH,Wang Y,Sun Z.The androgen receptor negatively regulates the expression of c-Met:implications for a novel mechanism of prostate cancer progression.Cancer Res 2007;67:967e75.
[64]Li L,Chang W,Yang G,Ren C,Park S,Karantanos T,et al.Targetingpoly(ADP-ribose)polymerase and the c-Mybregulated DNA damage response pathway in castrationresistant prostate cancer.Sci Signal 2014;7:ra47.
[65]Stenman G,Andersson MK,Andren Y.New tricks from an old oncogene gene fusion and copy number alterations of MYB in human cancer.Cell Cycle 2010;9:2986e95.
[66]Bohrer LR,Chen SA,Hallstrom TC,Huang HJ.Androgens suppress EZH2 expression via retinoblastoma(RB)and p130-dependent pathways:a potential mechanism of androgenrefractory progression of prostate cancer.Endocrinology 2010;151:5136e45.
[67]Varambally S,Dhanasekaran SM,Zhou M,Barrette TR,Kumar-Sinha C,Sanda MG,et al.The polycomb group protein EZH2 is involved in progression of prostate cancer.Nature 2002;419:624e9.
[68]Xu KX,Wu ZJ,Groner AC,He HH,Cai C,Lis RT,et al.EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent.Science 2012;338:1465e9.
[69]Miranda TB,Cortez CC,Yoo CB,Liang G,Abe M,Kelly TK,et al.DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation.Mol Cancer Ther 2009;8:1579e88.
[70]Sahu B,Laakso M,Pihlajamaa P,Ovaska K,Sinielnikov I,Hautaniemi S,et al.FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells.Cancer Res 2013;73:1570e80.
[71]Arora VK,Schenkein E,Murali R,Subudhi SK,Wongvipat J,Balbas MD,et al.Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade.Cell 2013;155:1309e22.
[72]Carver BS,Chapinski C,Wongvipat J,Hieronymus H,Chen Y,Chandarlapaty S,et al.Reciprocal feedback regulation of pi3k and androgen receptor signaling in PTEN-deficient prostate cancer.Cancer Cell 2011;19:575e86.
[73]Mulholland DJ,Tran LM,Li YF,Cai H,Morim A,Wang S,et al.Cell autonomous role of pten in regulating castration-resistant prostate cancer growth.Cancer Cell 2011;19:792e804.
[74]Nguyen HG,Yang JC,Kung HJ,Shi XB,Tilki D,Lara Jr PN,et al.Targeting autophagy overcomes enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model.Oncogene 2014;33:4521e30.
[75]Mizushima N.Autophagy:process and function.Genes Dev 2007;21:2861e73.
[76]Sui X,Chen R,Wang Z,Huang Z,Kong N,Zhang M,et al.Autophagy and chemotherapy resistance:a promising therapeutic target for cancer treatment.Cell Death Dis 2013;4:e838.
[77]Yang ZJ,Chee CE,Huang S,Sinicrope FA.The role of autophagy in cancer:therapeutic implications.Mol Cancer Ther 2011;10:1533e41.
[78]Schroeder A,Herrmann A,Cherryholmes G,Kowolik C,Buettner R,Pal S,et al.Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling.Cancer Res 2014;74:1227e37.
[79]Bishop JLSB,Gleave M,Zoubeidi A.Immune evasion strategies of neuroendocrine-like enzalutamide resistant prostate cancer.J Immunother Cancer 2013;1(S1):1.
[80]Svensson C,Ceder J,Iglesias-Gato D,Chuan YC,Pang ST,Bjartell A,et al.REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer.Nucleic Acids Res 2014;42:999e1015.
[81]Beltran H,Tomlins S,Aparicio A,Arora V,Rickman D,Ayala G,et al.Aggressive variants of castration-resistant prostate cancer.Clin Cancer Res 2014;20:2846e50.
*
.
E-mail address:timthomp@mdanderson.org(T.C.Thompson).
Peer review under responsibility of Chinese Urological Association and SMMU.
1S Karanika and T Karantanos contributed equally to this work.
http://dx.doi.org/10.1016/j.ajur.2015.04.004
2214-3882/ª2014 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier(Singapore)Pte Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Urology2014年1期
Asian Journal of Urology2014年1期
- Asian Journal of Urology的其它文章
- Systematic evaluation of a holmium:yttrium-aluminum-garnet laser lithotripsy device with variable pulse peak power and pulse duration
- Robotic assisted laparoscopic simple suprapubic prostatectomy e The Smith Institute for Urology experience with an evolving technique
- Current trends in urethral stricture management
- Prostate cancer in Asia:A collaborative report
- Evolution:Back to the future to understand and control prostate cancer
- Comment on the discovery of prostate specific antigen
