污泥发酵液对A2O脱氮除磷和微生物的影响
刘亚利,袁一星,李 欣,詹技灵,康晓荣,董林沛
(1.哈尔滨工业大学市政环境工程学院,150090哈尔滨;2.大连迈克环境科技有限公司,辽宁大连116000)
污泥发酵液对A2O脱氮除磷和微生物的影响
刘亚利1,袁一星1,李 欣1,詹技灵2,康晓荣1,董林沛1
(1.哈尔滨工业大学市政环境工程学院,150090哈尔滨;2.大连迈克环境科技有限公司,辽宁大连116000)
为研究剩余污泥发酵液作碳源对微生物群落结构的影响,将发酵液与市政污水按流量比1∶35回用于厌氧-缺氧-好氧反应器,在室温下运行90 d.聚类分析表明,发酵液明显改变了微生物群落结构,5~30 d和45~90 d的微生物属于不同的聚集区;微生物多样性分析表明,发酵液使Shannon-Wiener指数从2.6升高到3.1,系统运行稳定性增强;PCR-DGGE分析表明,发酵液对微生物群落具有一定的选择性,氨氧化菌Nitrosomonas sp.、硝化菌Betaproteobacteria和Nitrospira sp.、反硝化菌Comamonas sp.和聚磷菌Gammaproteobacteria得到富集,TN和TP去除率从64.5%和52.4%提高到84.7%和94.3%.
剩余污泥;脱氮除磷;碳源;生物群落;聚合酶链式反应-变性梯度凝胶电泳技术
最近的研究表明,剩余污泥厌氧发酵产生的挥发酸是脱氮除磷的良好碳源[1-2],且剩余污泥发酵液比乙酸盐更适合作为脱氮除磷的碳源[3]. Gao等[4]发现将剩余污泥发酵液应用于厌氧-缺氧-好氧(A2O)工艺后,TN和TP的去除率达80.1%和90.0%;Tong等[5]的研究表明,剩余污泥碱发酵液与市政污水按1∶35投入SBR反应器后,总氮(TN)和磷酸盐(PO43--P)的去除率分别由63.3%和44.0%提高到83.2%和92.9%.为进一步研究污泥发酵液提高污水脱氮除磷效果的机理,Ji等[6]采用荧光原位杂交(fluorescence in situ hybridization,FISH)技术研究污泥发酵液和乙酸盐对SBR中脱氮除磷功能菌的影响,发现污泥发酵液能够促进短程硝化-反硝化和反硝化除磷反应发生,节省碳源,提高合成废水的脱氮除磷效果.Zhu等[7]通过FISH技术研究发现:在厌氧-低溶解氧工艺中,剩余污泥碱发酵液能够增加将氧化二氮(N2O)直接还原为氮气(N2)的微生物量,减少N2O和一氧化氮(NO)产生,提高TP和TN去除效率,降低氧气消耗.
本实验从实际应用的角度出发,将剩余污泥发酵液作为内碳源与市政污水按比例混合后,回用于A2O反应器.考察投加发酵液对微生物群落结构的影响,分析微生物群落结构与工艺脱氮除磷效能之间的关系.同时采用PCR-DGGE技术分析投加发酵液前后脱氮除磷功能菌群的变化.
1 实 验
1.1 实验材料
剩余活性污泥取自哈尔滨某污水厂二沉池,经超声(0.6 W/mL;5 min)和碱(pH=12)联合预处理后厌氧发酵5 d,所得发酵液于10 000 r/min离心10 min,再通过鸟粪石法去除氮和磷[8].所得污泥发酵液的性质如下:COD 8 120 mg/L;TN 256.3 mg/L;氨氮(-N)38.1 mg/L;总磷(TP)47.2 mg/L;28.7 mg/L;挥发酸(VFAs)5 061 mg/L;溶解性蛋白279 mg/L;溶解性多糖91 mg/L.其中VFAs中乙酸、丙酸所占的质量分数分别为38.2%和30.6%.
1.2 实验装置
污水处理工艺流程如图1所示.A2O反应器的厌氧、缺氧和好氧池的水力停留时间分别为2,2和6 h.缺氧和好氧池的溶解氧分别控制在0.5~1.0和3.0~3.5 mg/L.混合液悬浮固体质量浓度(MLSS)为(4 000±500)mg/L,污泥停留时间为15 d.反应器在室温下连续运行90 d.从第20天开始将污泥发酵液与市政污水按1∶35[5]投加到反应器中,投加前后进水水质见表1.

