Molecular Dynamics Study on Conf i guration Energy and Radial Distribution Functions of Ammonium Dihydrogen Phosphates Solution
Kun Wng,Y-fn Zho,Gui-wu Lu,Yu-ling Wng,Ju-n Chen,De-zhi Su
a.Department of Basic Science,Naval Aeronautical and Astronautical University,Yantai 264001,China
b.College of Science,China University of Petroleum(Beijing),Beijing 102249,China
Molecular Dynamics Study on Conf i guration Energy and Radial Distribution Functions of Ammonium Dihydrogen Phosphates Solution
Kun Wanga∗,Ya-fan Zhaoa,Gui-wu Lub,Yu-liang Wanga,Ju-na Chena,De-zhi Sua
a.Department of Basic Science,Naval Aeronautical and Astronautical University,Yantai 264001,China
b.College of Science,China University of Petroleum(Beijing),Beijing 102249,China
Molecular dynamics simulations were carried out to study the conf i guration energy and radial distribution functions of mmonium dihydrogen phosphate solution at different temperatures. The dihydrogen phosphate ion was treated as a seven-site model and the ammonium ion was regarded as a five-site model,while a simple-point-charge model for water molecule.An unusually local particle number density f l uctuation was observed in the system at saturation temperature.It can be found that the potential energy increases slowly with the temperature from 373 K to 404 K,which indicates that the ammonium dihydrogen phosphate has partly decomposed.The radial distribution function between the hydrogen atom of ammonium cation and the oxygen atom of dihydrogen phosphate ion at three different temperatures shows obvious difference,which indicates that the average H-bond number changes obviously with the temperature.The temperature has an inf l uence on the combination between hydrogen atoms and phosphorus atoms of dihydrogen phosphate ion and there are much more growth units at saturated solutions.
Ammonium dihydrogen phosphates solution,Conf i guration energy,Radial distribution function,Molecular dynamics simulation
I.INTRODUCTION
As a type of nonlinear optical crystal like the potassium dihydrogen phosphate(KDP),ammonium dihydrogen phosphate(ADP)crystal is well known for its piezo-electric,non-linear optical and electro-optical properties and widely used in X-ray monochromators [1].Despite some differences in the chemical composition,ADP and KDP have a number of important properties in common.In particular,they are characterized by low symmetry of crystal lattice and possess complex unit cells.A common distinctive feature is the contrast between strong covalent chemical bonds inside these anionic groups and relatively weak ionic bonds between a cation and the corresponding anionic group.
ADP crystal has usually grown in an aqueous solution by the temperature reduction method,since growth from an aqueous solution is particularly amenable to optical visualization.Recently,ADP has been given considerable interest among the researchers to grow this important crystal from solutions with faster rates by adopting cooling rates[2].A lot of work has been done to study how to control the growth rates and growth habits of the ADP crystal.The surface micromorphology,defect substructure,defect concentration,surface conductivity and microhardness have been studied by Ramakrishna[3].The effect of EDTA on the growth kinetics,structure,optical and mechanical properties of ADP crystal has been discussed by Rahman et al. [4].The kinetics of electron tunneling transfer under the conditions of thermostimulated mobility of recombining reactants in nonlinear KDP and ADP crystals has been investigated by Ogorodnikov et al.[5]using the method of mathematical modeling.Structural characterization at the atomistic level of ADP solution is crucial for the quantification and prediction of crystal growth rate,growth morphology,and their physical,chemical and mechanical properties.Study on microstructure of ADP solution is an important step for exploring the stabilization of crystal growth solution and growth mechanism of ADP crystal.However,the structure of mother liquid and the structural characterization at the atomistic level now face challenges.We have investigated thermodynamic properties and solutions micro-structure of KDP[6].In this work,the solutions micro-structure of ADP and the effect of temperature on conf i guration energy and structural characterization at the atomistic level were studied in detail.
II.SIMULATION METHODS
Molecular dynamics simulations were carried out to study the conf i guration energy and the micro-structureof ADP solution at di ff erent temperatures.All calculations were performed in the framework of the MD,using the Material Studio 4.0 from Accelrys Inc.(formerly Molecular Simulation,Inc.)[7].In both energy optimization and MD simulations,the Discover package included in MS software was adopted and the condensedphase optimized molecular potentials for atomistic simulation studies(COMPASS)[8-10]force fi eld were used for all the components of the system.COMPASS which is based on the earlier class IICFF9X and PCFF forcefi eld is able to make accurate predictions of structural, conformation,vibrational,cohesive and thermophysical properties for a broad range of compounds both in isolation and in condensed phase.
