Fe-N/C-TsOH催化剂应用碱性介质催化氧还原的电催化活性
徐 莉 潘国顺,* 梁晓璐 罗桂海 邹春莉 罗海梅
(1清华大学摩擦学国家重点实验室,北京100084;2深圳清华大学研究院深圳市微纳制造重点实验室,广东深圳518057)
1 Introduction
In response to increasing awareness of environmental pollution and limiting energy source,great effort has been made worldwide to generate power in more efficient and environmental friendly ways.Fuel cells have attracted significant attention as a promising technology due to their advantages such as high energy density,high power density,and high energy conversion efficiency,as well as their low or zero emission.1However,there are two fundamental catalytic challenges remained at our current technology,including prohibitive cost and inadequate durability,hampered the commercialization of fuel cells.With respect to the high cost,Pt-based catalysts which have been regarded as the most electrocatalyst for oxygen reduction reaction(ORR)contributes over 50%of the total cost of a fuel cell stack.2One solution to overcome this predicament is to reduce the Pt content by a factor of ten by replacing the Pt-based catalysts with non-precious metal catalysts at the oxygen-reduction cathode.Fe-and Co-based electrocatalysts(M-Nx/C)for this reason have been developed for more than 50 years,but they were insufficiently active for the high efficiency and power density needed for applications such as stationary,portable,and automobile power supplies.3,4However,several breakthroughs occurred have improved the activity and durability of those kinds of catalysts,which can now be treated as potential competitors to Pt-based catalysts.
In addition,it has been recognized that the nitrogen sources in the catalyst precursors during the M-Nx/C synthesis play a crucial role in ameliorating ORR activity as well as stability.And this is the reason why several different types of nitrogencontaining macrocycles,inorganic salts,and organometallic compounds have been employed as the precursors to form metalnitrogen complexes.5-11
Polypyrrole(PPy),a conducting polymer with many pyrroletype N atoms,altering surface,and easy preparation and doping,has been widely used for the synthesis of M-Nx/C since it was first investigated by Yuasaet al.12in 2005.Yuasaet al.found that Co-PPy/C ORR activity enhanced after being pyrolyzed for the cobalt site forming four coordinate bonds with the nitrogen of the PPy rings(Co-N4).Bashyam and Zelenary3deposited PPy on carbon black to form a catalyst support(PPy/C)viaan oxidative polymerization process using hydrogen peroxide.After impregnating cobalt ions,a carbon-supported cobalt catalyst(Co-PPy/C)was generated.Research showed that sodium borohydride as reductive agent could improve the catalyst activity and stability and Co-N2may be active site.
Furthermore,thermal-treatment has been recognized as a crucial role and sometimes necessary step to further improve the activity and stability.Although the heat-treatment effect on catalysts has been well documented,the mechanisms of the catalyst reaction during the heat-treatment process and the resulting improvement in activity are complicated and not fully understood.Reviewing many papers,thermal activation has significant impact on the metal particle size and size distribution,particle surface morphology,and metal dispersion on the support for such catalyst.Other benefits of heat treatment are to remove any undesirable impurities and allow a uniform dispersion and stable distribution of the transition metal on the support,and,therefore,to improve the electrocatalytic activity.13In addition,when the catalysts are pyrolyzed at a desired high temperature in a flowing inert atmosphere(nitrogen or argon),M-N precursor is partially or completely decomposed,resulting in a catalyst with much better catalytic activity and stability than a untreated catalyst.14
To further improve the activity,dual-doped carbons with two different heteroatoms become one promising option for ORR by taking advantage of different heteroatoms in conjugated carbon backbone that can create new non-electron-neutral sites.In particular,the sulfur(or sulfo group),which has a close electronegativity to carbon,has been employed as a dualdopant in the preparation of M-Nxcatalysts.It was thought that the sulfur group doped to the M-Nx/C might be helpful for entrapping M ions in an environment rich in pyrrole-type nitrogen or pyridine-type nitrogen.For instance,N/S co-doped Vulcan XC-72R has been demonstrated to show superior ORR performance with excellent activity9and durability.14
Based on the above conception,p-toluenesulfonic acid(TsOH)and polypyrrole were used as dual-dopant(S and N)to synthesize a novel non-precious metal catalyst based on carbon-supported ferrum,polypyrrole,and TsOH complex(Fe-N/CTsOH).In particular,TsOH,an organic compound,is known as tosyl group and not only was used as the S precursor,but also used as an“organic-soluble”acid catalyst to promote the oxidation of pyrrole.And a comparative study was carried out through the systematic analyses of Fe-N catalysts prepared without and with TsOH dopant by cyclic voltammeters(CVs),rotating disk electrode technique(RDE)in oxygen-saturated alkaline solutions,and scanning electron microscope(SEM),X-ray diffraction(XRD),and X-ray photoelectron spectroscopy(XPS)are used to characterize these catalysts in terms of their structures and compositions.