图1 A2O处理工艺流程

表1 投加污泥发酵液前后进水水质
1.3 检测方法
1.4 数据分析
1.4.1 工艺运行稳定性分析
采用出水COD、TN和TP的标准差(Ds)来衡量不同阶段出水的波动,进而对工艺运行稳定性进行评价.Ds的计算式为
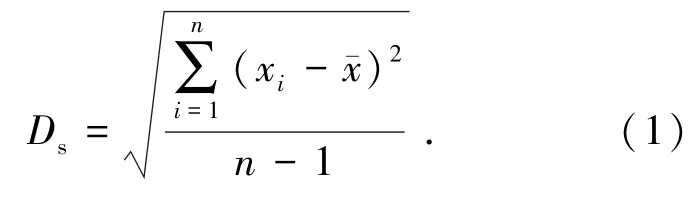
式中:Ds为COD、TN或TP标准差,mg/L;xi为第i个出水样品的COD、TN或TP质量浓度,mg/L;¯x为出水COD、TN或TP质量浓度平均值,mg/L;n为数据个数.
1.4.2 生物信息学分析
微生物种群多样性采用Shannon指数(Shannon-Wiener index,H)[12]表示,用来评价系统内微生物种群的丰富程度及分配均匀性,即
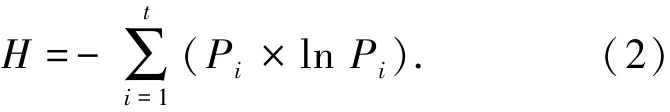
式中:Pi为条带i所占比例;t为条带数.
2 结果和讨论
2.1 工艺运行效果分析
投加发酵液前后,COD、TN和TP去除率随时间的变化如图2所示.与市政污水相比,投加发酵液使进水COD升高了220.4 mg/L,但COD去除率仍由86.7%提高到89.2%.这是因为增加的COD以VFAs、溶解性蛋白和多糖为主,能够在A2O反应器中得到完全降解.该结果与发酵液作为SBR反应器碳源所得的结论一致[5].在进水TN和TP质量浓度略有升高的条件下,发酵液使TN和TP去除率由64.5%和52.4%提高到84.7%和94.3%.这是因为:发酵液中富含的VFAs为聚磷菌提供了最佳碳源[14];发酵液使碳氮比由4.1升高到9.0,削弱了反硝化菌和聚磷菌对有限碳源的竞争[3].
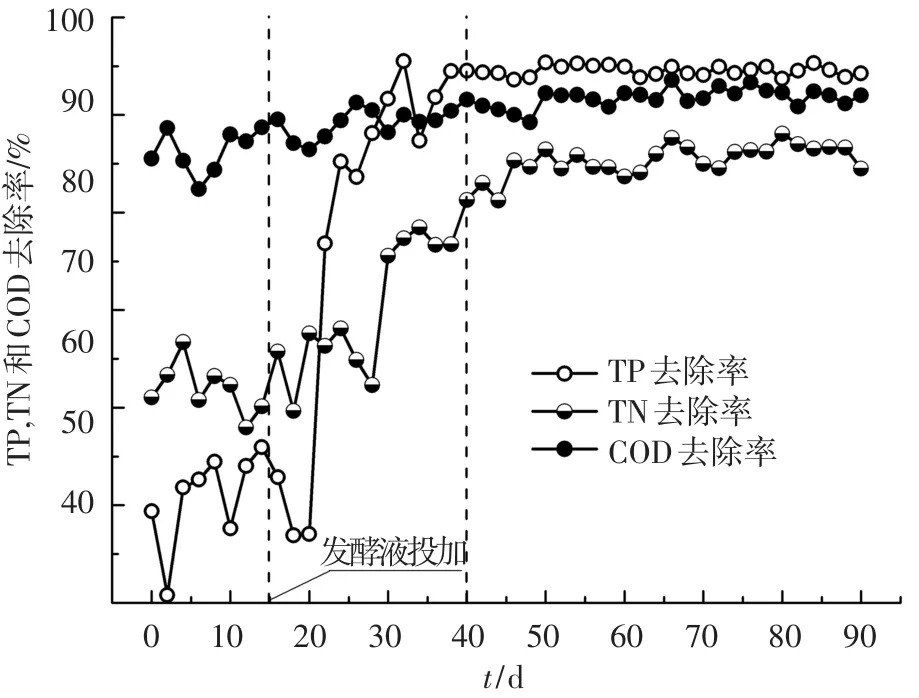
图2 COD、TN和TP去除率随时间的变化
投加发酵液前后,反应器出水COD、TN和TP的标准差如表2所示.发酵液使出水COD、TN和TP的标准差均呈现先升高后降低的趋势,表明工艺的运行稳定性先降低后提高.这是因为发酵液改变了进水水质,进而影响了微生物群落结构,经过25 d的驯化期后,适应新水质的微生物群落结构达到稳定,工艺运行稳定性提高.

