Characterization of expressed genes in the establishment of arbuscular mycorrhiza between Amorpha fruticosa and Glomus mosseae
Fuqiang Song · Jize Li · Xingxing Zhang
Introduction
Amorpha fruticosa, a perennial leguminous woody shrub plant, is distributed mainly north of the Yangtze and Huaihe Rivers in China. It is an excellent shrub species for soil and water conservation and its roots, stems and leaves contain Amorphigenin, a flavonoid glycoside with a variety of medicinal properties, one of which is production of apigenin by hydrolysis. Because of its medicinal uses, the demand for A. fruticosa seedlings has increased gradually in recent years.
Arbuscular mycorrhiza (AM) is an ancient (>460 million years BC) symbiotic association between plants and soil fungi called AM fungi (Malloch et al. 1980; Brundrett 2002; Bonfante and Genre 2008). AM symbiosis is based on bidirectional nutrient exchange, i.e., AM fungi support the plants with nutrients such as phosphorus and nitrogen, and in turn they receive photosynthetic carbon from the plants (Harrison 1998; Ohtomo and Saito 2005; Paszkowsk 2006). In addition, the AM fungi can confer protection to the host plants against biotic (e.g., root pathogens) and abiotic stress (Vallino et al. 2008; Chen et al. 2009a; Evelin et al. 2009; Wehner et al. 2010). In nature, AM plays a significant role in biodiversity, evolution of land plants, ecosystem restoration and reconstruction, as well as agricultural, forestry and horticultural applications (Miransari et al. 2009; Hausmann and Hawkes, 2009; Uibopuu et al. 2009).
AM symbiosis is a result of complex and fine-tuned signaling events that lead to morphological and physiological alterations in both symbiotic partners. This process recruits genes from existing plant functions and modifies their expression patterns to fulfill the needs of AM formation. This may have resulted in the expression of mycorrhiza-regulated genes typical of higher plants and new genes with mycorrhiza-specific functions (Brechenmacher et al. 2004; Parniske 2004; Küster et al. 2007; Reinhardt 2007). Many genes related to symbiosis establishment have recently been isolated. The plant signaling network consists of three essential components, a receptor-like kinase, a predicted ion-channel and a calmodulin-dependent protein kinase, such as LjSYM2, ENOD11, LjSYMRK, PsSYM36, which regulate root symbiosis (Kosuta et al. 2003; Grunwald et al. 2004; Kistner et al. 2005). In spite of this recent progress, a detailed morphological study of the symbiotic relationship is still needed. Being obligate biotrophs, AM fungi can not complete their life cycle without host roots, which hinder in-depth research on the signaling events in the establishment of symbiosis between fungi and host plants. To date, AM symbiosis research has focused on root nodule symbiosis (RNS) mainly in Medicago truncatula and Lotus japonicus (Antunes et al. 2006a). However, studies of mycorrhizal symbiosis in woody plants have rarely been reported. Amorpha is a genus of woody legumes, alfalfa plants, and lotus root, which can have different growth patterns and infection molecular pathways and can provide a theoretical basis for AM symbiosis research.
The transcriptome opens a window for insight into the symbiotic mechanism between AMF and A. fruticosa. Transcriptome, the total set of transcripts, is a widely used method nowadays, we can get thousands of papers in the database, so we did not describe it here. Previous research on symbiosis between AMF and A. fruticosa mainly on the physiology of the plant influenced by AMF, while transcriptome open the window for insight into the symbiotic mechanism between AMF and A. fruticosa. Much of our knowledge of AMF genetics, molecular biology and physiology is restricted to the species G. intraradices. The isolate DAOM197198 was chosen for the first genome sequencing project on AMF (Martin et al. 2008). The production of an assembled genome has proven to be an arduous challenge due to a gene space larger than expected, abundant transposons and a high level of polymorphism (Martin et al. 2008), as well as the difficulty of preparing sufficient pure genomic DNA. Initial sequence data (Martin et al. 2008) provided an indirect estimate of genome size of about 150 Mb, a value that has recently been confirmed experimentally (Sedzielewska et al. 2011). Nevertheless, the determination of the mitochondrial genome sequence of a G. intraradices isolate based on whole-genome shotgun sequencing, and the recent publication of the mitochondrial genome of two Gigaspora isolates (Pelin et al.; Nadimi et al. 2012), demonstrate that there is no longer any technical obstacle to obtaining sequence data from AMF.
