Optimizing Reactors Selection and Sequencing: Minimum Cost versus Minimum Volume
Rachid Chebbi*Department of Chemical Engineering, American University of Sharjah, Sharjah 26666, United Arab Emirates
Optimizing Reactors Selection and Sequencing: Minimum Cost versus Minimum Volume
Rachid Chebbi*
Department of Chemical Engineering, American University of Sharjah, Sharjah 26666, United Arab Emirates
The present investigation targets minimum cost of reactors in series for the case of one single chemical reaction, considering plug flow and stirred tank reactor(s) in the sequence of flow reactors. Using Guthrie’s cost correlations three typical cases were considered based on the profile of the reaction rate reciprocal versus conversion. Significant differences were found compared to the classical approach targeting minimum total reactor volume.
cost, optimization, flow reactor, reactors in series, reactor selection
1 INTRODUCTION
Sequencing of chemical reactors is a typical reaction engineering problem. For ideal reactors, steady-state mole balances yield the volumes of the CSTR (continuous stirred tank reactor) and PFR (plug flow reactor) required to increase conversion in the case of one single reaction occurring [1-3]. Levenspiel plots [1-3] are obtained by plotting the inlet flow rate of the limiting reactant over the rate of disappearance versus conversion. The CSTR volume is represented by the area of a rectangle, whereas the PFR volume is given by the area under the curve. Levenspiel plots can be used to find the optimum reactors sequencing that provides the minimum total area (volume). An analytical approach [4] was proposed to resolve the optimum reactors sequencing problem using the same criterion: minimum total reactor volume. This led to results consistent with those using Levenspiel plots.
Different computational methods have been formulated to synthesize reactor networks. Superstructures incorporate anticipated process options and interconnections. A review was given by Smith [5], and complex superstructures were considered by Kokossis and Floudas [6, 7]. Reaching adequate superstructure complexity cannot be guaranteed; a concept, initially introduced by Horn [8], defined the attainable region (AR) as the points in concentration space that can be attained through reaction and mixing. The infinite dimensional state space (IDEAS) was introduced to find the AR and to synthesize reactor networks [9, 10]. Recent reviews and formulation of cost-oriented synthesis was given by Bedenik et al. [11] and Zhou and Manousiouthakis [12]. Use of a superstructure to minimize/maximize the total annual cost/profit was considered [13-15]. The concept of economic region (ER) obtained from concentration attainable region (AR) was introduced by Bedenik et al. [11], leading to a hybrid approach for mixed-integer nonlinear programming (MINLP) superstructure synthesis based on the ER concept. A cost minimization methodology using the IDEAS framework was introduced for isothermal reactor networks synthesis by Zhou and Manousiouthakis [12]. To avoid mathematical complication, a geometric approach based on areas comparison was given by Chen and Feng [16] for synthesizing reactors network targeting waste reduction. A combination of superstructure-based optimization with semantic models and analytical tools was introduced by Labrador-Darder et al. [17] to provide a progressive change in the superstructure along with a simplified design and interpretation. To reduce hydrogen waste in a refinery, Khajehpour et al. [18] used heuristic rules to reduce the superstructure followed by a genetic algorithm to solve the MINLP problem. A combination of linear programming and stochastic optimization was used by Jin et al. [19] for a superstructure composed of CSTRs and PFRs in order to reduce the complexity of solving the non-linear programming problem. The network considered here is less general and consists of PFRs/CSTRs in series with one chemical reaction taking place; however, a simple methodology is reached. The cost of manufacture is a function of fixed capital investment, costs of utilities, operating labor, raw materials and water treatment [20]. The cost of raw materials is the same for the different selection and sequencing options, and the only costs that are expected to change significantly are the fixed capital investment and cost of utilities. Since intercooling or inter-heating between the adiabatic reactors is not included in the present work and is deferred to further investigations, the objective is reactor cost minimization. Guthrie’s cost correlation [21] is used to estimate the cost of the installed reactors. The objective is different from minimization of the total reactor volume [1, 4]. Both approaches are compared in the following cases: constant f(X) (example: isothermal zero-order reaction), increasing function f (X) (example: isothermal positive order reaction) and function f(X) showing a minimum (example: autocatalytic and adiabatic exothermic reactions).
2 COSTING FORMULATION
The volumes of the CSTR and PFR required to increase conversion from 0 to X are given by [1-3]

where subscripts C and P represent CSTR and PFR, and the following function f(X) is used to simplify the notations