2 Experimental
2.1 Preparation of carbon black-supported Fe-N-S catalyst
For preparation of carbon black supported Fe-N-S catalyst(Fe-N/C-TsOH),120 mg carbon black(Vulcan XC-72R with a BET surface area of 235 m2·g-1,purchased from Cabot)dispersed in 10 mL methanol(analytically pure)and then added 50 mg pyrrole(chemically pure),followed by 10 min of ultra-sonication.0.25 mL 30%H2O2and 50 mg TsOH(analytically pure,purchased from Guoyao)were added into this ultrasonication suspension while milling in a mortar for some time to obtain a slurry.10 mL methanol with 149 mg FeSO4·7H2O(analytically pure)was added into the mortar while followed by constant grinding for another 45 min,which was then dried in a vacuum at 60°C for 1 h to obtain a powder.This powder was further processed by thermal treatment under N2atmosphere at 200,400,600,and 700°C,respectively,for 2 h to optimize the heat-treatment temperature with respect to ORR electrolytic activity,forming the final carbon-supported Fe-N-S catalyst.To elucidate the effect of TsOH alone,a baseline sample of carbon loaded with TsOH-free Fe-N was also prepared under the same conditions depicted above,and is named as Fe-N/C-None in this paper.
2.2 Electrochemical measurements
Electrochemical measurements were conducted using a rotating glassy carbon disk electrode(glassy carbon electrode with a geometric surface area of 0.19625 cm2,purchased from Gamry Instruments).According to the electrode preparation method described by Qiaoet al.,1410 μL of catalyst ink,which consists of 2.0 mg catalyst per mL,was pipetted onto the glassy carbon(GC)disk electrode.The loading of catalysts was 101 μg·cm-2.A saturated calomel electrode(SCE)and Pt wire were used as the reference and counter electrodes,respectively.All measured potentials were converted to the standard hydrogen electrode(SHE).
A 0.1 mol·L-1KOH aqueous solution was used as the electrolyte.For cyclic votammograms(CVs),the electrode was scanned at a scan rate of 50 mV·s-1in the potential range between-0.80 and 0.30 V to measure the surface behavior of the catalyst in N2-saturated KOH solution,and the ORR activity of the catalyst in O2-saturated KOH solutions,respectively.For more quantitative measurements of ORR activity,linear sweep voltammetry(LSV)was conducted on the catalyst-coated RDE in the potential range between-0.76 and 0.15 V in O2-saturated KOH solution at various rotation rates from 300 to 2100 r·min-1.All measured potentials(vsSCE)in this work were converted to the standard hydrogen electrode(SHE),as shown in the relevant text and figures.
2.3 Material characterization
The structure and phase analyses of the catalyst samples were performed using X-ray diffraction(XRD)with CuKαradiation(λ=0.15406 nm)and operating at 40 kV and 40 mA.The morphology and particle size of the catalyst samples were studied using a scanning electron microscope with a Bruker TESCAN.Surface characterization of the catalyst samples was conducted by X-ray photoelectron spectroscopy on a PHI Quantera Scanning X-ray MicroprobeTM5300 system(ULVAC-PHI.INC)with AlKX-ray anode source(hv=1486.6 eV)at 300 W and 15.0 kV.
3 Results and discussion
3.1 Electrochemical activity of Fe-N/C-TsOH catalysts towards ORR
CVs of the catalysts pyrolized at 600°C prepared without and with TsOH were measured in O2-saturated 0.1 mol·L-1KOH solutions with a scan rate of 50 mV·s-1and the results are presented in Fig.1.In this figure′s legends,“600H”indicated the sample pyrolyzed at 600°C.There are obvious redox peaks appeared for two catalysts with a characteristic reduction peak associated with the ORR suggesting that Fe-N/C catalyst has ORR activity regardless of whether they are decorated with TsOH or not.However,more excellent positive peak potential value and higher peak current density were obtained for Fe-N/C-TsOH-600H catalyst than those for Fe-N/C-None-600H catalyst,indicting a better ORR activity after TsOH-doping.That is to say,the addition of TsOH leads to a considerable activity enhancement of the catalysts relative to the TsOH-free catalyst,which makes us resolutely believe that it is the TsOH or sulfur that plays an important role in enhancing the electrocatalytic performance of carbon materials for the ORR.Yanget al.15confirmed that the dopant would break the electroneutrality of carbon materials because the different electroneutrality between carbon and dopant would create favorable positive charged sites for the side-on O2surface adsorption.Pyrolysis of the catalyst in the presence of sulfur could lead to amorphous carbon,resulting in an increased catalyst porosity and in turn enhanced catalyst performance.16Besides,larger capacitance current over the potential range for Fe-N/C-TsOH-600H indicated a larger catalyst surface area.