In the absence of a complete sequence, our knowledge of the G. intraradices DAOM197198 genome has recently been expanded by the publication of genome-wide transcriptomic data (Tisserant et al. 2012). A rather large and highly diversified gene repertoire was inferred. The uniqueness of Glomus, and most probably of the AMF lineage, is reflected in the fact that 58.2% of the transcripts have no match in public nucleotide databases. A striking feature of the Glomus transcript data set is an abundance of sequence polymorphisms. This was not unexpected, since sequence variants have been described for a number of genes (Sanders et al. 2010), but the fact that these variants appear as expressed sequences implies the possibility of divergent functional roles.
Stable genetic transformation of AMF has not yet been achieved but Helber et al. (2008), recently used HIGS (host-induced gene silencing) as a tool to silence AMF genes expressed in planta. Since RNA silencing is based on the movement of RNA molecules, the success of HIGS with AMF raises the question of whether horizontal gene transfer events (HGT) have occurred at the plant–fungus interface, where there has been intimate contact between the partners for more than 400 million years. The parasitic plant Striga hermonthica, which forms an invasive organ called haustorium to allow transfer of nutrients form the host plant, recently provided an example of eukaryotic–eukaryotic HGT (Yoshida et al. 2010).
Materials and methods
AM fungi (AMF)
The AMF used in this study, Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe was obtained from the Chinese Academy of Agriculture Sciences (CAAS), Beijing, China. Inocula containing Sudan grass (Sorghum sudanese Staff) mycorrhizal root fragments, spores, and mycelia were obtained from a 1-year-old sterilized Sudan grass culture. The propagule was prepared with fungi suspension before inoculation. The concentration of the propagule in the suspension was about 80 spores/g-inocula for G. mosseae. All experiments were conducted in the Laboratory of Microbiology, Heilongjiang University, China (Song 2009).
Plant material
Amorpha fruticosa seeds were sterilized with 0.3% K2MnO4for 20 min, and then germinated at 25°C for 60 h in an incubator after soaking for 24 h. The germinated seeds were then planted in pre-sterilized mixed matrix and grown under conditions of 25/18°C (day/night) temperature, 50% relative humidity, 14h photoperiod and 500 μmol·m-2·s-1photosynthetic photon flux density. The culture matrix was a mixture of peat soil, sand and vermiculite at a ratio of 5:2:3, was wrapped in brown paper and autoclaved at 121°C, 0.1 MPa for 1.5 h, and then air dried one week before the start of the experiment. The plants were divided into two groups. One was inoculated with G. mosseae, whereas another was set as a control, i.e., inoculated with sterilized inocua (5% v/v). The inocula consisted of thoroughly mixed rhizosphere samples containing spores, hyphae and mycorrhizal root fragments. The plants in the greenhouse were collected after 14 days of growth and sampled every two days thereafter. Roots were washed extensively and carefully to separate them from the soil, quickly dried with paper, and either stained immediately, stored at 4°C for endophyte analysis or at -20°C for molecular analysis.
Root colonization analysis
Roots were stained with 0.12% cotton blue in lactic acid for about 20 h and then de-stained four times with lactic acid. The roots were cut into small pieces (1 cm each) and mounted onto microscope slides with lactic acid. For each root sample, 200 pieces were observed for a total of 600 root pieces. The intensity of fungal colonization into the root cortex and the presence of arbuscules were determined as previously described (Trouvelot et al. 1986). Root fungal colonization was determined for each plant. Non-inoculated controls showed 0% AMF colonization in all cases.
RNA isolation
For the mRNA differential display analysis, root tissue from 3 biological replicates (G. mosseae- or mock-inoculated plants) were ground in liquid nitrogen using a preprocessed mortar and pestle. Total RNA was extracted from plant roots with AMF-colonized and non-colonized by Biozol using a RNA isolation kit which is Tiangen, Beijing, China. The RNA was suspended in a total volume of 100 μL double-distilled water. Agarose gel electrophoresis was used to test the integrity and a UV spectrophotometer was used to determine the purity of the RNA by monitoring the absorbance ratio at 260/280/230 nm.
