Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid
LI Fu-An BAI Su-Zhen
LI Xing-Wua CHEN Quanb①
a (College of Chemistry and Chemical Engineering,Pingdingshan University, Pingdingshan 467000, China)
b (Chemical Engineering and Pharmaceutics School, Henan University of Science and Technology, Luoyang 471023, China)
1 INTRODUCTION
The construction of new supramolecular coordination polymers attracts fast growing interest in the fields of coordination chemistry, not only for the creation of various interesting structures, but also for the potential applications of new functional materials[1-5]. Tactical selection of the organic ligand is a key factor for designing metal-organic coordination polymers. In general, structural motifs of the coordination polymers are closely related to the geometry and the number of coordination sites provided by organic ligands, and some characteristics of the supramolecules are related to noncovalent interactions such as hydrogen bonding[6-7]interaction.Therefore, both structure of the organic ligand and the presence of noncovalent interactions are very important factors for the construction of supramolecular coordination polymers.
Since carboxylate groups can adopt different binding modes to construct exciting networks, organic aromatic polycarboxylate ligands have been extensively employed in the preparation of coordination polymers[8]. As an important family of multidentate O-donor ligands, 3-(4-hydroxyl-pyridinium-1-yl)phthalic acid (H2dpa)is a very good candidate for building supramolecular coordination polymers.Firstly, the abundant functional groups on this ligand can adopt various coordinate modes such as terminal monodentate, chelating one metal ion, bridging two metal ions in synsyn, syn-anti and anti-anti, and in a tridentate form to build interesting polymer architectures, as well as act as hydrogen-bond acceptor to give rise to supramolecular networks; Secondly, this organic ligand has a good water-solubility and is beneficial to investigate the nature of this ligand in hydrothermal conditions. Thirdly, only a few coordination polymers constructed from H2dpa have been reported by the literatures[9], and much work is still necessary to understand the coordination chemistry of H2dpa.
One good approach for the construction of coordination polymers is to perform reactions with mixed-ligands such as polycarboxylate and N-containing ligands in the same system, and a great number of new intriguing structures have been obtained[10],which indicates that the introduction of N-containing auxiliary ligands into a metal-polycarboxylate system often leads to structural changes and affords new networks. From this point of view, we focus on the self-assembly of Ni(II)and carboxylate incorporating bpe as an auxiliary ligand to intrigue structure and obtained a new metal-organic framework,{[Ni(dpa)(bpe)(H2O)]2}n(1), and its thermal analysis and UV-vis absorption spectra have also been investigated. In this paper, we should report the coordination polymer 1.

2 EXPERIMENTAL
2. 1 Materials and measurements
3-(4-Hydroxypyridinium-1-yl)phthalic acid was synthesized according to the literature[11]. The single crystal of complex 1 was obtained through hydrothmaerl method in a Teflon-lined stainless steel container. Other starting materials were of reagent grade and obtained commercially without further purification. Elemental analyses for C, H and N were performed on a Perkin-Elmer 240 elemental analyzer. Thermal analysis was carried out on a SDT 2960 thermal analyzer from room temperature to 800 ℃ at a heating rate of 20 ℃/min under nitrogen flow. The FT-IR spectra were recorded from KBr pellets in the range of 4000~400 cm−1on a Nicolet NEXUS 470-FTIR spectrometer. Solid UV-visible spectra were obtained in the 200~800 nm range on a JASCO UVIDEC-660 spectrophotometer.
2. 2 Synthesis of complex 1
Complex 1 was synthesized hydrothermally in a Teflon-lined stainless steel container by heating a mixture of H2dpa (0.0130 g, 0.05 mmol), bpe(0.0091 g, 0.05 mmol), Ni(NO3)2·6H2O (0.0145 g,0.05 mmol)and KOH (0.0056 g, 0.1 mmol)in 7 mL of distilled water at 150 ℃ for 3 d. Subsequent cooling to room temperature yielded block crystals of 1 (75% yield based on nickel). C25H19N3NiO6(516.14): calcd. (%)C, 58.17; H, 3.71; N, 8.14.Found (%): C, 58.25; H, 3.64; N, 8.23. IR/cm−1(KBr): 3427(m), 2924(w), 1637(s), 1607(s), 1584(s),1559(s), 1384(s), 1339(w), 1285(w), 1191(m), 1114(s), 893(w), 839(w), 618(w).