To further clarify the effect of thermal treatment on the ORR activity,the polarization curves were measured using RDE technique with the electrode rotation rate of 1500 r·min-1in O2-saturated 0.1 mol·L-1KOH solution for catalysts pyrolyzed from 200 to 700°C,with the unpyrolyzed catalyst sample(Fe-N/C-TsOH-RT)(“RT”means untreated or unpyrolyzed catalyst)for comparison.And the results are given in Fig.2.The pyrolyzed catalysts show better activities than the unpyrolyzed catalyst.There is general agreement in the literature that a heattreatment step has beneficial effects on the activity.11,13,14,17And the optimal treating temperature is 600°C.The ORR onset potential(Eonset)and the potential at the current density of-1.5 mA·cm-2for Fe-N/C-TsOH-600H are 30 and 170 mV,respectively,more positive than Fe-N/C-TsOH-RT.In addition,the diffusion-limiting current for the Fe-N/C-TsOH-600H is also slightly higher than the rest catalysts and shows well-defined plateaus which means that the ORR kinetics catalyzed by Fe-N/C-TsOH-600H is fast enough to exhaust the O2concentration at the electrode surface.When the thermal temperature is below 600°C,with increasing heat-treatment temperature,more Fe-Nxactive sites could be produced.However,when temperature is higher than 600 °C,such as 700 °C,not only the structure of the material was collapsed under exceedingly high temperature,which results in the material morphology obviously changed,but also the undesired formation of secondary species can be increased,which could lead to a reduced concentration of Fe-Nxmoieties on the catalyst surface,as shown in the following SEM images and XRD patterns.

Fig.1 CVs of catalysts doped with and without TsOH in O2-saturated 0.1 mol·L-1KOH solution
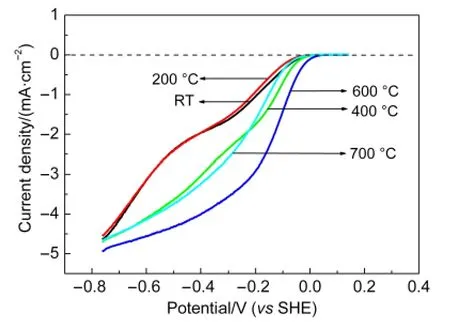
Fig.2 Polarization curves for Fe-N/C-TsOH catalysts pyrolyzed at different heat-treatment temperatures