表2 出水COD、TN和TP标准差随发酵液的变化mg·L-1
2.2 微生物相似性分析
投加发酵液前后,反应器内活性污泥样品的DGGE图谱如图3所示.可以看出,发酵液导致条带数量和强度均发生了明显改变.20~45 d时条带数减少,45~90 d时条带数增加.同时,随着发酵液的投加,条带11,12,14,15,16,17,18和19明显增强;条带4和8逐渐减弱,直至消失.

图3 PCR产物变性梯度凝胶电泳
为进一步研究微生物群落之间的关系,采用聚类分析对污泥样品中的微生物相似性进行分析.由图4可见,不同进水条件的微生物大致分为3类:接种污泥样本(1 d)聚为一类;市政污水作为进水时的污泥样本(5~30 d)聚为一类;发酵液和市政污水混合液作为进水时的污泥样本(45~90 d)聚为一类.这说明进水水质对微生物群落具有一定的选择性,进水水质发生改变,种群相似性明显降低.

图4 污泥样品的DGGE图谱的聚类分析
2.3 微生物多样性分析
投加发酵液前后,微生物Shannon-Wiener指数的变化如图5所示.可以看出,投加发酵液后,Shannon-Wiener指数呈先降低后升高的趋势,这是进水水质对细菌种群筛选的结果.一方面,不能适应水质变化的种群被淘汰,Shannon-Wiener指数降低;另一方面,投加发酵液前未检出的部分种群(<1%)逐渐适应水质变化,随工艺运行得到积累,Shannon-Wiener指数升高.
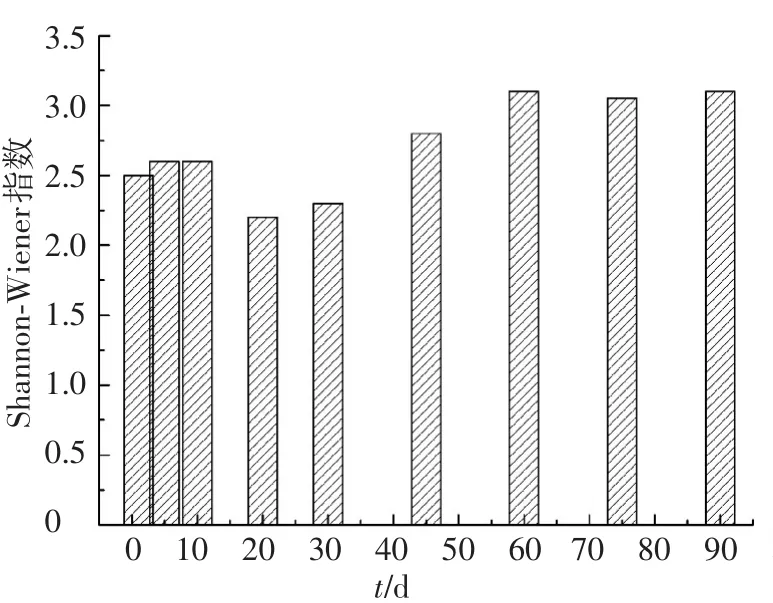
图5 Shannon-Wiener指数随发酵液的变化
结合图2、表2和图5发现,微生物多样性影响工艺的脱氮除磷效能和运行稳定性[14].与0~20 d相比,45~90 d时Shannon-Wiener指数从2.6升高至3.1,TN和TP去除率由64.5%和52.4%提高到84.7%和94.3%.同时,45~90 d时Shannon-Wiener指数的变化幅度仅为0.06,TN和TP的标准差降至0.61和0.05,工艺运行稳定性提高.