The notations FA0andAr− denote the inlet molar flow rate to reactor and the rate of disappearance of the limiting reactant A, respectively. Levenspiel plots [1-3] are obtained by plotting f(X) versus conversion X.
Guthrie’s cost correlation [21] providing the installed reactor cost is used

where Fcis a factor accounting for pressure and material of construction and IM&Sis the Marshall and Swift equipment cost index.
Using the aspect ratio kR(R=C or P), defined as length (H) over diameter D (PFR case) or height H over D (CSTR case) along with the relation between volume V, H and D gives upon substitution into Eq. (4)

The plug flow reactor aspect ratio kPis obviously larger than kC.
3 REACTOR VOLUMES AND COSTS

Figure 1 Plots of f(X) versus X for Cases 1 (a), 2 (b) and 3 (c)

Table 1 PFR and CSTR volumes for specific functions f(X)
Figures 1-3 show different profiles for f(X) versus X. Fig. 1 (a) is a case in which f(X) is constant with a typical case of isothermal zero order reaction. Fig. 1 (b) is a more common case where f(X) increases with conversion X, corresponding for instance to the casewhere a decrease in reactant(s) concentration at constant temperature, which leads to smaller reaction rate and increasing f(X) with X. Fig. 1 (c) is typical for autocatalytic and adiabatic exothermic reactions [2].
For the purpose of calculations, more specific equations for f(X) need to be considered. They are shown in Table 1 along with the corresponding PFR and CSTR volumes. To simplify the analysis, costs are written in the following compact form:

4 ANALYSIS FOR SMALL CONVERSION X
Near X=0, PFR and CSTR volumes are given by Maclaurin series expansions

The ratio kC/kPis smaller than 1; therefore, a CSTR is less costly for small X. Typically, X is high and more analysis is required. In Eqs. (11)-(13), f(0) appears in the denominator. The case f(0)=0 cannot occur since the reaction rate cannot be infinite.
5 REACTORS SEQUENCING
The analysis close to X=0 suggests the first reactor is a CSTR.
5.1 PFR following CSTR
Whether a PFR following the CSTR provides a lower total cost is investigated using the total cost obtained as a function of the intermediate conversion Xi

Eq. (16) is a non-linear equation that has only one unknown Xiafter substituting for VP,iand VC,iusing Eq. (15). A better way is to plot the total cost CS=CC,i+CP,ifor a given final conversion X versus Xi, tackling a more general problem: global optimum and cases where the optimum is not in the interior of the interval for Xi.
Based on the volumes shown in Table 1, the results for the specific cases considered are as follows for a ratio kC/kPof 1.5/10 taken as an example.
Case 1
For f(X)=constant, the CSTR and PFR volumes are equal, and the CSTR cost is smaller. The two costs are given by
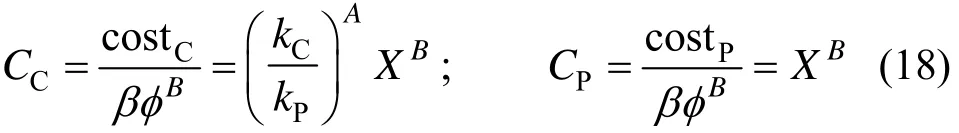
Obviously, no PFR following a CSTR is needed in this case. The results are shown in Fig. 2. For a constant reaction rate (Case 1), the CSTR cost is less compared to a PFR, while both require equal volumes for a given required conversion.

Figure 2 Plots of reactor cost versus X (Case 1)
Case 2
Using the volumes from Table 1, the reactor costs are given by