For a more quantitative evaluation of the ORR catalytic activity of the catalysts developed in our work,RDE voltammetry measurements were also carried out at different electrode rotation rates(ω)from 300 to 2100 r·min-1,which was demonstrated in Fig.3.The parameters obtained about the two catalysts are listed in Table 1,The difference in diffusion-limiting currents between Fe-N/C-TsOH-600H and Fe-N/C-None-600H may suggest that the ORR mechanisms catalyzed by these catalysts are different,particularly in terms of the overall electron transfer number.The ORR catalyzed by pyrolyzed Fe-N/C-TsOH may have more electron numbers(or fewer two-electron processes for peroxide production)than that of the pyrolyzed Fe-N/CNone.The overall electron transfer number(n)during the ORR can be evaluated from the Koutecky-Levich equation.1The peroxide production yield(y,%)is calculated from the ring-disk measurements according to the following equation:18,19

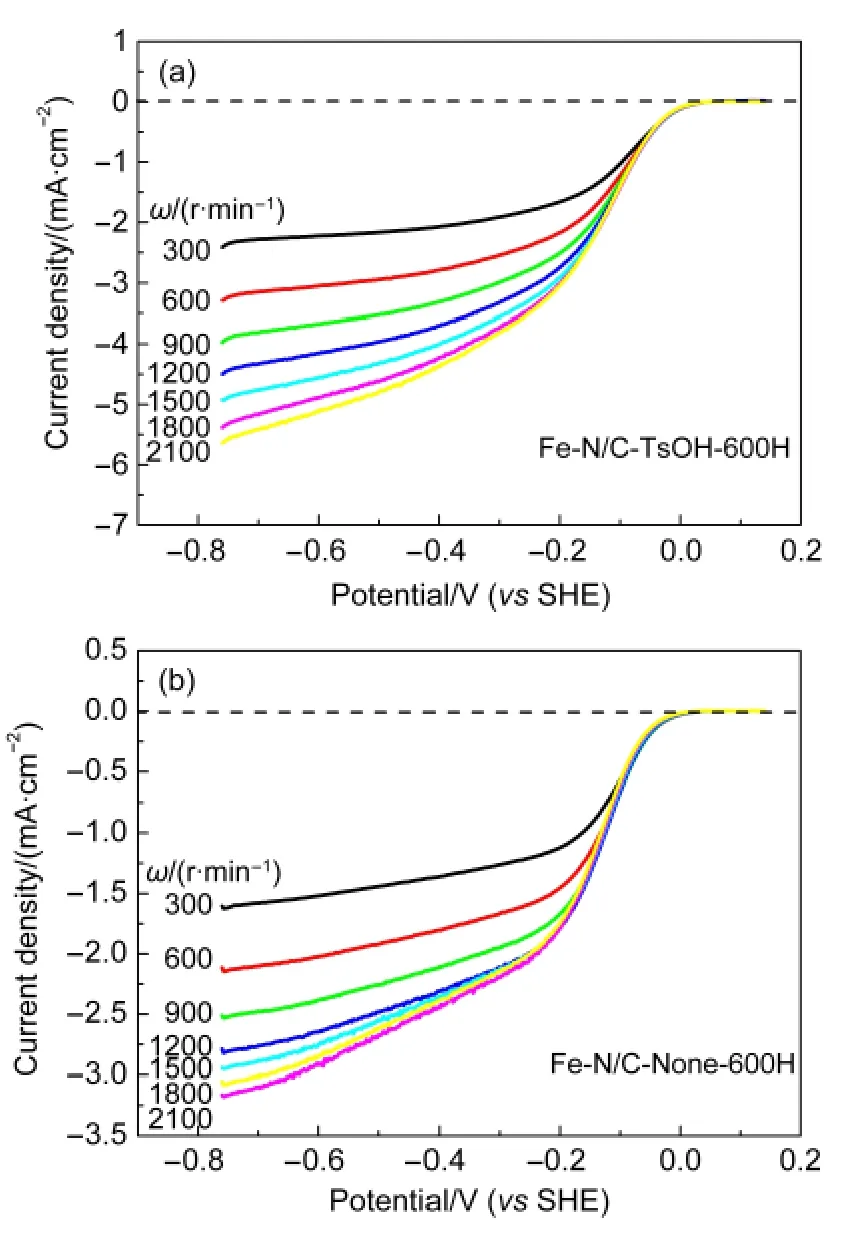
Fig.3 Polarization curves for ORR on Fe-N/C-TsOH-600H and Fe-N/C-None-600H catalysts in O2-saturated 0.1 mol·L-1KOH electrolytes at various rotation rates
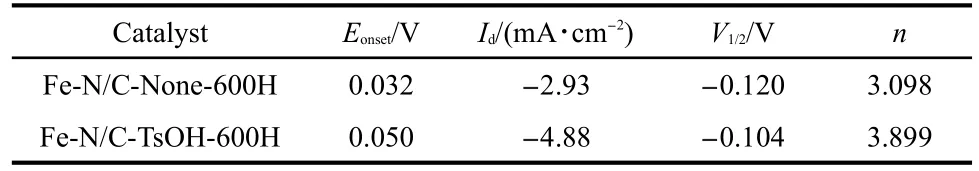
Table 1 Comparison of electrochemical data for Fe-N/C-TsOH-600H and Fe-N/C-None-600H catalysts
where,jis the current density at specific potential,such as-0.6 V,-0.5 V,etc,jdis the disk ORR current density,jkis the kinetic current density,nis the overall number of electrons transferred per molecule of O2reduced,Fis Faraday′s constant(F=96485 C·mol-1),COis the concentration of oxygen dissolved(1.1×10-6mol·cm-3),DOis the diffusion coefficient of O2in the bulk solution(1.9×10-5cm2·s-1),andνis the kinetic viscosity of the solution(1.0×10-2cm2·s-1).The Koutecky-Levich plots are shown in Fig.4.Based on those plots,we can see that:the averagenvalues were determined to be 3.098 for Fe-N/CNone-600H,while 3.899 for Fe-N/C-TsOH-600H,which is very close to 4,suggesting that the catalysts doped with TsOH have a higher overall ORR electron number than the catalysts without TsOH,indicating a very difference in the mechanisms catalyzed by these two catalysts.Accordingly,the percentageof H2O2produced in the potential range for Fe-N/C-TsOH-600H was less than 15%,while 50%for Fe-N/C-None-600H.In other words,the ORR catalyzed by Fe-N/C-TsOH-600H is a 4-electron transfer process from O2to H2O,whereas Fe-N/CNone-600H catalysts generally tend to catalyze the ORR through a(2+2)-electron pathway,producing H2O2,which is capable of oxidizing and splitting active sites.