2.4 测序结果分析
对19条主要条带进行提取、扩增、克隆和测序,将所得的基因序列与NCBI中已鉴定同源性最接近的序列进行比对,其同源性达97%~100%,如表3所示.结合图3和表3发现,条带8对应的氨氧化菌Nitrosospira sp.不能适应进水水质变化而被淘汰,而条带15对应的氨氧化菌Nitrosomonas sp.则随污泥发酵液投加而增强,这与前人的研究结果一致[6].据报道,当污泥发酵液使SBR反应器中腐殖酸达70.5 mg/g时,会造成亚硝酸盐积累,抑制硝化菌Nitrospira sp.生长[6],而本实验中条带12和17所对应的硝化菌Betaproteobacteria和Nitrospira sp.逐渐增加,表明反应器中未发生明显的亚硝酸盐积累.随着进水被转化为硝酸盐,条带11所对应的反硝化菌Comamonas sp.[15]得以生长,提高了TN的去除效率.投加发酵液后,条带18所对应的聚磷菌Gammaproteobacteria[16]发生积累,而条带4所对应的聚糖菌CandidatusCompetibacter phosphatis则逐渐消失,表明除磷效果增强.这可能是因为进水中含有乙酸和丙酸,且其比例为5∶4,更有利于促进聚磷菌积累[17];另外,聚糖菌因缺少充足的亚硝酸盐而失去与聚磷菌的竞争力[18],与Ji等[6]的研究不同的是:条带1所对应的聚磷菌Candidatus Accumulibacter sp.在反应器运行稳定时消失,这一方面是因为本实验进水为市政污水而非合成废水,水质复杂、波动大[19];另一方面是因为该细菌更易以亚硝酸盐而非硝酸盐作为电子受体进行代谢[20].同时,条带16和19的相似菌Actinobacteria和Trichococcus sp.能够在厌氧条件下分别将蛋白和多糖降解为乙酸和丙酸,有利于强化生物除磷过程[21].在本实验中,条带14对应的Sphingobacteriaceae随发酵液的应用而逐渐增强.该细菌已被鉴定为生物强化除磷工艺(EBPR)中的反硝化聚磷菌[22],但其在本实验中的代谢机理和功能需要通过FISH等更精确的分子生物学手段来探索.
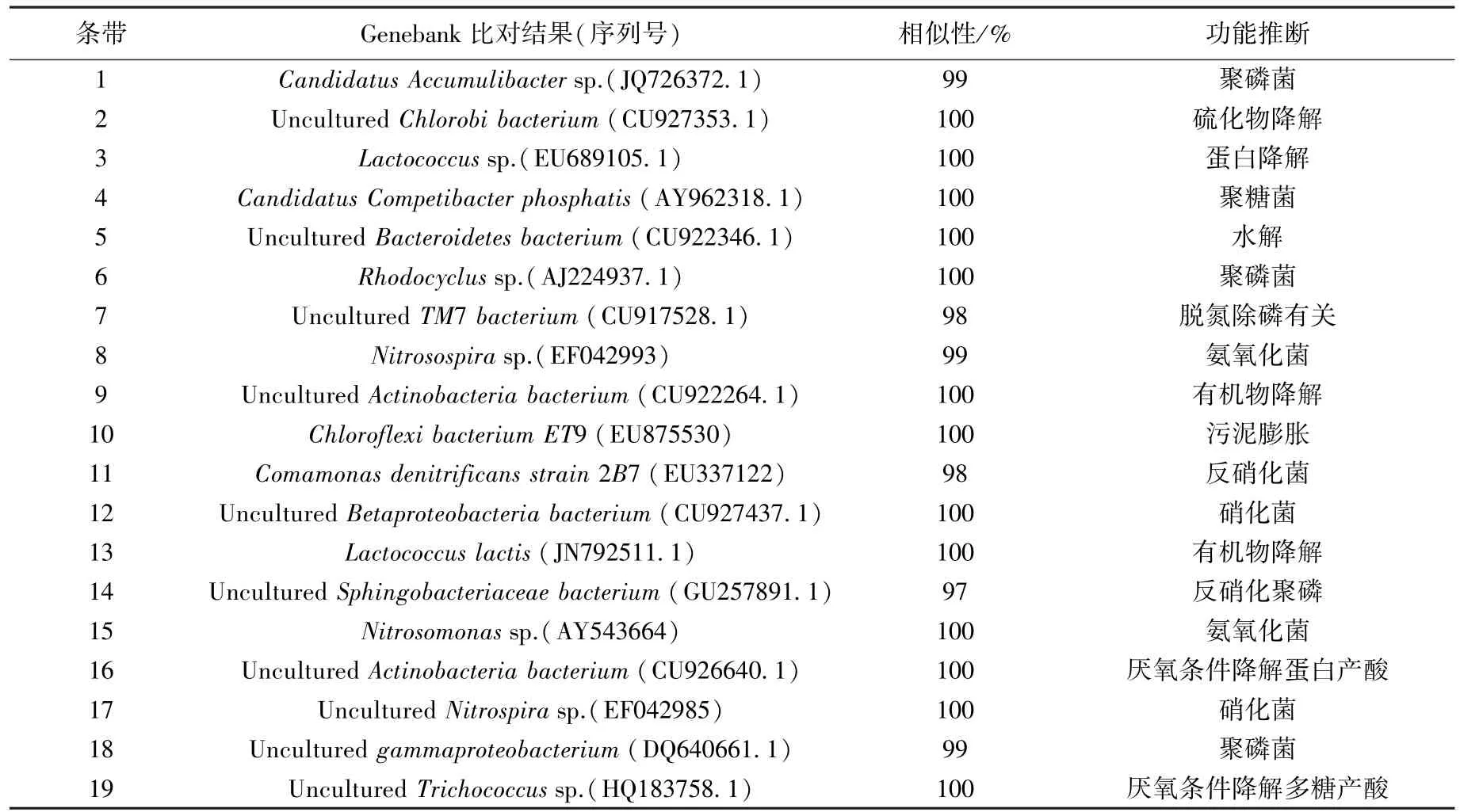
表3 细菌克隆在NCBI库最为相似的细菌种类
3 结 论
1)发酵液明显改变了微生物群落结构,5~30 d和45~90 d的微生物属于不同的类群.
2)发酵液使Shannon-Wiener指数从2.6升至3.1,出水TN和TP的标准差降至0.61和0.05,工艺运行稳定性提高.
3)发酵液对微生物群落具有一定的选择性,氨氧化菌Nitrosomonassp.、硝化菌Betaproteobacteria和Nitrospira sp.、反硝化菌Comamonas sp.和聚磷菌Gamma proteobacteria得到富集,TN和TP去除率从64.5%和52.4%提高到84.7%和94.3%.
参考文献
[1]COKGOR E U,OKTAY S,TAS D O,et al.Influence of pH and temperature on soluble substrate generation with primarysludgefermentation[J].Bioresource Technology,2009,100(1):380-386.
[2]彭晶,郭泽冲,侯玲玲,等.热碱预处理对剩余污泥发酵产酸效能提升的影响[J].哈尔滨工业大学学报,2012,44(8):43-47.