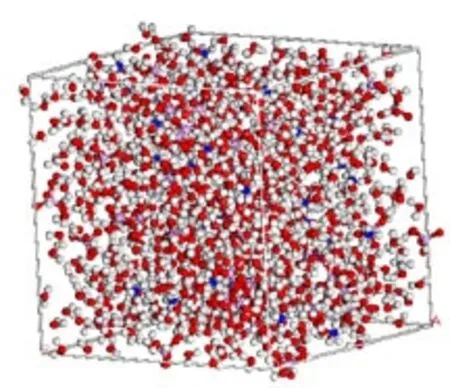
FIG.1 Atomic structure model of saturated solution of ammonium dihydrogen phosphate at 293 K after 200 ps relaxation.
Thesystemsizeofthesimulatedsolutionis 2.69 nm×2.69 nm×2.69 nm.In order to allow the system to exchange heat with the environment at a controlled temperature,the canonical ensemble(NVT) molecular dynamics simulations were adopted while applying the periodic condition.The ammonium ion and dihydrogen phosphate ion were treated as a five-site and seven-site model,while the water molecule was treated as simple-point-charge(SPC)model respectively.The system contains 854 water molecules,50 ammonium cations and 50 dihydrogen phosphate anions,which corresponds to the solubility 37.4%of ADP saturated solution at 293 K.Both molecular species,water and ammonium dihydrogen phosphate,were kept rigid throughout the simulations,and equations of motions were solved using leapfrog scheme with a timestep of 1 fs.The pre-equilibrium period was performed for 200 ps.The atomic structure model of saturated solution of ADP at 293 K after 200 ps relaxation is shown in Fig.1.
III.RESULTS AND DISCUSSION
A.Conf i guration energy
The conf i guration energy corresponding to different temperatures is shown in Table I.The relationship of ADP solution between kinetic,potential,total energy and temperature is shown in Fig.2,respectively.It is seen from Table I that the potential and total energyof ADP solution basically increase with temperature, which indicates that the long-range attractive interaction dominates.The kinetic energy increases with the temperature,which is in agreement with tradition thermodynamic theory.
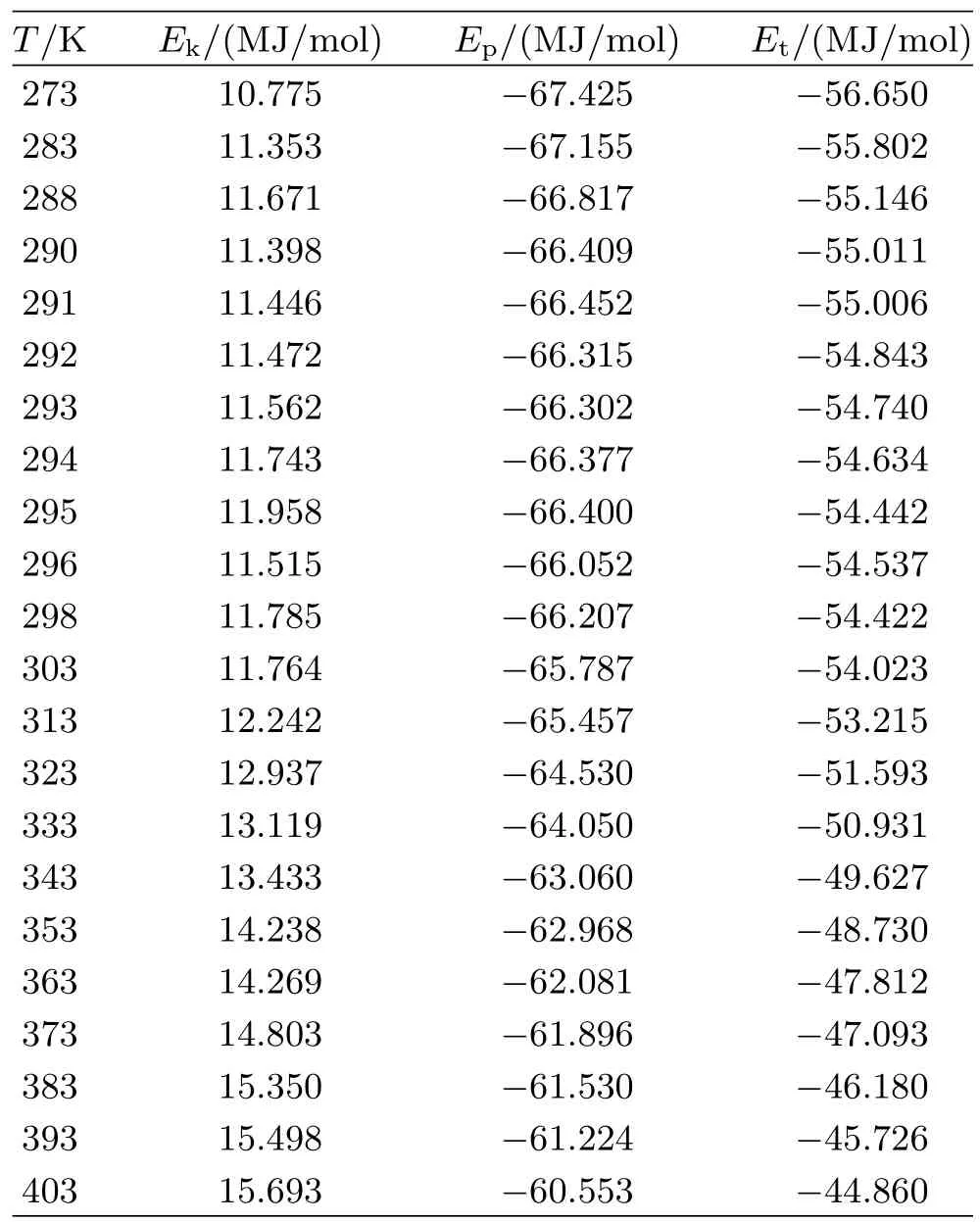
TABLE I Kinetic energy Ek,potential energy Ep,total energy Etat different temperature T.
At saturation temperature 293 K,it can be seen from Fig.2 that the kinetic,potential and total energy have an obvious f l uctuation,which indicates an unusually local particle number density f l uctuation in the system. Thus we can conclude that the solution experience a crystallization process below saturation temperature. From Fig.2(b),it can be observed that the potential energy increases slowly with the temperature from 373 K to 404 K,which means that the ammonium dihydrogen phosphate has partly decomposed.
B.Structure of ADP solution