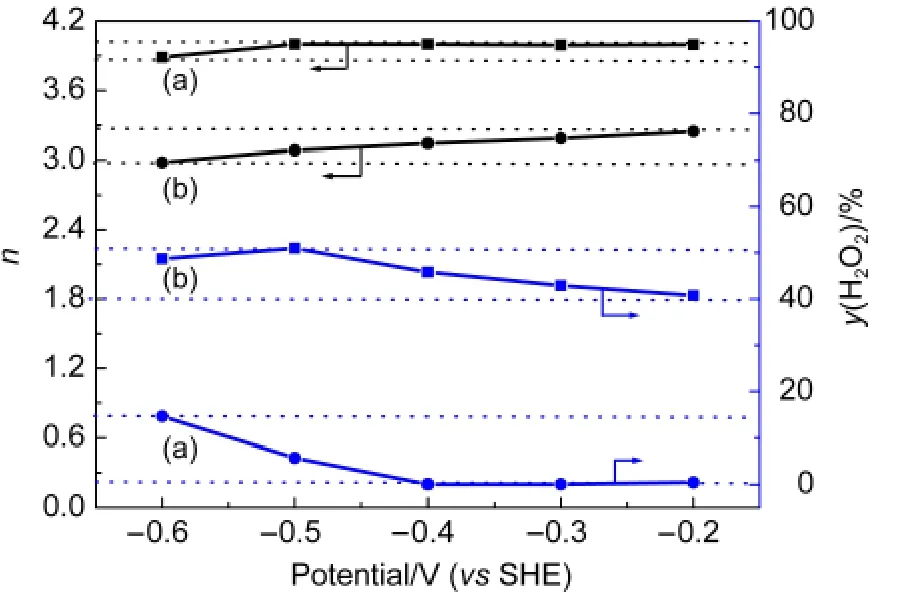
Fig.4 Overall electron transfer number(n)and the amount of peroxide produced during the ORR on the disk electrode and calculated by Eq.(3)for(a)Fe-N/C-TsOH-600H and(b)Fe-N/C-None-600H catalysts in O2-saturated 0.1 mol·L-1 KOH at the potentials between-0.6 and-0.2 V
3.2 Morphology and structure of the prepared catalysts
The morphologies of the samples were examined by SEM.As displayed in Fig.5,we can see that the temperature of thermal treatment has a direct influence on the morphology and crystal structure of the catalysts.In our study,the catalyst without thermal treatment shows some perfect carbon spheres with a smooth surface and a narrow diameter range from 40 to 200 nm(Fig.5(a)).After being heat-treated at 600°C,the shape of the materials looks like deformed spheres and the aggregate size decreases.Whatʹs more,there are many small pores on the surfaces of the particles,which help to increase BET surface area of the materials(Fig.5(b)).For the catalyst sample pyrolyzed at 700°C,the material morphology has obviously changed,not only the diameter of the sample increased,but also the pores on the surface of the particle decreased(Fig.5(c)).It confirmed that the structure of the material was collapsed under exceedingly high temperature.
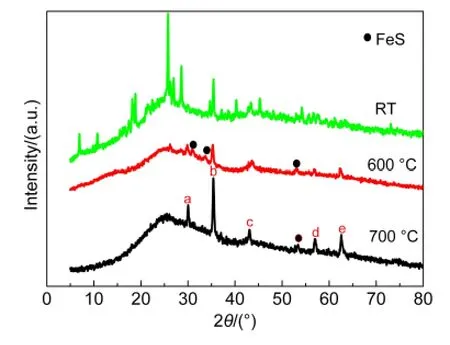
Fig.6 XRD patterns of non-pyrolyzed and pyrolyzed Fe-N/C-TsOH catalysts at 600 and 700°C
In order to make clear of pyrolysis effect on the ORR activity,Fe-N/C-TsOH-RT,Fe-N/C-TsOH-600H,and Fe-N/C-TsOH-700H samples were chosen for further XRD measurements.The representative diffractograms are shown in Fig.6.A large broad peak located at about 2θ=25°in all the XRD patterns is assigned to the(002)carbon planes of the carbon support with a remarkable disordered structure.From Fig.6,it can be seen that Fe-N/C-TsOH-RT shows some quite strong diffraction peaks due to the crystalline nature of FeSO4·7H2O.However,those peaks disappeared after heat treatment.Instead,some additional diffraction signals were formed at 600 and 700°C,which can be due to the generation of metallic iron(α-Fe),iron carbide(Fe3C),and iron oxide like magnetite(Fe3O4)as well as FeS.These results indicate that the structure of the Fe(II)-PPy precursor complex may have decomposed during the heat-treatment process.And those species could be a kind of ORR active sites with much less activity than that expected for Fe-Nxsites.It is noted that the diffraction signals for Fe-N/C-TsOH-700H are much stronger and sharper than those for Fe-N/C-TsOH-600H.Due to the formation of these less ORR activity species,the quantity of ORR active Fe-Nxactive sites would be reduced,resulting in a less ORR activity of the Fe-N/C-TsOH-700H.20
The chemical nature of Fe-N/C-TsOH was further investigated by XPS analysis.The N 1sand S 2pcore levels of the catalyst material were recorded for both the unpyrolyzed and pyro-lyzed Fe-N/C-TsOH samples.As a typical candidate,the catalyst pyrolyzed at 600°C was selected as the target analysis material.Fig.7(a,b)shows the N 1sbinding energy region,where the peaks of N 1sat 398.9 and 400.5 eV can be attributed to pyridinic-N and pyrrolic-N(or pyridone-N),respectivity.8,21It should be