The results presented in Fig. 3 show that the cost of a PFR is higher than the cost of CSTR up to a conversion of about 0.61, which is in consistency with the local analysis in Section 4. A PFR is less costly at higher conversions. Using a CSTR followed by a PFR does not yield a lower reactor cost as seen from Fig. 3. For a decreasing reaction rate with conversion (Case 2), a PFR is less costly at sufficiently high conversion and a CSTR is more economic at lower conversion, whereas the volume minimization approach favors a PFR in all cases.
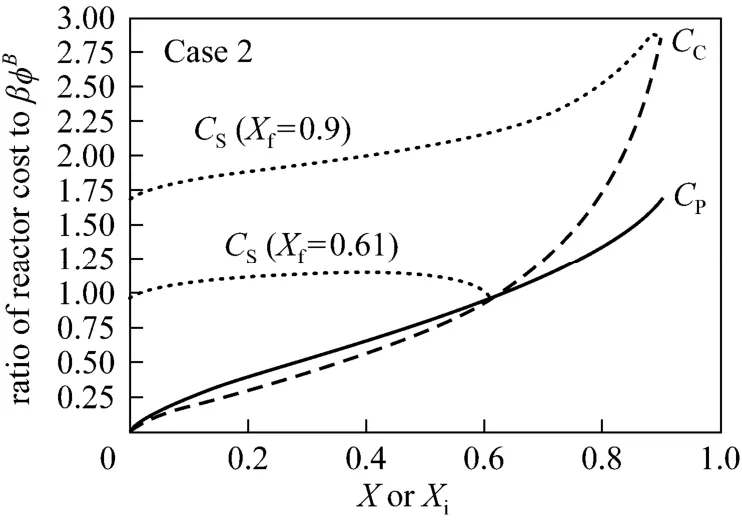
Figure 3 Plots of reactor cost versus X (Case 2)
Case 3

Using for simplicity a quadratic function of X, f(X) showing a minimum at Xm, to approximate autocatalytic and exothermic adiabatic reactions, it is possible to get an insight regarding the optimum sequencing features. From the reactor volumes given in Table 1, it is possible to get the costs as Using two values of ω, 1 (Xm=0.5) and 5 (Xm=0.1), along with a typical final conversion Xf=0.9 as an example, the results are shown in Fig. 4. Similar results to the ones for ω=1 are obtained when ω is changed to 2 (Xm=0.25), which indicates that minimum reactor cost is achieved by a PFR for sufficiently small Xmand by a CSTR for most values of ω (intermediate to large values of Xm).

Figure 4 Plots of reactor cost versus X (Case 3)
In the case where reaction rate increases up to X=Xmand then decreases with conversion (Case 3) approximated by a quadratic function, a CSTR is less costly in most cases. However when Xmis sufficiently small, a PFR is more economic. The results are different from the one using minimum volume as the criterion, leading to the following selection: a CSTR achieving an intermediate conversion of Xm, followed by a PFR to reach the final conversion [1, 4]. Using PFRs in series does not change the total volume required [1], but the cost is higher. For another subcase of interest in Case 3, when a CSTR is a better option than a PFR (Xmnot small), two CSTRs in series were found to enhance the reactor cost.
5.2 Multiple smaller reactors in series versus one larger reactor of the same type
Constant Rate or PFRs in Series
In this case, the volume of a reactor V achieving a given conversion is equal to the sum of the N reactors in series. This is well known in the case of PFRs in series, but it is also valid in the case of CSTRs in series if the reaction rate is constant. In both cases V is given by

where Vn(n=1, N) is the volume of the small reactor n.
The cost function of V being concave downward, we have

meaning that one large reactor is less costly than the Nreactors in series of the same type.
Non-Constant Rate and CSTRs in Series
Equation (23) is no longer valid and more analysis is required. For two CSTRs in series C1 and C2 achieving a final conversion X and an intermediate conversion Xi, the total cost is

The results are shown in Fig. 5 for a selected final conversion Xfof 0.9 and Xm=0.5 (ω=1), showing clearly that one CSTR is less costly than two CSTRs in series.
6 CONCLUSIONS
Using Guthrie’s cost correlation, it has been shown that minimizations of the total reactor volume and installed cost do not necessarily concur for the three typical cases considered. When space is a concern, minimizing the total volume can be considered as a proper objective, otherwise, minimum cost can be considered as a more appropriate target.
Temperature effect on reaction rate is embedded in function f(X). However, when the reactors are not adiabatic the coolers or heaters in the cost analysis require additional theoretical analysis to account for the additional cooling/heating cost.
NOMENCLATURE
Superscripts

