11例瘤源性骨软化症的临床诊治分析
杨云建,陈继营,杨 帆,张振东,李 恒
解放军总医院 骨科,北京 100853
11例瘤源性骨软化症的临床诊治分析
杨云建,陈继营,杨 帆,张振东,李 恒
解放军总医院 骨科,北京 100853
目的 分析瘤源性骨软化症(tumor-induced osteomalacia,TIO)的临床特征、诊断及治疗规律,提高对TIO的识别及早期诊治能力。方法我院2010年4月- 2013年4月收治11例确诊TIO患者,分析其手术前、后临床症状及血生化变化情况。结果男性5例,女性6例;年龄19 ~ 54岁,平均37.7岁。发病到确诊的时间2 ~ 15年,平均5.5年。11例均有骨痛和肌无力症状,血磷低而尿磷高,血钙正常,碱性磷酸酶(alkaline phosphatase,ALP)升高。3例发现皮下软组织包块。8例行奥曲肽扫描(99mTc-OCT)均提示有生长抑素受体高表达病变。11例手术治疗后均获得病理确诊,术后10例血磷恢复正常,所有患者骨痛及肌无力症状明显缓解。结论TIO临床症状典型,99mTc-OCT及其他影像学检查有助于定位肿瘤。手术切除病灶后血磷可恢复正常,症状改善明显。
瘤源性骨软化症;低血磷;奥曲肽扫描
瘤源性骨软化症(tumor-induced osteomalacia,TIO)是以低血磷、高尿磷、骨骼矿化障碍为特点的一种少见代谢性骨病。该肿瘤分泌多种调磷因子,可以使肾小管磷排泄显著增加,导致低血磷[1-2]。由于临床医生对TIO认识不足,导致长期漏诊、误诊。一旦确诊,手术治疗能够获得很好的效果。因此本文收集TIO患者相关资料,分析其诊治特点,以提高对本病的认识。
资料和方法
1 资料 2010年4月- 2013年4月,我院收治11例确诊TIO患者,均无阳性家族史,其中男性5例,女性6例;年龄19 ~ 54岁,平均37.7岁。发病到确诊时间为2 ~ 15年,平均5.5年。
2 方法 收集11例患者的临床资料,包括临床表现、实验室和影像学检查、诊断和治疗经过、病理资料、手术前后临床及血生化变化情况等。
结 果
1 症状与体征 11例均有全身骨痛、双下肢乏力及活动困难,多累及腰背部、双下肢等负重部位。3例伴有明显的身高缩短,分别为6 cm、10 cm、15 cm。5例因严重骨痛和双下肢肌无力不能行走。
2 实验室检查 11例均明显低血磷,血磷范围0.17 ~ 0.58 mmol/L(正常参考值:0.89 ~ 1.6 mmol/L),尿磷排出相对增多,血钙正常或轻度下降,碱性磷酸酶(alkaline phosphatase, ALP)不同程度升高,最高达875 U/L,全段甲状旁腺激素7例轻度升高,4例正常。
3 影像学检查 骨骼X线提示11例均存在骨小梁模糊、骨纹理不清、骨密度普遍降低及骨皮质变薄等骨质疏松和软骨病表现,其中3例骨折、2例假性骨折。双能X线吸收测量法(dual energy X-ray absorptiometry,DXA)示均有骨质疏松;ECT全身骨扫描均提示全身多处骨骼放射性异常浓聚灶,结合病史符合骨软化症表现。3例病灶位于皮下,易于发现,行彩色超声明确结节性质和血流情况,手术切除后活检病理证实为TIO。其他9例行99mTc-OCT检查,均提示身体某一部位生长抑素受体高表达病灶,经CT或MRI进一步明确病变部位、大小、毗邻关系后,手术完整切除并行病理检查以明确病理性质。