mentioned that pyridinic-N is a type of nitrogen that contributes onep-electron to the aromaticp-system and has a lone electron pair in the plane of the carbon matrix.The pyridinic-N can be found on the edge of a carbon plane and a carbon vacancy.Since the pyridinic-N has a lone electron pair in the plane of the carbon matrix,this can increase electrondonor property of the catalyst,and thus weaken the O―O bondviathe bonding between oxygen and nitrogen and/or the adjacent carbon atom,and facilitate the reduction of oxygen.In other words,the pyridinic N species may have converted the ORR reaction mechanism from a 2e-dominated process to a 4e-dominated process and improved the ORR onset potential.While pyrrolic-N atoms are incorporated into five-membered heterocyclic rings,where each N atom is bonded to two carbon atoms and contributes twop-electrons to theπsystem.Although some progresses toward the identity and role of the electrocatalytic active center have been made,the precise relationship between catalytic activity and nitrogen species,is still unclear.22From the Fig.7(a,b),we know that increasing the thermal treatment temperature transforms more of the pyridinic nitrogen to pyrrolic nitrogen,where Fe-N/C-TsOH heat-treated at 600°C has larger fraction of the pyrrolic nitrogen groups compared to the pyridinic nitrogen groups,and it shows the higher activity than the catalyst unpyrolyzed as CVs and RDE results showed(Fig.1 and Fig.2).This indicates that the pyrrolic nitrogen group is more active for oxygen reduction and the catalyst pyrolyzed at 600°C has more active sites(pyrrolic nitrogen)to facilitate oxygen adsorption.The result agrees well with the reports that pyrrolic nitrogen is responsible for the unique activity of Fe-containing catalysts which was claimed earlier.23In addition,the preferred protonation of pyridinic sites may explain why higher heat-treatment temperature,which leads to more pyrrolic nitrogen and less pyridinic nitrogen,24results in more stable catalysts.25Fig.7(c)shows the S 2pXPS spectra measured for unpyrolyzed catalyst samples.It can be seen that the catalyst without thermal treatment shows a large band at 167.0-171.0 eV,which could be assigned to sulfate in catalyst precusor.14,16After pyrolyzed at 600°C,the deconvolution of the S signals gave two bands with binding energies of 163.8 and 168.8 eV,as shown in Fig.7(d).These peaks could be attributed to the binding sulfurs in C―Sn―C(n=1,2)bonds and oxidized―SOn―bonds,which are expected to occur at the edges of carbon.26,27Guoet al.28found that―C―S―C―plays the key role in promoting the ORR.The fact may explain the result that the catalyst pyrolyzed at 600°C shows better catalytic activity for the ORR as demonstrated in this work.It is believed that pyrolysis of the catalyst in the presence of sulfur could lead to amorphous carbon,resulting in an increased catalyst porosity and in turn enhanced catalyst performance.16,29,30Besides,sulfur group doped into the M-Nx-C might be helpful for entrapping M ions in an environment rich in pyrrole-type nitrogen.Thus,it was surmised that not only nitrogen doping but also sulfur doping of carbon play a key role in im-proving electrocatalytic activity of the ORR.