2. 3 Crystallographic data collection and structure determination
Single-crystal X-ray diffraction data of 1 were collected on a Bruker Smart Apex CCD diffractometer[12]equipped with graphite-monochromatized MoKα radiation (λ = 0.71073 Å)at room temperature using the ω-scan technique. Empirical absorption corrections were applied to the intensities using the SADABS program[13]. The structure was solved by direct methods using SHELXS-97[14]computer program and refined by full-matrix least-squares methods on F2with the SHELXL-97[15]program package. All non-hydrogen atoms were subjected to anisotropic refinement. The hydrogen atoms of organic ligands and the coordination water mole-cules were included in the structure factor calculation at the idealized positions using a riding model and refined isotropically. The selected bond distances and bond angles for complex 1 are listed in Table 1.
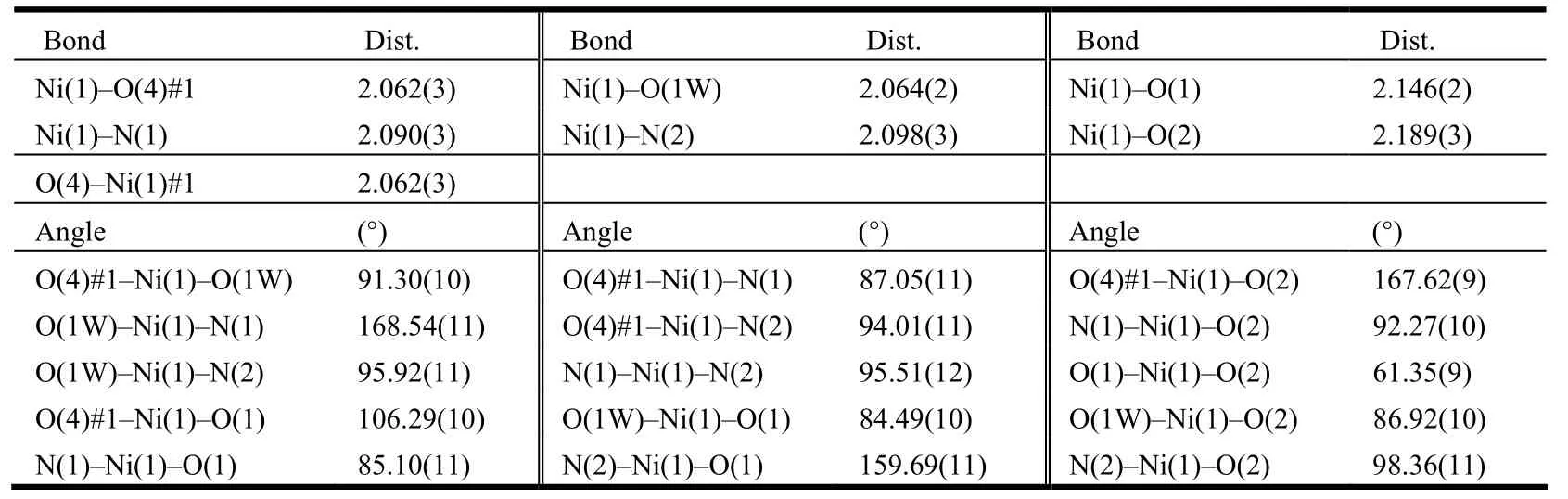
Table 1. Selected Bond Lengths (Å)and Bond Angles (°)
3 RESULTS AND DISCUSSION
3. 1 Crystal structure description
In 1, {[Ni(dpa)(bpe)(H2O)]2}n, the asymmetric unit consists of one Ni center, one bpe (two one-half BPE)molecule, one coordination water and one completely deprotonated dpa2−anion (Fig. 1). As illustrated in Fig. 2, the coordinated environment around the Ni(II)center can be described as a distorted octahedron coordination geometry [NiN2O4],where each Ni ion is six-coordinated by three carboxylate oxygen atoms (O(1), O(2)and O(4#1))from two different dpa2−ligands, one coordinated water molecule (O(1W)and two nitrogen atoms(N(1)and N(2))from two different bpe ligands. The Ni−N bond lengths are 2.090(3)and 2.098(3)Å, and the Ni−O bond lengths fall in the range of 2.062(3)~2.189(3)Å, which are in good agreement with those reported in other Ni(II)complexes with N,O-mixed ligands[16]. As shown in Fig. 2, the carboxylate groups adopt the chelating bidentate and monodentate coordination modes to connect the two Ni ions and form a dinuclear unit with the Ni··Ni distance about 5.267(2)Å. The dinuclear units are linked by the bpe ligands to give rise to a metal-organic layer (Fig. 3).