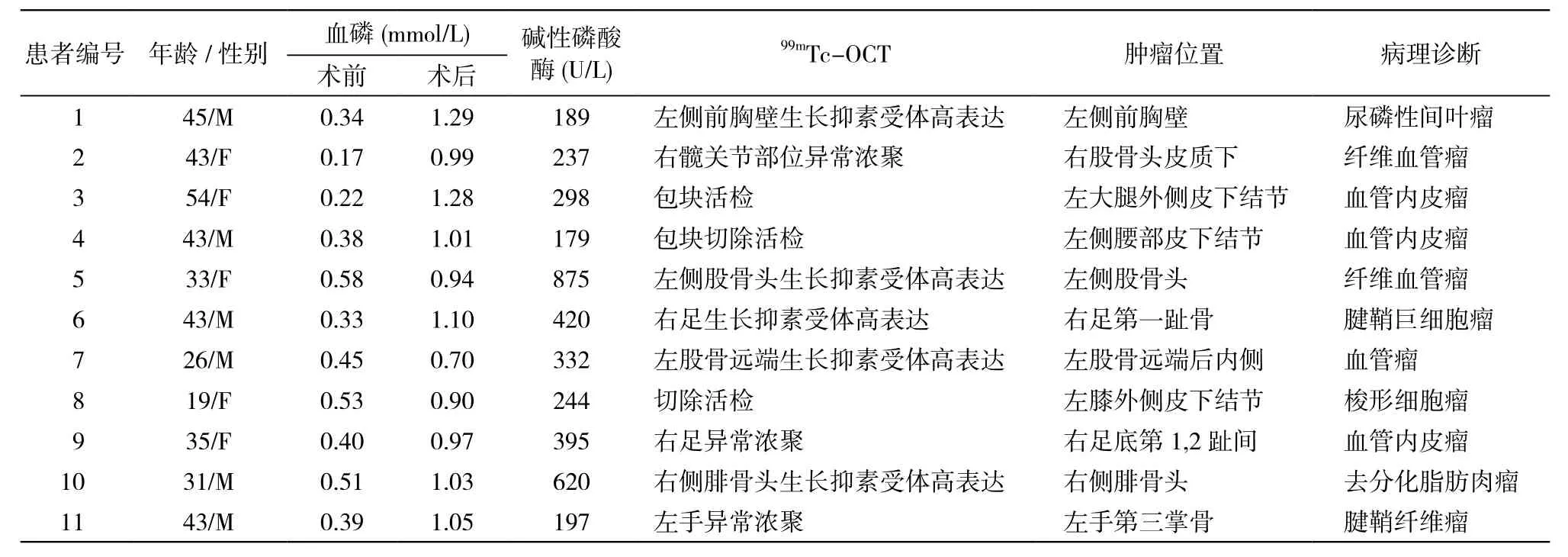
表1 11例TIO患者临床资料及病理结果Tab. 1 Clinical data and pathological diagnosis of 11 patients with TIO
4 治疗 患者入院后予以口服中性磷溶液,每次8 ml为起始量,5次/d;碳酸钙600 mg口服,1次/d;骨化三醇0.25 U口服,2次/d。患者骨痛稍缓解,血磷上升,但仍低于正常水平。11例定位诊断明确后行手术治疗,术前及术后监测临床表现和血磷变化情况。具体肿瘤部位及术后病理结果见表1。病例2前两次入院虽明确低血磷性骨软化症诊断,但经CT、MRI和99mTc-OCT等相关检查均未找到病因,直到第3次入院经99mTc-OCT检查才查明病灶部位,明确TIO的诊断,可能与肿瘤生长缓慢和隐匿有关,前因两次肿瘤太小或隐匿一直未检出。病例5共行两次手术,第1次因病变部位较小、患者年轻,要求行左侧股骨头病灶扩大切除术加骨移植,术后血磷短暂上升后又下降,第2次手术将病灶彻底清除,行左股骨头切除、人工全髋关节置换术,术后血磷恢复正常,骨痛症状明显改善,说明对于TIO肿瘤一定要彻底切除,不可心存侥幸。病例7行99mTc-OCT检查提示左股骨远端后内侧靠近关节面部位骨质密度稍高,生长抑素受体表达异常增高,行病灶彻底切除、左膝人工关节置换术,术后骨痛症状改善明显,血磷小幅升高,但仍低于正常,可能该患者其他部位存在隐匿病灶。
5 病理诊断 11例手术后都送病理检查,血管内皮瘤3例,纤维血管瘤3例,尿磷性间叶瘤1例,腱鞘巨细胞瘤和腱鞘纤维瘤各1例,梭形细胞瘤1例,去分化脂肪肉瘤1例。见表1。
讨 论
低血磷性骨软化症是一类成人罕见的骨代谢性疾病,分为遗传性和获得性两类。获得性低血磷性骨软化症包括TIO和病因不明者。Mccance[3]于1947年首先报道TIO,目前国内外仅报道300余例[4]。本病致病机制尚不完全清楚。肿瘤可分泌成纤维细胞生长因子(fibroblast growth factor-23,FGF23),其过度释放影响肾小管中磷的重吸收,使血磷下降、尿磷升高,同时使肾1-α羟化酶活性降低,1,25(OH)2D3产生减少,影响钙磷代谢,导致骨骼矿化障碍[5-6]。但是否还有其他调磷因子参与其中影响钙磷代谢,仍在研究之中[7-9]。
TIO好发于成人,临床表现主要为肌无力、全身骨痛,严重时出现骨骼畸形、骨折或假骨折。典型的疾病特点:1)骨痛逐渐加重,多开始于负重关节;2)明显低血磷,尿磷排泄显著增加,血钙一般正常或轻度降低;3)ALP升高,1,25(OH)2D3下降,少数患者可伴有继发性甲状旁腺功能亢进;4)需要大剂量补充中性磷溶液和活性维生素D才能明显改善骨痛和血磷水平;5)通过相关检查可以发现肿瘤,常体积小且较隐匿,切除肿瘤后骨痛症状和血磷水平常迅速明显改善[10-11]。低血磷性软骨症一般满足前4项,满足全部5项可诊断TIO。