The cDNA was synthesized from 5 μg of total RNA using the“First-strand Synthesis System for RT-PCR” kit and OligodT12GC (TTTTTTTTTTTTGC). mRNA differential display was carried out as described in the Experiment Guide of Molecular Cloning (Sambrook et al. 1989; Liu et al. 2007; Fabi et al. 2009; Pandit et al. 2010). The anchored primers (H-T11A: 5′-AAGCTTTTTTTTTTTA-3′, H-T11G: 5′-AAGCTTTTTTTTTTTG-3′ and H-T11C: 5′-AAGCTTTTTTTTTTTC-3′) were synthesized by Sangon, In a 0.5 mL micro-centrifugation tube, 2 μL of 10 × RT mixtures, 2 μL of 25 μM dNTP mixtures, and 2 μL of 10-μM Oligo-dT15were added. The mixture was set to a volume of 19 μL by adding diethylpyrocarbonate-treated water. The tube was heated in a water bath at 37°C for 5 min, and then cooled on ice. Then, 0.5-μL total RNA and 1-μL Quant Reverse Transcriptase (200 U/μL) were added, maintained at 42°C for 60 min and then cooled on ice. The reverse transcription products were stored at 4°C for immediate use or at -20°C for later use. PCR amplification of the reverse transcription products was carried out in combination with one of the twenty arbitrary primers H-AP synthesized by Sangon (Table 1). The samples were denatured at 95°C for 1 min and then amplified as follows: 94°C for 15s, 45°C for 30 s, 72°C for 1.5 min for 40 cycles, and finally 72°C for 5 min. The amplified cDNA populations transcribed by 60 different mRNA pairs and one of the arbitrary primers with every anchored primer were size-fractionated by electrophoresis in 6% non-denaturing polyacrylamide gel. Silver staining of the polyacrylamide gel was performed according to the Bio-Rad Silver Stain Handbook.
Re-amplification
The gel bands of the differentially displayed cDNA fragments (DDF) were excised, diluted with sterile water, and boiled for 15 min. The eluted DNA was re-amplified in an Eppendorf tube using the same pair of primers as in the differential display. The PCR conditions were similar to those described above. The re-amplified DDFs were resolved using agarose gel electrophoresis and purified using the PCR DNA and Gel Band Purification kit, TIANquick Midi Purification Kit, Tiangen Biotech Co. Ltd., China.

Table 1: List of twenty arbitrary primers and three anchored primers
Reverse northern blot
Probe labeling and detection of hybridization signals were carried out using DNA DIG labeling and DIG detecting kits, respectively, according to manufacturer protocols (Boeechringer-Mannheim Company, Germany). Sub-samples of 2 μL of amplified cDNA from the treated and control samples were denatured at 70°C for 5 min and then blotted onto a nylon membrane (Hybond-N+, Amersham, UK). The DNA on the membrane between the two sheets of filter paper was fixed in an oven at 80°C for 2 h. Probe labeling and hybridization conditions were as described in the instructions of the Northern direct HRP labeling and detection kit, which is Biotin Chomogenic Detection Kit, MBI, Shanghai, China.
Cloning
Positive DDFs were cloned into a pMD18-T vector (Invitrogen), allowing blue-white selection in Escherichia coli DH-5α. Inserted DNA sequences of the plasmid clones were determined by automated sequencing by using ABI3330, Invitrogen, Shanghai, China. All sequences were aligned with the sequences in the non-redundant GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST).
Verification of the origin of EST with PCR
Primers were designed based on the sequence information with Primer 3. Specific primers for the 11 differentially expressed ESTs were designed to give a total of 22 primers, of which Group 1 (AfSM1, AfSM3, AfSM6, AfSM7, AfSM9, AfSM10) had similar TM temperature, Group 2 (AfSM2, AfSM4, AfSM5, AfSM8) had similar TM values, and Group 3 (AfSM11) had the lowest TM values. The product sizes of AfSM1-AfSM11 were 192bp, 200bp, 205bp, 232bp, 253bp, 191bp, 183bp, 265bp, 192bp, 202bp and 202bp, respectively. Annealing temperatures were based on TMs during runs of the PCR program. Table 2 and Table 3 shows the amplification reaction system and amplification procedure of PCR.