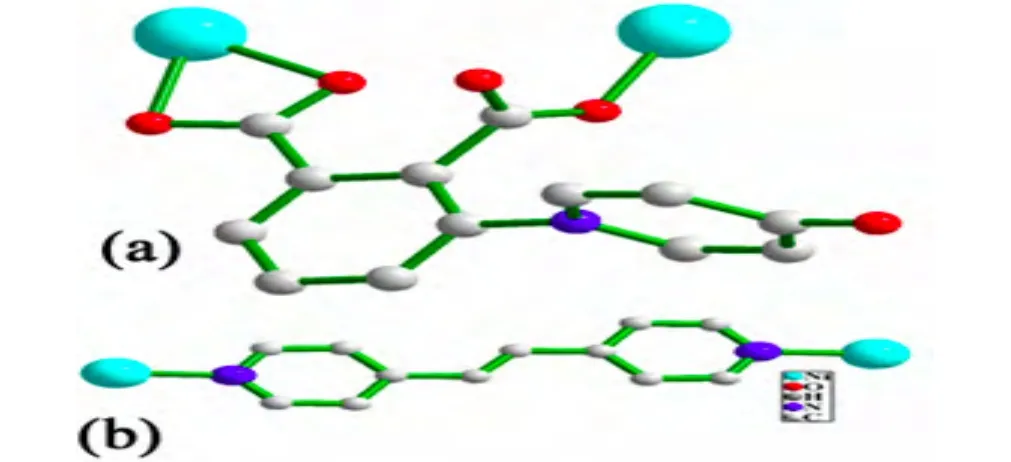
Fig. 1. (a)Coordination modes of dpa2- ligand. (b)Coordination modes of the bpe ligand. All of the hydrogen atoms are omitted for clarity
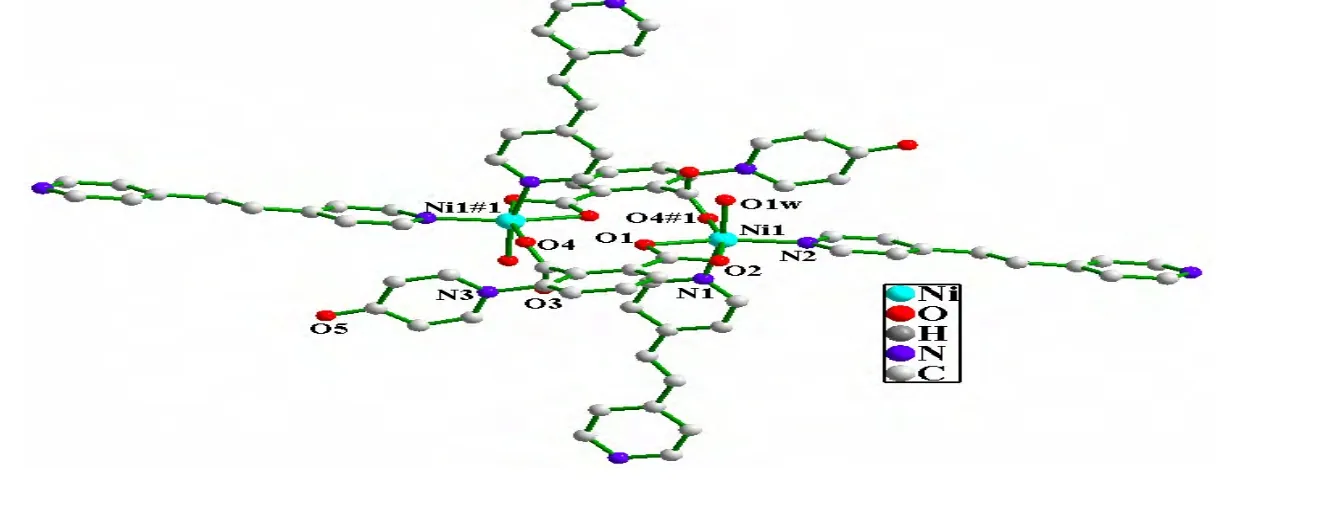
Fig. 2. Coordination environment of Ni(II)ion and the binuclear structure unit in complex 1.The hydrogen atoms are omitted for clarity. Symmetric code: #1: –x+1, –y+2
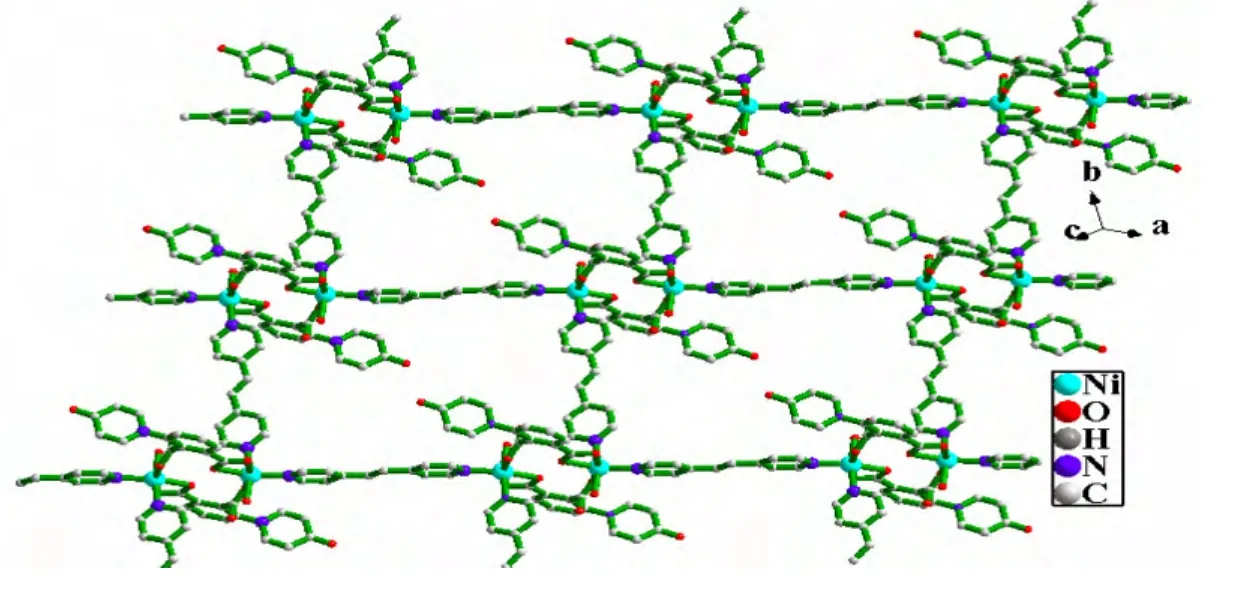
Fig. 3. Two-dimensional structure of complex 1. The hydrogen atoms are omitted for clarity
Hydrogen bonding interactions are generally very important for generating supramolecular architectures[17]. In 1, the coordinated water molecule O(1W)is hydrogen-bonded to carboxyl group O(3)#1 through the O(1W)··O(3)#1 to consolidate the 2D sheet, and intersheet hydrogen bonding O(1W)··O(2)#4 further extends the 2D sheet into a 3D supramolecular framework (Fig. 4, Table 2).