TIO肿瘤大多为间充质组织起源,可来源于间叶组织、血管源性及纤维组织[4,12]。近年来有学者认为,大多数肿瘤是独立的病理组织类型即磷酸盐尿性间质肿瘤,混合组织亚型[13-14]。能引起TIO的肿瘤类型很多,血管瘤或血管内皮瘤多见,占50%以上,其次是纤维瘤或纤维肉瘤[15-16]。TIO无相关家族史,肿瘤多为良性,体积较小,隐匿且生长缓慢,约10%为恶性,若彻底切除,病情可以得到明显改善[17]。肿瘤多位于四肢,其次是头颈部和颌面部,可位于软组织浅层或深层,也可位于骨骼表面或内部。本组中1例位于上肢,2例位于躯干部,8例位于下肢。
研究发现放、化疗对TIO无效,药物治疗主要是补充中性磷溶液和活性维生素D,有一定效果,但是不能将血磷恢复到正常水平。手术治疗不论良、恶性,只要完整、彻底切除肿瘤就可以很快治愈。如果肿瘤切除后血磷未恢复正常水平,应考虑是否存在隐匿病灶或其他原因。手术前应准确掌握肿瘤的数量、位置、大小及毗邻关系,做好术前计划,术中应完整切除肿瘤,以防止复发和再发。本研究中除1例术后血磷未恢复正常外,其余10例切除肿瘤后血磷均很快上升到正常范围,骨痛及肌无力显著缓解。
明确TIO肿瘤的位置是治疗的前提和关键。引起TIO的肿瘤多生长缓慢、体积较小、部位隐匿,除部分位于体表外,其他部位不易发现。1996年Reubi等[18]发现这类间叶组织来源肿瘤表达生长抑素受体,因此可用生长抑素类似物奥曲肽与其结合显像来寻找肿瘤,显著提高了定位诊断的有效性和阳性率[19]。本研究中8例行99mTc-OCT检查发现全身不同部位生长抑素受体高表达病灶,另外3例因体表软组织包块经B超进一步明确肿瘤位置。因此,对于低血磷性骨软化症患者,可行全身99mTc-OCT筛查TIO,有阳性发现者再行影像学检查进一步定位。有学者利用TIO肿瘤分泌FGF-23因子的特点,分段取全身多部位静脉血测FGF-23浓度来进行定位,取得很好的效果[20-22]。F-18 FDG PET/CT利用肿瘤糖利用增加或血供丰富的特点,也被用于TIO肿瘤的定位诊断[23-24]。
TIO起病隐匿,病情发展缓慢,单纯补磷,血磷难以恢复正常水平,由于对其认识不足加上肿瘤定位困难,其实际发病率可能更高,常造成长时间的误诊、误治。临床上对于青春期后起病,无家族史的低血磷性骨软化症患者,需要警惕TIO的可能。对于病程初期未发现病灶的可疑TIO患者,仍需不断寻找肿瘤病灶。99mTc-OCT、PET-CT及分段取静脉血测FGF23对于发现病灶具有重要意义,B超、骨扫描、CT和MRI对于诊断及定位也有一定价值。治疗上应首选手术,力求病灶切除完整和彻底,术后应定期随访和加强监测,以防复发和再发。
1 Nanes MS. Phosphate wasting and fibroblast growth factor-23[J]. Curr Opin Endocrinol Diabetes Obes, 2013, 20(6): 523-531.