[3]SOARE A,KAMPAS P,MAILLARD S,et al.Comparison between disintegrated and fermented sewage sludge for production of a carbon source suitable for biological nutrient removal[J].Journal of Hazardous Materials,2010,175(1/2/3):733-739.
[4]GAO Yongqing,PENG Yongzhen,ZHANG Jingyu,et al.Biological sludge reduction and enhanced nutrient removal in a pilot-scale system with 2-step sludge alkaline fermentation and A2O process[J].Bioresource Technology,2011,102(5):4091-4097.
[5]TONG Juan,CHEN Yinguang.Recovery of nitrogen and phosphorus from alkaline fermentation liquid of waste activated sludge and application of the fermentation liquidtopromotebiologicalmunicipalwastewater treatment[J].Water Research,2009,43(12):2969-2976.
[6]JI Zhouying,CHEN Yinguang.Using sludge fermentation liquidtoimprovewastewatershort-cutnitrificationdenitrification and denitrifying phosphorus removal via nitrite[J].Environmental Sscience&Ttechnology,2010,44(23):8957-8963.
[7]ZHU Xiaoyu,CHEN Yinguang.Reduction of N2O and NO generation in anaerobic-aerobic(low dissolved oxygen)biological wastewater treatment Process by using sludge alkaline fermentation liquid[J].Environmental Science&Technology,2011,45(6):2137-2143.
[8]佟娟.剩余污泥碱性发酵产生的短链脂肪酸作为生物脱氮除磷碳源的研究[D].上海:同济大学,2008.
[9]魏复盛,国家环境保护总局,水和废水监测分析方法编委会.水和废水监测分析方法[M].北京:中国环境科学出版社,2002.
[10]LOWRY O H,ROSEBROUGH N J,FARR A L,et al. Protein measurement with the Folin phenol reagent[J].J Bbiol Chem,1951,193(1):265-275.
[11]HERBERT D,PHILIPPS P J,STRANGE R E. Carbohydrate analysis[J].Methods Enzymol B,1971,5:265-277.