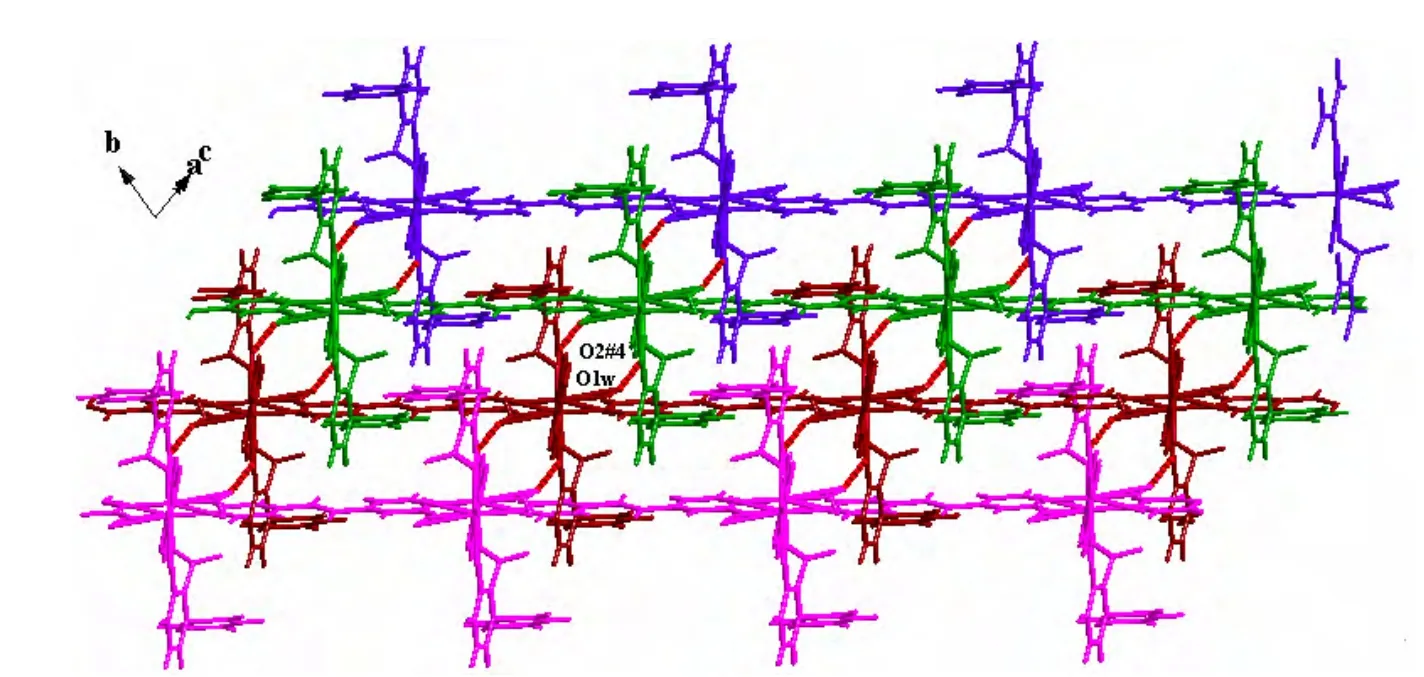
Fig. 4. 3D net connected by hydrogen bonding (dotted lines)interactions. Symmetry code: #4: –x, 2–y, –z
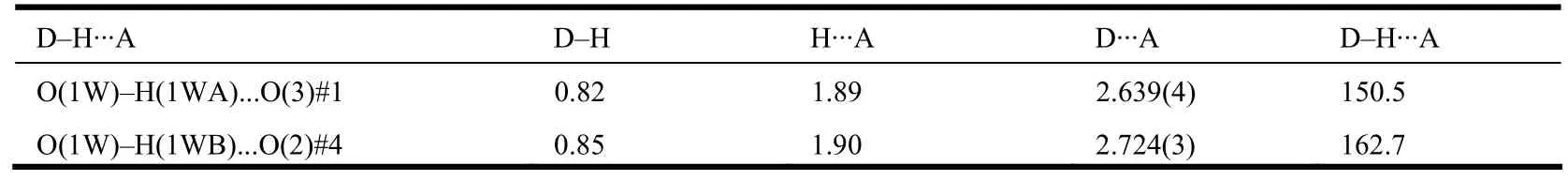
Table 2. Hydrogen-bond geometry (Å, °)
3. 2 Thermal analysis
Thermogravimetric analysis (TGA)was conducted to study the thermal stability of complex 1,which is an important aspect of metal-organic framework. TGA was performed on crystalline samples of 1 in the range of 40~800 ℃, as depicted in Fig. 5.The framework of 1 can be stable up to 80 ℃. The compound loses its coordinated water molecules from 80 to 190 ℃ (obsd. 4.42%, calcd. 3.49%). Then the compound begins to rapidly decompose at 310℃, as shown in Fig. 5.