Table 2: The amplification reaction system of PCR
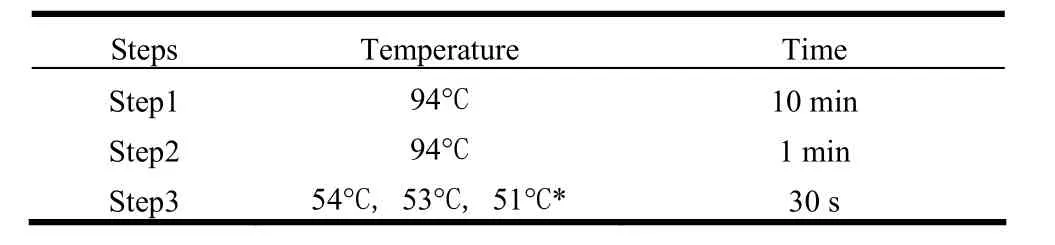
Table 3: The amplification reaction procedure of PCR
Results
Mycorrhizal development
At different stages of inoculating A. fruticosa and G. mosseae, we selected 200 inoculated AMF roots samples for determining the extent of root fungal colonization using a microscope. The 0% of infection rate, no G. mosseae arbuscule, was observed in the root systems of A. fruticosa before the 15th day. Mycorrhizal plants had colonization levels of 12% at day 22, 31% at day 28, 53% at day 32, and 98% at day 35. AM development required time for colonizing. After colinization, symbiosis quickly took place. AM development can be divided into two stages, the early stage or initial 15 days, and the late stage, after 28 days, when colonization exceeded 30% (Fig. 1). We used these stages for isolating the symbiosis-related genes by differential display analyses.
The growth of inoculated (GM) was significantly better than for unvaccinated (CK) plants after culture in the greenhouse for 15 days (Fig. 2). Fig. 3 shows the vesicular and arbuscules of AMF colonized inside the roots at midanaphase colonization.
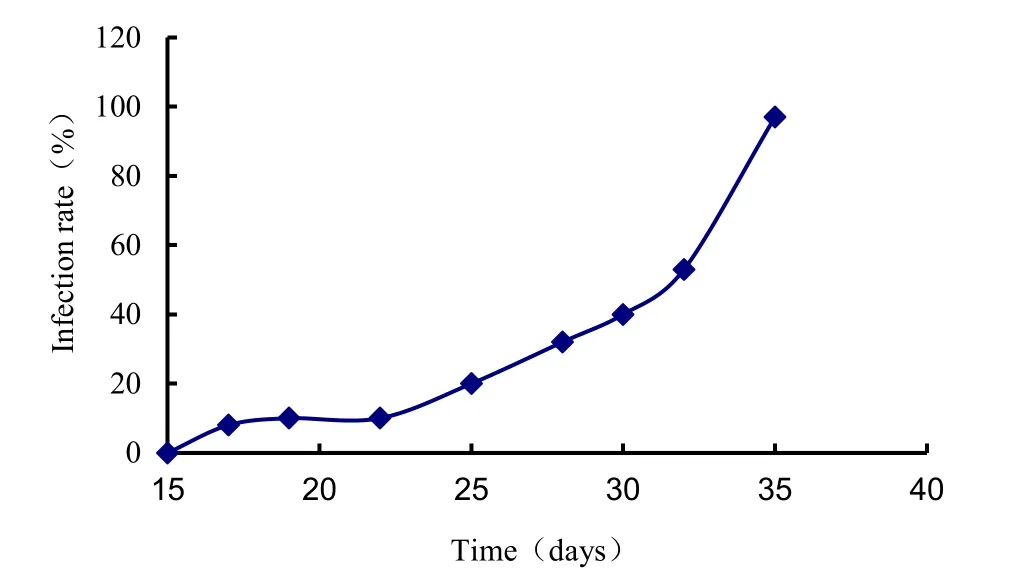
Fig. 1: Effect of seedling age on the colonization of arbuscular mycorrhiza fungi.

Fig. 2: Growth of GM and CK plants.

Fig. 3: The vesicular and arbuscules in the roots.
AM development
Total root RNA was extracted by Biozol and purified. The total RNA had high purity suitable for subsequent differential display analysis.
In total, 60 primer combinations of 3 anchor primers and 20 arbitrary primers were amplified. These PCR products were separated on 6% non-denaturing polyacrylamide gels. Twenty to 50 bands were found in each lane. More differential fragments were found in the late stage (after day 28) than in the early stage (before day 14) (Fig. 4). Other samples had some bands representing both the early and late stages, and were expressed in preand post-mycorrhizal development. Some bands were obtained in the control but not in the samples of inoculated AMF, indicating that these genes may be associated with defense. Interestingly, some bands appeared or disappeared in the late or early stages of the symbiosis, indicating that the expression of the genes might stop and other genes might start to express at different stages. In addition, the expression of the same band may be strong or weak, indicating that gene expression levels vary at different stages of mycorrhizal formation.