The most important information about the structure of ADP solution is calculated in the form of atom-atom radial distribution functions,gi-j(r).These functions give the probability of f i nding a pair of atom i and j with a distance r apart,relative to the probability expected for a completely random distribution of atoms at the same temperature[11].
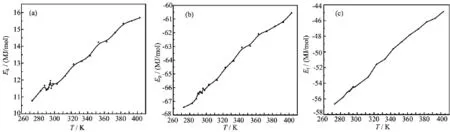
FIG.2 The relationship of ADP solution between(a)kinetic energy Ek,(b)potential energy Ep,(c)total energy Etand temperature.
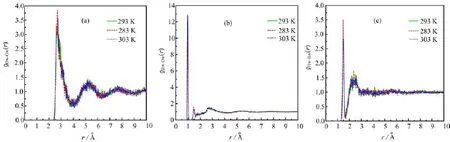
FIG.3 Radial distribution functions for water-water of ADP solution at 283,293 and 303 K.(a)Ow-Ow,(b)Hw-Ow, and(c)Hw-Hw.
Symbols Ow,Hw,and Op,Hp,hereafter,represent oxygen atoms and hydrogen atoms of water and dihydrogen phosphate ion respectively,Hn represents hydrogen atoms of ammonium cation.The water-water radial distribution function gOw-Ow(r),gHw-Ow(r),and gHw-Hw(r)at 283,293,and 303 K,are shown in Fig.3.
The peaks of the distribution which represent the fi rst,second and third coordinate zone diminish with the increasing of r.The rapid attenuation of the peaks indicates the order arrangement of water molecule disappears with the increasing of r.A pronounced maximum of gOw-Ow(r),gHw-O˚w(r),and gHw-Hw(r)ap-pears near 2.8,1.0,and 1.5A,respectively(see Fig.4). The fi rst peak of gHw-Ow(r)at three di ff erent temperatures(see Fig.4(b))shows obvious di ff erence,which indicates the average H-bond number changes obviously with temperature.And comparing with gHw-Ow(r) functions at three temperatures we can fi nd that the fi rst peak at 293 K is higher than that at 283 and 303 K which indicates that the H-bond number of saturated solution is maximal.
The water-ion radial distribution functions at 283, 293 and 303 K are shown in Fig.4 and Fig.5.Figure 4 shows that the radial distribution function approach to zero while the distance r is less than the e ff ective collision diameter 1.3˚A between water molecule and ammonium dihydrogen phosphate molecule.And we can fi nd that the radial distribution function˚ approach to onewhile the distance r is greater than 3A,which indicates that the local partial number density is equal to the average partial number density at this p˚oint.The fi rstpeak of gHw-Op(r)appears near 1.45A(see Fig.4(e)) followed a broader second maximum due to second hydration shell which is not involved in the hydrogen bond with the dihydrogen phosphate ion[12].The maxima˚lpeaks of gHw-Hn(r)and gHw-Hp(r)appear near 1.5A (see Fig.4(b)and(d))which indicates that the water molecule has a strong interaction with the ammonium dihydrogen phosphate molecule.