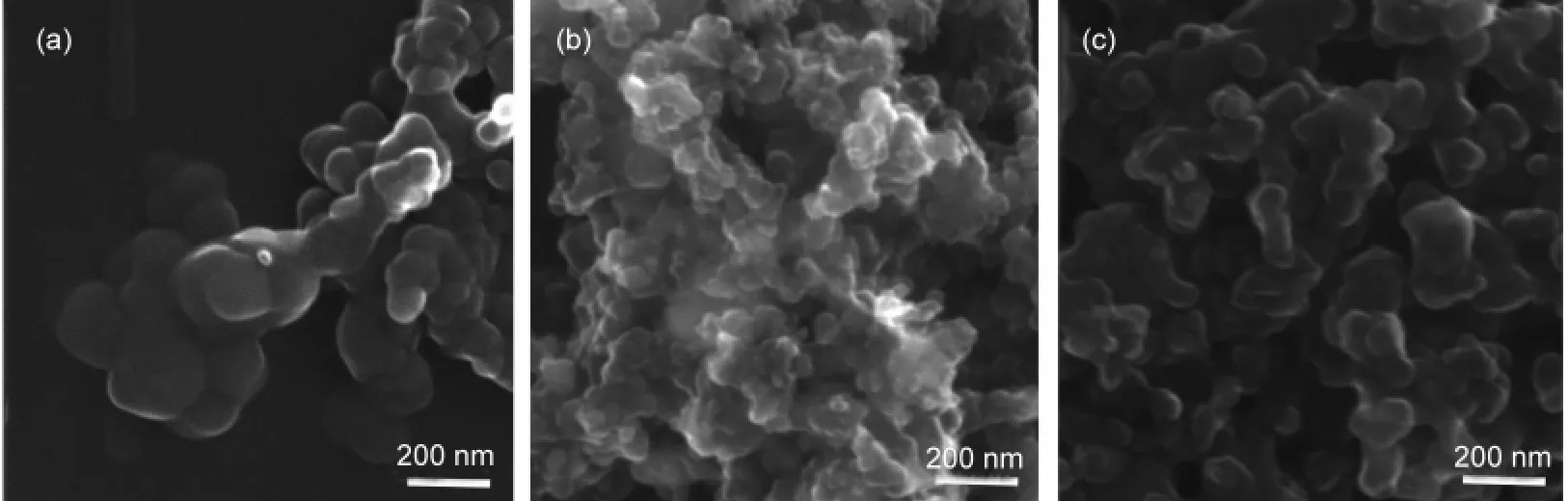
Fig.5 SEM images of Fe-N/C-TsOH(a)without thermal treatment,(b)pyrolyzed at 600 °C,and(c)pyrolized at 700 °C

Fig.7 XPS spectra of deconvoluted(a,b)N 1s and(c,d)S 2p for Fe-N/C-TsOH catalyst
4 Conclusions
In short,non-noble metal Fe-N/C electrocatalysts were prepared without and with TsOH dopant.The dual-doped catalysts show better catalytic activity for the oxygen reduction in alkaline solution than the catalysts doped only with N in terms of on-set potential,half-wave potential,as well as limited current potential values.The percentage of H2O2produced in the potential range for Fe-N/C-TsOH were less than 15%,while 50%for Fe-N/C-None,indicating a difference in the mechanisms catalyzed by these two catalysts.Furthermore,the catalytic activity strongly depended on the thermal temperature for the catalyst synthesis,and the best ORR performance was obtained at 600°C.In particular,Eonsetand the potential at the current density of-1.5 mA·cm-2for Fe-N/C-TsOH-600H are 30 and 170 mV more positive than Fe-N/C-TsOH-RT,respectively.Instrumental analysis and the SEM,XRD,and XPS results all showed that most of the active sites were formed after the samples were pyrolyzed at 600°C.
(1) Qiao,J.L.;Xu,L.;Ding,L.;Shi,P.H.;Zhang,L.;Baker,R.;Zhang,J.J.Int.J.Electrochem.Sci.2013,8,1189.
(2) Kromera,M.A.;Joseck,F.;Rhodes,T.;Guernsey,M.;Marcinkoski,J.Int.J.Hydrog.Energy2009,34,8276.doi:10.1016/j.ijhydene.2009.06.052
(3) Bashyam,R.;Zelenary,P.Nature2006,443,63.doi:10.1038/nature05118
(4) Lee,K.;Zhang,L.;Lui,H.;Hui,R.;Shi,Z.;Zhang,J.Electrochim.Acta2009,54,4704.doi:10.1016/j.electacta.2009.03.081
(5) Baker,R.;Wilkinson,D.P.;Wilkinson,J.Electrochim.Acta2008,53,6906.doi:10.1016/j.electacta.2008.01.055
(6) Xu,Z.;Li,H.;Cao,G.;Zhang,Q.;Li,K.;Zhao,Z.J.Mol.Catal.A:Chem.2011,335,89.doi:10.1016/j.molcata.2010.11.018
(7) Ding,L.;Qiao,J.L.;Feng,X.;Zhang,J.;Tian,B.Int.J.Hydrog.Energy2012,37,14103.doi:10.1016/j.ijhydene.2012.07.046
(8) Li,X.;Liu,G.;Popov,B.N.J.Power Sources2010,195,6373.doi:10.1016/j.jpowsour.2010.04.019
(9) Qiao,J.;Xu,L.;Xu,P.;Shi,J.;Wang,H.Electrochim.Acta2013,96,298.doi:10.1016/j.electacta.2013.02.030
(10) Jaouen,F.;Goellne,V.;Lefèvre,M.;Herranz,J.Proietti,E.;Dodelet,J.P.Electrochim.Acta2013,87,619.doi:10.1016/j.electacta.2012.09.057
(11) Charreteur,F.;Ruggeri,S.;Jaouen,F.;Dodelet,J.P.Electrochim.Acta2008,53,6881.doi:10.1016/j.electacta.2007.12.051
(12)Yuasa,M.;Yamaguchi,A.;Itsuki,H.;Tanaka,K.;Yamamoto,M.;Oyaizu,K.Chem.Mater.2005,17,4278.doi:10.1021/cm050958z
(13) Hinds,G.Preparation and Characterisation of PEM Fuel Cell Electrocatalysts:a Review.InNPL Report DEPC-MPE 019;National Physical Laboratory:Teddington,Middlesex,United Kingdom,2005;p 10.