Subscripts

REFERENCES
1 Levenspiel, O., Chemical Reaction Engineering, Wiley, New York (1972).
2 Villermaux, J., Chemical Reaction Engineering—Conception and Operation Reactors, TEC & DOC-Lavoisier, Paris, France (1993).
3 Fogler, H.S., Elements of Chemical Reaction Engineering, Prentice Hall, Upper Saddle River, NJ (2006).
4 Chebbi, R., “Chemical reactors sequencing”, Computer Applications in Engineering Education, DOI: 10.1002/cae.20545 (2011).
5 Smith, R., Chemical Process Design and Integration, Wiley, Chichester (2005).
6 Kokossis, A.C., Floudas, C.A., “Optimization of complex reactor networks: Isothermal operation”, Chemical Engineering Science, 45, 595-614 (1990).
7 Kokossis, A.C., Floudas, C.A., “Optimization of complex reactor networks: Nonisothermal operation”, Chemical Engineering Science, 49, 1037-1051 (1994).
8 Horn, F., “Attainable regions in chemical reaction technique”, In: the Third European Symposium on Chemical Reaction Engineering, Pergamon Press (1964).
9 Burri, J.F., Wilson, S.D., Manousiouthakis, V.I., “Infinite dimensional state-space (IDEAS) approach to reactor network synthesis: Application to attainable region construction”, Computers & Chemical Engineering, 26, 849-862 (2002).
10 Zhou, W., Manousiouthakis, V.I., “Variable density fluid reactor network synthesis construction of the attainable region through the IDEAS approach”, Chemical Engineering Journal, 129, 91-103 (2007).
11 Bedenik, N.I., Ropotar, M., Kravanja, Z., “MINLP synthesis of reactornetworks in overall process schemes based on a concept of time-dependent economic regions”, Computers & Chemical Engineering, 31, 657-676 (2007).
12 Zhou, W., Manousiouthakis, V.I., “Global capital/total annualized cost minimization of homogeneous and isothermal reactor networks”, Industrial & Engineering Chemistry Research, 47, 3771-3782 (2008).
13 Kokossis, A.C., Floudas, C.A., “Synthesis of isothermal reactorseparator-recycle Systems”, Chemical Engineering Science, 46, 1361-1383 (1991).
14 Luyben, M.L., Luyben, W.L., “Design and control of a complex process involving two reaction steps, three distillation columns, and two recycle streams”, Industrial & Engineering Chemistry Research, 34, 3885-3898 (1995).
15 Bedenik, N.I., Pahor, B., Kravanja, Z., “An integrated strategy for the hierarchical multilevel MINLP synthesis of overall process flowsheets using the combined synthesis/analysis approach”, Computers & Chemical Engineering, 28, 693-706 (2004).
16 Chen, Q., Feng, X., “Reactor network synthesis for waste reduction using instantaneous value of environmental index”, Chinese Journal of Chemical Engineering, 16 (1), 155-158 (2008).
17 Labrador-Darder, C., Cecelja, F., Kokossis, A.C., Linke, P., “Integration of superstructure-based optimization and semantic models for the synthesis of reactor networks”, Computer Aided Chemical Engineering, 26, 865-870 (2009).
18 Khajehpour, M., Farhadi, F., Pishvaie, M.R., “Reduced superstructure solution of MINLP problem in refinery hydrogen management”, International Journal of Hydrogen Energy, 34 (22), 9233-9238 (2009).
19 Jin, S., Li, X., Tao, S., “Globally optimal reactor network synthesis via the combination of linear programming and stochastic optimization approach”, Chemical Engineering Research and Design, 90 (6), 808-813 (2012).
20 Turton, R., Baillie, R.C., Whiting, W.B., Shaeiwitz, J.A., Analysis, Synthesis, and Design of Chemical Processes, Prentice Hall, New Jersey (2003).
21 Douglas, J.M., Conceptual Design of Chemical Processes, McGraw-Hill, New York (1988).
2012-06-24, accepted 2013-01-20.
* To whom correspondence should be addressed. E-mail: rchebbi@aus.edu
 Chinese Journal of Chemical Engineering2014年6期
Chinese Journal of Chemical Engineering2014年6期
- Chinese Journal of Chemical Engineering的其它文章
- Roles of Biomolecules in the Biosynthesis of Silver Nanoparticles: Case of Gardenia jasminoides Extract*
- Modeling and Optimization for Short-term Scheduling of Multipurpose Batch Plants*
- Symbiosis Analysis on Industrial Ecological System*
- Phase Behavior of Sodium Dodecyl Sulfate-n-Butanol-Kerosene-Water Microemulsion System*
- Photochemical Process Modeling and Analysis of Ozone Generation
- Unified Model of Purification Units in Hydrogen Networks*