Figure 5 shows the corresponding radial distribution functions between the oxygen atoms of water molecule and ions.The radial distribution function between the oxygen atom of water molecule and the hydrogen atom of the dihydrogen phosphate ion(gOw-Hp(r))shows a very strong hydr˚ogen bond structure with the fi rstmaximum at 1.65A(see Fig.5(d))compared with ˚thefi rst peak of gOw-P(r)and gOw-Op(r)(2.7 and 2.68A).
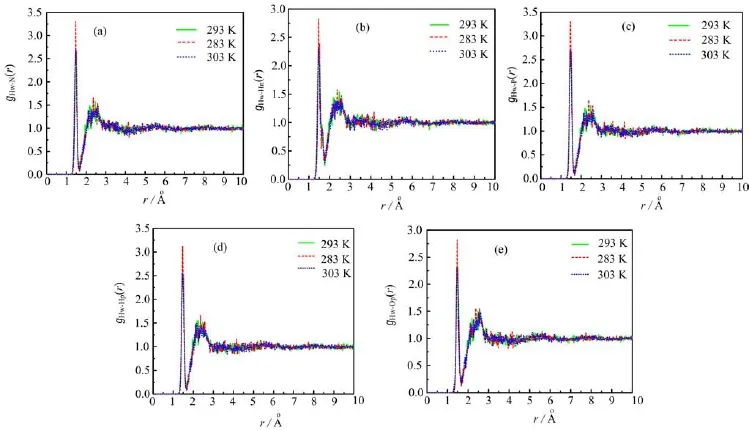
FIG.4 Radial distribution functions between the ion sites and hydrogen of water molecule at 283,293,and 303 K.(a)Hw-N, (b)Hw-Hn,(c)Hw-P,(d)Hw-Hp,and(e)Hw-Op.

FIG.5 Radial distribution functions between the ion sites and oxygen of water molecule at 283,293,and 303 K.(a)Ow-N, (b)Ow-Hn,(c)Ow-P,(d)Ow-Hp,and(e)Ow-Op.
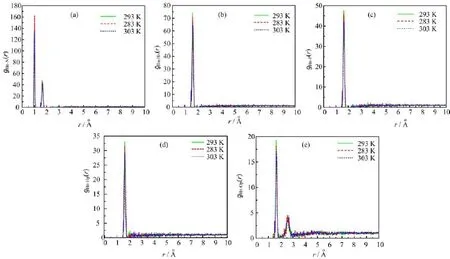
FIG.6 Radial distribution functions for(a)Hn-N,(b)Hn-Hn,(c)Hn-P,(d)Hn-Hp,(e)Hn-Op pairs at 283,293,and 303 K.
The fi rst peak of gOw-Hn(r)appears near 1.7˚A with a broader second sharp peak(see Fig.5(b))due to second hydration which is not involved in the hydrogen bond with the dihydrogen phosphate ion.From gOw-Hp(r) and gOw-Hn(r)functions at di ff erent temperatures we can fi nd that the second peak at 283 K is higher than that at 293 and 303 K,which indicates that the second hydration is inclined to occur at over-saturated solutions.
Figure 6 shows the radial distribution functions for Hn-N,Hn-Hn,Hn-P,Hn-Hp,Hn-Op pairs respectively at 283,293 and 303 K.The fi rst peak o˚fgHn-Hn(r),gHn-P(r)and gHn-Hp(r)appears near 1.7A compared with the fi rst peak of gHn-N(r)(1.0˚A),which indicates that the hydrogen atom of ammonium cation has a strong interaction with the nitrogen a˚tom.Thefi rst peak of gHn-Op(r)appears near 1.7A followed by a second maximum at 2.1˚A(see Fig.6(e)),which means that the second hydrogenation also occurs between the hydrogen atom of ammonium cation and oxygen atom of dihydrogen phosphate anion.The fi rst peak of gHn-Op(r)at three di ff erent temperatures(see Fig.6(e))shows obvious di ff erence,which indicate that the average H-bond number changes obviously with the temperature.