Of the 12 positive ESTs, one (GW327873) did not show homologous sequences, whereas the other 11 ESTs can be divided into two types. One group (AfSM4, AfSM7, and AfSM9) was similar to bacterial proteins or fungi genes such as in Penicillium or Ajellomyces capsulatus, which may be vital for completing the fungal life cycle and the exchange of nutrients between A. fruticosa and G. mosseae at AM development. The other group (AfSM1, AfSM2, AfSM3, AfSM5, AfSM8, AfSM10, AfSM11, and AfSM12) was similar to plant proteins such as L. japonicas or Ricinus communis that may be specifically induced by the fungi and have an important role in AM establishment.
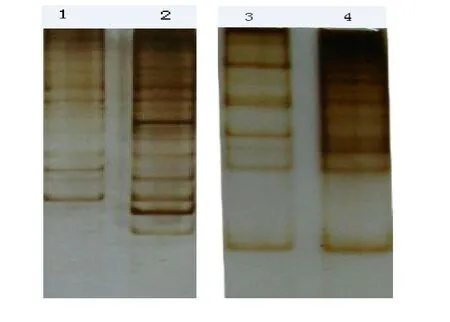
Fig. 4: Differentially expressed cDNA of agarose gel electrophoresis (1% agarose). Lanes 1 and 2 contain total RNA samples from control roots and Amorpha fruticosa colonized roots at early stage (14 days after infection), respectively; Lanes 3 and 4 contain total RNA extracted from control and colonized roots at late stage (28 days after infection).
DDRT-PCR of differentially expressed cDNAs from the samples
In total, we isolated 30 expressed fragment appearances Initial screening of the cDNAs to discard false positives was performed by reverse northern hybridization, and 12 fragments were isolated. Five of the 12 gene fragments were AM-related genes obtained from the A. fruticosa-G. mosseae in the early-stage interaction. Whereas, seven genes were isolated at the late stage of mycorrhizal development. Three ESTs corresponded to the origin of the fungi, whereas eight ESTs corresponded to plant genes. Putative functions were confirmed and sequence similarities were determined by Blastx analysis (Table 4).

Table 4: Sequence analysis of differentially expressed cDNAs by DDRT-PCR
PCR results of the agarose gel electrophoresis (1% agarose)
Fig. 5A shows the consequence of gDNA of Amorpha fruticosa inoculated with AM fungi as a template, amplified by application six pairs of primers in Group 1. Fig. 5B shows the result of gDNA of Amorpha fruticosa without AM fungi as a template, amplified by application six pairs of primers in Group 1. Fig. 5C shows the consequence of gDNA of Amorpha fruticosa inoculated with AM fungi as a template, amplified by application four pairs of primers in Group 2. Fig. 5D shows the result of gDNA of Amorpha fruticosa without AM fungi as a template, amplified by application four pairs of primers in Group 2. Fig. 5E shows the consequence of gDNA of Amorpha fruticosa inoculated with AM fungi as a template in first lane and gDNA of Amorpha fruticosa without AM fungi in second lane, amplified by application primers in Group 3. The size of the marker was 2000 bp.
After amplification by PCR, the origins of AfSM 4, AfSM 7, AfSM 9 were determined to belong to Glomus mosseae as determined by evaluation of the photograph of agarose gel electrophoresis. The rest of expressed EST sequences were derived from Amorpha fruticosa. This consequence coincided with the results mentioned-above in Table 4, in which BLAST was used to search the EST sequences against GeneBank for functional annotation. The results also proved that the 3 different ESTs are important for fungi. We can also infer that AfSM 11 is derived from Amorpha fruticosa even though the band is not clear in Fig. 5E. This is because of the low expression or the annealing temperature.