(14) Qiao,J.;Xu,L.;Ding,L.;Zhang,L.;Baker,L.;Dai,X.;Zhang,J.Appl.Catal.B:Environ.2012,125,197.doi:10.1016/j.apcatb.2012.05.050
(15)Yang,Y.;Jiang,S.;Zhao,Y.;Zhu,L.;Chen,S.;Wang,X.;Wu,Q.;Ma,J.;Ma,Y.;Hu,Z.Angew.Chem.Int.Edit.2011,50,7132.doi:10.1002/anie.v50.31
(16) Kramm,U.I.;Herrmann,I.;Fiechter,S.;Zehl,G.;Zizak,I.;Abs-Wurmbach,I.;Radnik,J.;Dorbandt,I.;Bogdanoff,P.ECS Trans.2009,25,659.
(17) Cheng,H.;Yan,W.;Scott,K.Fuel Cells2007,7,16.
(18) Paulus,A.U.;Schmidt,H.A.;Gasteiger,R.J.;Behm,R.J.Electroanal.Chem.2001,495,134.doi:10.1016/S0022-0728(00)00407-1
(19) Bezerra,C.W.B.;Zhang,L.;Lee,K.;Liu,H.;Zhang,J.;Shi,Z.;Marques,A.L.B.;Marques,E.P.;Wu,S.;Zhang,J.Electrochim.Acta2008,53,7703.doi:10.1016/j.electacta.2008.05.030
(20) Jaouen,F.;Dodelet.J.P.Electrochim.Acta2007,52,5975.doi:10.1016/j.electacta.2007.03.045
(21) Subramanian,N.P.;Li,X.;Nallathambi,V.;Kumaraguru,S.P.;Colon-Mercado,H.;Wu,G.;Lee,J.W.;Popov,B.N.J.Power Sources2009,188,38.doi:10.1016/j.jpowsour.2008.11.087
(22)Wang,H.;Maiyalagan,T.;Wang,X.ACS Catal.2012,2,781.doi:10.1021/cs200652y
(23)Wu,G.;Chen,Z.;Artyushkova,K.;Garzon,F.H.;Zelenay,P.ECS Trans.2008,16,159.
(24)Kundu,S.;Nagaiah,T.C.;Xia,W.;Wang,Y.;Dommele,S.V.;Bitter,J.H.;Santa,M.;Grundmeier,G.;Bron,M.;Schuhmann,W.;Muhler,M.J.Phys.Chem.C2009,113,14302.doi:10.1021/jp811320d
(25)Wu,G.;Artyushkova,K.;Ferrandon,M.;Kropf,J.;Myers,D.;Zelenay,P.ECS Trans.2009,25,1299.
(26) Bubnova,O.;Khan,Z.U.;Malti,A.;Braun,S.;Fahlman,M.;Berggren,M.;Crispin,X.Nat.Mater.2011,10,429.doi:10.1038/nmat3012
(27) Paraknowitsch,J.P.;Wienert,B.;Zhang,Y.;Thomas,A.Chem.Eur.J.2012,18,15416.doi:10.1002/chem.v18.48
(28)Wang,H.;Bo,X.;Zhang,Y.;Guo,L.Electrochim.Acta2013,108,404.doi:10.1016/j.electacta.2013.06.133
(29) Herrmann,I.;Kramm,U.I.;Radnik,J.;Fiechter,S.;Bogdanoff,P.J.Electrochem.Soc.2009,156,1283.doi:10.1149/1.3185852
(30) Grabke,H.J.;Moszynski,D.;Muller-Lorenz,E.M.;Schneider,A.Surf.Interface Anal.2002,34,369.
——纪念摩擦学创始人乔斯特博士诞生100周年