Figure 7 shows the radial distribution functions for Op-Hp,Op-Op,Op-N,Op-P,P-P pairs at 283, 293,and 303 K,respectively.The radial distribution function between the oxygen atom and the hydrogen of the dihydrogen phosphate ion(gOp-Hp(r))shows a very hydrogen bond structure with the f i rst peak at 1.0˚A followed by a broader second maximum due to the second hydration shell,which is not involved in the hydrogen bond with the dihydrogen phosphate ion.The f i rst peak of gOp-P(r)(see Fig.7(d))at 303 K is much higher than that at 283 and 293 K,which illustrates that temperature has an inf l uence on the combination between oxygen atoms and phosphorus atoms of dihydrogen phosphate ion.There is no instinct peak in gP-P(r)(see Fig.7(e)),indicating there is no direct interaction between phosphorus atom.
Figure 8 shows the structural pair correlations for Hp-Hp,Hp-N,Hp-P,N-P,N-N pairs at 283,293, and 303 K,respectively.It can be seen that the f i rst peak of gHp-P(r)is located at 2.0˚A.The f i rst peak at 293 K is much higher than that at 283 and 303 K(see Fig.8(c)),which means temperature has an inf l uence on the combination between hydrogen atoms and phosphorus atoms of dihydrogen phosphate ion and there are much more growth units at saturated solutions.In the ADP solution there is hardly any structure to be observed between N-P,N-N,Hp-N and Hp-Hp which illustrates that there is no direct interaction between them.
IV.CONCLUSION

FIG.7 Radial distribution functions for(a)Op-Hp,(b)Op-Op,(c)Op-N,(d)Op-P,and(e)P-P pairs at 283,293, and 303 K.

FIG.8 Radial distribution functions for(a)Hp-Hp,(b)Hp-N,(c)Hp-P,(d)N-P,and(e)N-N pairs at 283,293,and 303 K.
Molecular dynamics simulations were carried out to study the conf i guration energy and radial distributionfunctions of ammonium dihydrogen phosphate(ADP) solutions at different temperatures.By simulating relatively large systems for long sampling periods,quantitatively useful results have been obtained.Firstly, an unusually local particle number density f l uctuation was observed in the system at saturation temperature, and the potential energy increases slowly with the temperature from 373 K to 404 K,which indicates that the ammonium dihydrogen phosphate has partly decomposed.Secondly,the radial distribution function between the hydrogen atom of ammonium cation and the oxygen atom of dihydrogen phosphate ion at three different temperatures shows obvious difference,which indicate that the average H-bond number changes obviously with the temperature.Finally,the temperature has an inf l uence on the combination between hydrogen atoms and phosphorus atoms of dihydrogen phosphate ion and there are much more growth units at saturated solutions.
[1]N.P.Rajesh,V.Kannana,P.S.Raghavan,and C.W. Lan,Mater.Chem.Phys.76,181(2002).
[2]K.Srinivasan,K.Gottifried,and J.Wurzelova,J. Cryst.Growth.6,151(1970).
[3]M.Ramakrishna and E.Venkateshwar,India.J.Phys. 84,193(2010).
[4]A.Rahman and J.Podder,India.J.Phys.86,15 (2012).
[5]I.N.Ogorodnikov and M.S.Kiseleva,Phys.Solid. State.54,273(2012).
[6]K.Wang,G.W.Lu,G.G.Zhou,and H.W.Yang, Chin.J.Chem.Phys.23,160(2010).
[7]Materials Studio 4.0,Discover/Accelrys,San Diego, CA:(2005).
[8]H.Sun,J.Phys.Chem.B 102,7338(1998).
[9]H.Sun,P.Ren,and J.R.Fried,Comput.Theor.Polym. Sci.8,299(1998).
[10]D.Rigby,H.Sun,and B.E.Eichinger,Polym.Int.44, 311(1998).
[11]J.Eggebrecht and P.Ozler,J.Chem.Phys.93,2004 (1990).
[12]G.W.Lu,Y.F.Li,and W.Sun,Chin.J.Chem.Phys. 20,22(2007).
ceived on March 11,2014;Accepted on June 11,2014)
∗Author to whom correspondence should be addressed.E-mail:sindy5674580@sina.com
 CHINESE JOURNAL OF CHEMICAL PHYSICS2014年4期
CHINESE JOURNAL OF CHEMICAL PHYSICS2014年4期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Exchange Bias Effect in Phase Separated La0.33Pr0.34Ca0.33MnO3Thin Films
- Elasticity and Thermodynamic Properties of EuS Related to Phase Transition
- Corrosion Study on Tantalum in Anhydrous Ethanol
- Kinetics Study on O2Adsorption and OHadDesorption at Pt(111),Its Implication to Oxygen Reduction Reaction Kinetics
- Phase Transition Behaviour of VO2Nanorods
- Effect of Molybdenum Doping on Oxygen Permeation Properties and Chemical Stability of SrCo0.8Fe0.2O3-δ