2 Ward LM, Rauch F, White KE, et al. Resolution of severe,adolescentonset hypophosphatemic rickets following resection of an FGF-23 producing tumour of the distal ulna [J] . Bone, 2004, 34(5): 905-911.
3 Mccance RA. Osteomalacia with Looser’s nodes (Milkman’s syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years[J]. Q J Med, 1947, 16(61): 33-46.
4 Jiang Y, Xia WB, Xing XP, et al. Tumor-induced osteomalacia:an important cause of adult-onset hypophosphatemic osteomalacia in China: Report of 39 cases and review of the literature[J]. J Bone Miner Res, 2012, 27(9): 1967-1975.
5 Hu FK, Yuan F, Jiang CY, et al. Tumor-induced osteomalacia with elevated fibroblast growth factor 23: a case of phosphaturic mesenchymal tumor mixed with connective tissue variants and review of the literature[J]. Chin J Cancer, 2011, 30(11): 794-804.
6 Xia WB, Jiang Y, Li M, et al. Levels and dynamic changes of serum fibroblast growth factor 23 in hypophosphatemic rickets/osteomalacia[J]. Chin Med J (Engl), 2010, 123(9): 1158-1162.
7 Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism[J]. Pediatr Nephrol, 2010, 25(4): 591-601.
8 Imanishi Y, Hashimoto J, Ando W, et al. Matrix extracellular phosphoglycoprotein is expressed in causative tumors of oncogenic osteomalacia[J]. J Bone Miner Metab, 2012, 30(1): 93-99.
9 Carpenter TO. The expanding family of hypophosphatemic syndromes[J]. J Bone Miner Metab, 2012, 30(1): 1-9.
10 Jan de Beur SM, Levine MA. Molecular pathogenesis of hypophosphatemic rickets[J]. J Clin Endocrinol Metab, 2002, 87(6):2467-2473.
11 Ledford CK, Zelenski NA, Cardona DM, et al. The phosphaturic mesenchymal tumor: why is definitive diagnosis and curative surgery often delayed?[J]. Clin Orthop Relat Res, 2013, 471(11):3618-3625.
12 Drezner MK. Tumor-induced osteomalacia[J]. Rev Endocr Metab Disord, 2001, 2(2):175-186.
13 Komínek P, Stárek I, Geierová M, et al. Phosphaturic mesenchymal tumour of the sinonasal area: case report and review of the literature[J]. Head Neck Oncol, 2011:3-16.
14 Mori Y, Ogasawara T, Motoi T, et al. Tumor-induced osteomalacia associated with a maxillofacial tumor producing fibroblast growth factor 23: report of a case and review of the literature[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2010, 109(3):e57-e63.
15 Serafini EM, Pisarevsky AA, Plumet Garrido J, et al. Tumor-induced osteomalacia: rhinosinusal hemangiopericytoma[J]. Medicina (B Aires), 2013, 73(1): 39-42.
16 Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia[J]. J Clin Endocrinol Metab, 2006, 91(6): 2055-2061.