[12]WANG Aijie,SUN Dan,CAO Guangli,et al.Integrated hydrogen production process from cellulose by combining dark fermentation,microbial fuel cells,and a microbial electrolysis cell[J].Bioresource Technology,2011,102(5):4137-4143.
[13]KANG Xiaorong,ZHANG Guangming,CHEN Lin,et al. EffectofinitialpHadjustmentonhydrolysisand acidification of sludge by ultrasonic pretreatment[J]. Industrial&Engineering Chemistry Research,2011,50(22):12372-12378.
[14]OEHMEN A,LOPEZ V C M,CARVALHO G,et al. Modellingthepopulationdynamicsandmetabolic diversity of organisms relevant in anaerobic/anoxic/aerobicenhancedbiologicalphosphorusremoval processes[J].Water Research,2010,44(15):4473-4486.
[15]ZHANG Bin,SUN Baosheng,JI Min,et al.Quantification and comparison of ammonia-oxidizing bacterial communities in MBRs treating various types of wastewater[J]. Bioresource Technology,2010,101(9):3054-3059.
[16]LIU Xinchun,ZHANG Yu,YANG Min,et al.Analysis of bacterial community structures in two sewage treatment plants with differentsludgepropertiesandtreatment performance by nested PCR-DGGE method[J].Journal of Environmental Sciences,2007,19(1):60-66.
[17]GUERRERO J,GUISASOLA A,BAEZA J A.The nature of the carbon source rules the competition between PAOanddenitrifiersinsystemsforsimultaneous biological nitrogen and phosphorus removal[J].Water Research,2011,45(16):4793-4802.
[18]TAYA C,GARLAPATI V K,GUISASOLA A,et al. The selective role of nitrite in the PAO/GAO competition[J].Chemosphere,2013,93(4):612-618.
[19]WONG M T,MINO T,SEVIOUR R,et al.In situ identificationandcharacterizationofthemicrobial community structure of full-scale enhanced biological phosphorous removalplantsinJapan[J].Water Research,2005,39(13):2901-2914.
[20]GUISASOLA A,QURIE M,VARGAS M DEL M,et al. Failure of an enriched nitrite-DPAO population to use nitrateasanelectronacceptor[J].Process Biochemistry,2009,44(7):689-695.
[21]WU Guangxue,SORENSEN K,RODGERS M,et al. Microbial community associated with glucose-induced enhanced biological phosphorus removal[J].Water Science&Technology,2009,60(8):2105-2113.
[22]李伟光,田文德,康晓荣,等.强化生物除磷工艺微生物种群结构分析[J].化工学报,2011,62(12): 3532-3538.
(编辑 刘 彤)
The effect of sludge fermentation liquid on nutrient removal performances and microbial community structure in A2O process
LIU Yali1,YUAN Yixing1,LI Xin1,ZHAN Jiling2,KANG Xiaorong1,DONG Linpei1
(1.School of Municipal and Environmental Engineering,Harbin Institute of Technology,150090 Harbin,China;2.Dalian MEC Environmental Technology&Engineering Co.,Ltd,116000 Dalian,Liaoning,China)
To analyze the effect of sludge fermentation liquid,using as internal carbon source,on microbial community structure in anaerobic-anoxic-aerobic process,three-month-long operational experiment was conducted at flow ratio of fermentation liquid and domestic wastewater 1∶35 at room temperature.The clustering analysis indicated that the microbial community structure was changed significantly by fermentation liquid,and the microbes of 5-30 d and 45-90 d had quite different homology.The microbial diversity analysis demonstrated that the Shannon-Wiener index increased from 2.6 to 3.1,resulting in the enhancement of operational stability.Meanwhile,fermentation liquid appeared to be selective for ammonia-oxidizing bacteria Nitrosomonas sp.,nitrifying bacteria Betaproteobacteria and Nitrospira sp.,denitrifying bacteria Comamonas sp.and phosphorus-accumulating bacteria Gammaproteobacteria,which led to the TN and TP removal efficiency improved from 64.5%and 52.4%to 84.7%and 94.3%,respectively.
waste activated sludge;nutrient removal;carbon source;bacterial community;PCR-DGGE
TU992.3
A
0367-6234(2014)10-0042-05
2013-09-12.
国家高技术研究发展计划(863计划)资助项目(2012AA063503-02).
刘亚利(1982—),女,博士研究生;
袁一星(1957—),男,教授,博士生导师.
袁一星,yyx1957@163.com;
李 欣,lixinwindows@163.com.