3. 3 Electronic spectra
The diffuse-reflectance UV-vis spectra reveal the absorption features of complex 1, the ligand H2dpa and auxiliary ligand bpe (Fig. 6), and the three spectra consist of absorption components in the UV and Vis regions. In the three cases, the intense absorption peaks at 235, 266 and 262 nm for 1, the ligand H2dpa and auxiliary ligand bpe can be ascribed to π-π* transitions of the ligands, whereas the main UV absorption bands at 331 nm for 1 can be attributed to ligand-to-metal charge transfer(LMCT). In the case of complex 1, additional clear peaks in the visible region are observed at 647 nm,which probably respectively originate from the d-d spin-allowed transition of the Ni2+(d8)ion in a distorted octahedral coordination configuration. The patterns of coordination polymer 1 correspond well with other Ni(II)complexes with N,O-mixed ligands[18].
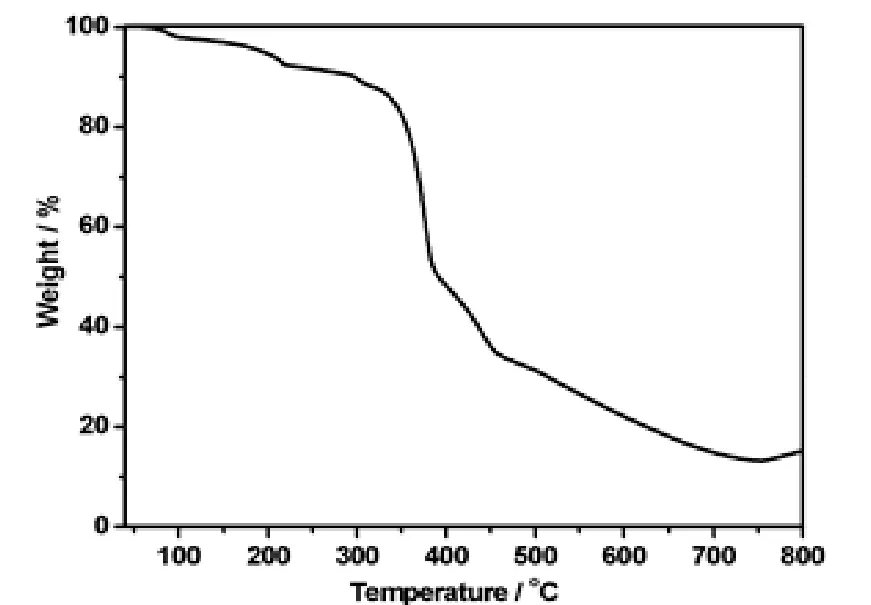
Fig. 5. TGA curve of the title complex

Fig. 6. UV-vis absorption spectra for complex 1 and the H2dpa and bpe ligands
ACKNOWLEDGEMENTS
The authors express thanks to Key Laboratory of Applied Chemistry of Pingdingshan University for financial support.
(1)(a)Eddaoudi, M.; Moler, D. B.; Li, H. L.; Chen, B. L.; Reineke, T. M.; O’Keeffe, M.; Yaghi, O. M. Modular chemistry:secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319−330. (b)James, S. L.Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276−288.