17 Pettifor JM. What’s new in hypophosphataemic rickets?[J]. Eur J Pediatr, 2008, 167(5): 493-499.
18 Reubi JC, Waser B, Laissue JA, et al. Somatostatin and vasoactive intestinal peptide receptors in human mesenchymal tumors: in vitro identification[J]. Cancer Res, 1996, 56(8): 1922-1931.
19 Jing H, Li F, Zhuang H, et al. Effective detection of the tumors causing osteomalacia using [Tc-99m]-HYNIC-octreotide (99mTc-HYNIC-TOC) whole body scan[J]. Eur J Radiol, 2013, 82(11):2028-2034.
20 Tarasova VD, Trepp-Carrasco AG, Thompson R, et al. Successful treatment of tumor-induced osteomalacia due to an intracranial tumor by fractionated stereotactic radiotherapy[J]. J Clin Endocrinol Metab, 2013, 98(11): 4267-4272.
21 Nasu T, Kurisu S, Matsuno S, et al. Tumor-induced hypophosphatemic osteomalacia diagnosed by the combinatory procedures of magnetic resonance imaging and venous sampling for FGF23[J]. Intern Med, 2008, 47(10): 957-961.
22 Ogura E, Kageyama K, Fukumoto S, et al. Development of tumorinduced osteomalacia in a subcutaneous tumor, defined by venous blood sampling of fibroblast growth factor-23[J]. Intern Med,2008, 47(7): 637-641.
23 Chong WH, Andreopoulou P, Chen CC, et al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia[J]. J Bone Miner Res, 2013, 28(6): 1386-1398.
24 Khadgawat R, Singh Y, Kansara S, et al. PET/CT localisation of a scapular haemangiopericytoma with tumour-induced osteomalacia[J]. Singapore Med J, 2009, 50(2): e55-e57.
Clinical diagnosis and treatment of tumor-induced osteomalacia: An analysis of 11 cases
YANG Yun-jian, CHEN Ji-ying, YANG Fan, ZHANG Zhen-dong, LI Heng
Department of Orthopedic, Chinese PLA General Hospital, Beijing 100853,China
CHEN Ji-ying. Email:chenjiying_301@163.com
ObjectiveTo improve the clinical diagnosis and treatment level of tumor-induced osteomalacia (TIO) by analyzing the clinical features, diagnosis and treatment of 11 cases with TIO.MethodsClinical data (including clinical presentation, management, perioperative clinical status and biochemical changes) of 11 patients with TIO admitted to our hospital from April 2010 to April 2013 were retrospectively analyzed.ResultsThere were 5 males and 6 females with an average age of 37.7 years (range 19-54 years). The average time interval between onset and proven TIO was 5.5 years (range, 2-15 years). All the 11 patients presented with bone pain and muscle weakness, hypophosphatemia, hyperphosphatemia, normal serum calcium and increased alkaline phosphatase (ALP). Subcutaneous soft tissue mass was found in 3 cases,99mTc-OCT was performed in another 8 cases and it showed high expression of somatostatin receptor on different lesions of different patients. All the patients were confrmed by histopathology after operation. Serum Pi levels returned to normal in 10 patients after resection of tumors, symptoms of bone pain and muscle weakness were all improved obviously.ConclusionTIO is an extremely rare acquired disease with typical clinical features. Identifying the responsible tumors is clinically essential for the treatment of TIO,99mTc-OCT and other imaging examinations are also helpful to locate the tumors. After complete excision of the tumors, the patients’ serum phosphate levels and clinical symptoms are greatly improved.
tumor-induced osteomalacia; hypophosphatemia;99mTc-octreotide scintigraphy
R 738.1
A
2095-5227(2014)07-0707-04
10.3969/j.issn.2095-5227.2014.07.017
时间:2014-04-04 11:30
http://www.cnki.net/kcms/detail/11.3275.R.20140404.1130.001.html
2014-03-06
杨云建,男,在读硕士。研究方向:关节外科。Email:yunjianyang0611@126.com
陈继营,男,教授,博士生导师。Email:chenjiying_301@163.com