(2)(a)Ma, L. Q.; Abney, C.; Lin, W. B. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248−1256. (b)Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353−1379.
(3)Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: opportunities for catalysis. Angew. Chem., Int. Ed. 2009, 48, 7502−7513.
(4)(a)Ruben, M.; Rojo, J.; Romero-Salguero, F. J.; Uppadine, L. H.; Lehn, J. M. Grid-type metal ion architectures: functional metallosupramolecular arrays. Angew. Chem., Int. Ed. 2004, 43, 3644−3662. (b)Zeng, M. H.; Zhang, W. X.; Sun, X. Z.; Chen, X. M. Spin canting and metamagnetism in a 3d homometallic molecular material constructed by interpenetration of two kinds of cobalt(ii)-coordination-polymer sheets. Angew. Chem., Int. Ed.2005, 44, 3079−3082.
(5)(a)Zhou, Y. F.; Hong, M. C.; Wu, X. T. Lanthanide-transition metal coordination polymers based on multiple N- and O-donor ligands. Chem.Commun. 2006, 135−143. (b)Du, M.; Jiang, X. J.; Zhao, X. J.; Cai, H.; Ribas, J. Novel metallosupramolecular networks constructed from CuII, NiII,and CdIIwith mixed ligands: crystal structures, fluorescence, and magnetism. Eur. J. Inorg. Chem. 2006, 1245−1254. (c)Oisaki, K.; Li, Q.; Furukawa,H.; Czaja, A. U.; Yaghi, O. M. A metal-organic framework with covalently bound organometallic complexes. J. Am. Chem. Soc. 2010, 132,9262−9264.
(6)(a)Jeffrey, G. A. An Introduction to Hydrogen Bonding. Oxford University Press: Oxford, U. K. 1997. (b)Goldberg, I. Metalloporphyrin molecular sieves. Chem. Eur. J. 2000, 6, 3863−3870.
(7)Telfer, S. G.; Wuest, J. D. Metallotectons: comparison of molecular networks built from racemic and enantiomerically pure tris(dipyrrinato)cobalt(iii)complexes. Cryst. Growth Des. 2009, 9, 1923−1931.
(8)(a)Hijikata, Y.; Horike, S.; Tanaka, D.; Groll, J.; Mizuno, M.; Kim, J.; Takata, M.; Kitagawa, S. Differences of crystal structure and dynamics between a soft porous nanocrystal and a bulk crystal. Chem. Commun. 2011, 47, 7632−7634. (b)Mihalcea, I.; Henry, N.; Clavier, N.; Dacheux, N.;Loiseau, T. Occurrence of an octanuclear motif of uranyl isophthalate with cation-cation interactions through edge-sharing connection mode. Inorg.Chem. 2011, 50, 6243-6249. (c)Lama, P.; Sanudo, E. C.; Bharadwaj, P. K. Coordination polymers of Mn2+and Dy3+ions built with a bent tricarboxylate: metamagnetic and weak anti-ferromagnetic behavior. J. Chem. Soc., Dalton Trans. 2012, 41, 2979-2985.
(9)(a)Sun, X. L.; Song, W. C.; Zang, S. Q.; Du, C. X.; Hou, H. W.; Mak, T. C. W. Hierarchical assembly of a homochiral triple concentric helical system in a novel 3D supramolecular metal-organic framework: synthesis, crystal structure, and SHG properties. Chem. Commun. 2012, 48,2113–2115. (b)Sun, X. L.; Wang, Z. J.; Zang, S. Q.; Song, W. C.; Du, C. X. A series of Cd(II)and Zn(II)coordination polymers with helical subunits assembled from a versatile 3-(4-hydroxypyridinium-1-yl)phthalic acid and n-donor ancillary coligands. Cryst. Growth Des. 2012, 12, 4431−4440.
(10)(a)LaDuca, R. L. Aliphatic and aromatic carboxylate divalent metal coordination polymers incorporating the kinked and hydrogen-bonding capable tethering ligand 4,4΄-dipyridylamine. Coord. Chem. Rev. 2009, 253, 1759−1792. (b)Yao, X. Q.; Cao, D. P.; Hu, J. S.; Li, Y. Z.; Guo, Z. J.; Zheng, H.G. Chiral and porous coordination polymers based on an n-centered triangular rigid ligand. Cryst. Growth Des. 2011, 11, 231−239. (c)Li, B.; Zang, S.Q.; Ji, C.; Du, C. X.; Hou, H. W.; Mak, T. C. W. Syntheses, structures and properties of two unusual silver-organic coordination networks: 1D→1D tubular intertwinement and existence of an infinite winding water chain. J. Chem. Soc., Dalton Trans. 2011, 40, 788−792. (d)Fang, S. M.; Hu, M.;Zhang, Q.; Du, M.; Liu, C. S. Ag(I)and Zn(II)coordination polymers with a bulky naphthalene-based dicarboxyl tecton and different 4,4΄-bipyridyl-like bridging co-ligands: structural regulation and properties. J. Chem. Soc., Dalton Trans. 2011, 40, 4527−4541. (e)Gu, J. Z.; Gao, Z.Q.; Tang, Y. pH and auxiliary ligand influence on the structural variations of 5(2΄-carboxylphenyl)nicotate coordination polymers. Cryst. Growth Des. 2012, 12, 3312−3323.
(11)Silva, A. M. G.; Leite, A.; Andrade, M.; Gameiro, P.; Brandão, P.; Felix, V.; Castro, B.; Rangel, M. Microwave-assisted synthesis of 3-hydroxy-4-pyridinone/naphthalene conjugates. Structural characterization and selection of a fluorescent ion sensor.Tetrahedron 2010, 66, 8544−8550.
(12)SMART and SAINT. Area Detector Control and Integration Software, Siemens Analytical X-ray Systems, Inc.: Madison, WI (USA)1996.
(13)Sheldrick, G. M. SADABS 2.05, University of Göttingen, Germany.
(14)Sheldrick, G. M. SHELXS-97, Program for the Solution of Crystal Structures, University of Göttingen, Göttingen (Germany)1997. See also:Sheldrick, G. M. Acta Crystallogr. A46 1990, p467.
(15)Sheldrick, G. M. SHELXL-97, Program for the Ref i nement of Crystal Structures. University of Göttingen, Göttingen (Germany)1997. See also:Sheldrick, G. M. Acta Crystallogr. A64 2008, p112.
(16)(a)Xu, Z. Q.; Thompson, L. K.; Milway, V. A.; Zhao, L.; Kelly, T.; Miller, D. O. Self-assembled dinuclear, trinuclear, tetranuclear, pentanuclear, and octanuclear Ni(II)complexes of a series of polytopic diazine based ligands:structural and magnetic properties. Inorg. Chem. 2003, 42, 2950−2959.(b)Horikoshi, R.; Mochida, T.; Kurihara, M.; Mikuriya, M. Supramolecular isomerism in self-assembled complexes from 4,4΄-dipyridyl disulfide and m(hfac)2:coordination polymers (M = Mn)and metallamacrocycles (M = Co, Ni). Cryst. Growth Des. 2005, 5, 243−249. (c)Grubel, K.; Fuller,A. L.; Chambers, B. M.; Arif, A. M.; Berreau, L. M. O2-dependent aliphatic carbon-carbon bond cleavage reactivity in a Ni(II)enolate complex having a hydrogen bond donor microenvironment; comparison with a hydrophobic analogue. Inorg. Chem. 2010, 49, 1071–1081.
(17)Krische, M. J.; Lehn, J. M. The utilization of persistent H-bonding motifs in the self-assembly of supramolecular architectures. Struct. Bond. 2000, 96, 3−29.
(18)Wen, L. L.; Zhao, J. B.; Lv, K. L.; Wu, Y. h.; Deng, K. J.; Leng, X. K.; Li, D. F. Visible-light-driven photocatalysts of metal-organic frameworks derived from multi-carboxylic acid and imidazole-based spacer. Cryst. Growth Des. 2012, 12, 1603−1612.
- 结构化学的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid