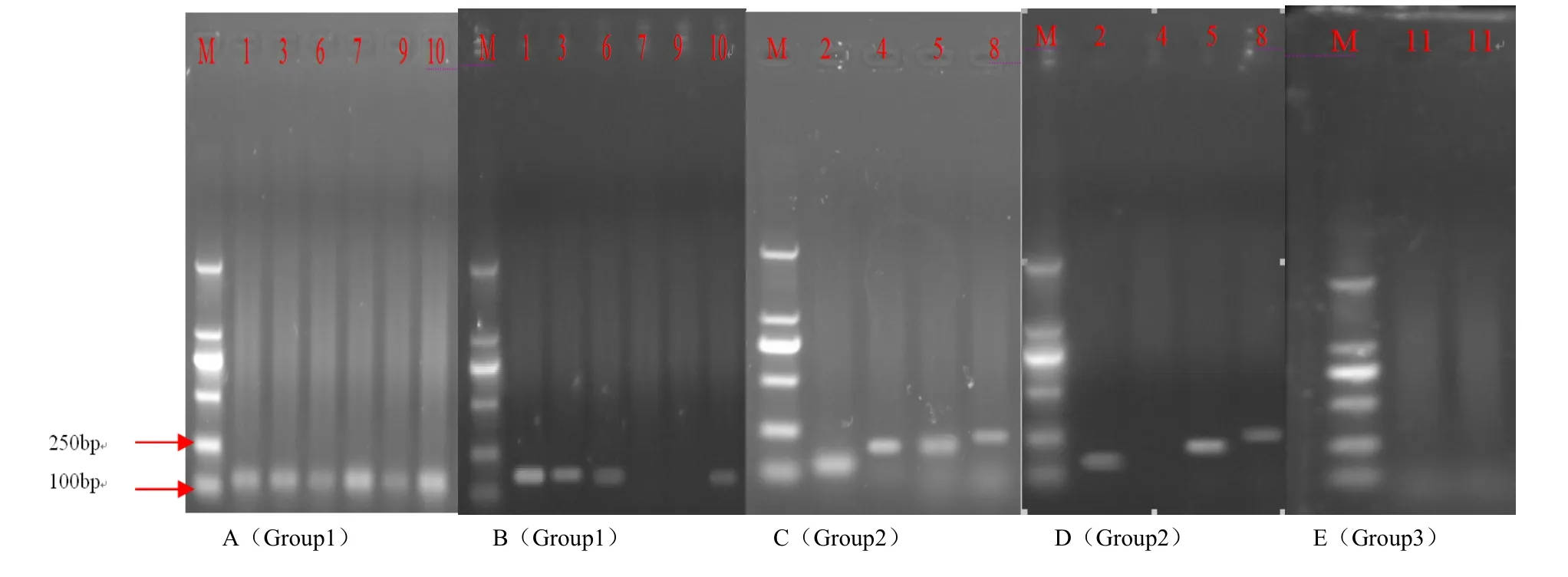
Fig. 5: PCR results of the agarose gel electrophoresis (1% agarose) to find the origins of 11 ESTs.
Discussion
In this study, the use of a well-controlled experimental mycorrhization system coupled with defined sampling times allowed us to attribute most of the 163 expressed genes to plant processes related to arbuscule development. After reverse northern hybridization, 11 genes were used to search for similarities to predict putative functions. For both symbionts, the period before the physical contact involved recognition and attraction of the appropriate partners and other events promoting mycorrhizal formation. The identified AfSM2 (A. fruticosa symbiosis) was similar to L. japonicus class III HD-Zip protein as it signals in response to external environmental stress and plays important regulatory roles in the unique developmental process of plants. The expression of AfSM2 is present both in the early and late stages, indicating that it plays an important role in the formation of AM. It is certainly an important mycorrhizal symbiosis-related gene that is required for increased AM fungi response to biotic and abiotic stress. AfSM3, similar to the ATP-binding cassette sub-family E member, belongs to a widespread transport system that moves metabolites, ions, sugars, amino acids, lipids, steroids, drugs and other substrates through the cell membrane. Many molecular components such as gas, sugar and secondary metabolites are transported across membranes so that plant and fungi can recognize each other for the pre-AM symbiosis (Fitter et al. 2011; Smith et al. 2011). AfSM4, similar to Penicillium marneffei hypothetical protein, is found mostly at the early stages. AM fungi form extensively branching hyphae called arbuscules in the cortical cells of the root due to the high levels of fungal gene expression (Bonfante and Genre 2010; Schüßler et al. 2010). AfSM5 is related to the respiratory oxidase gene and is highly expressed since there is a great increase in respiration and energy consumption at the early stage of mycorrhizal symbiosis (Krüger et al. 2012).
The similarity of AfSM8 with the acetyltransferase GNAT family protein indicates protein acetylation may be important for protein activity and structure. AMF can promote absorption by host plants of nutrients through the periarbuscular membrane in the mycorrhizal roots. Some protein transporters are used for exchange of N, P, and other minerals. These transporters are activated by acetylation (Lee et al. 2010; Rasmussen et al. 2008; Paungfoo-Lonhienne et al. 2010). AfSM7 and AfSM9, similar to the genes of Flavobacterium johnsoniae and A. capsulatus hypothetical proteins, might play an important role in the late stage of AM development to promote fungi-formed arbuscular structures in plant cells. These genes are connected to series genes to function in providing mineral nutrients for plants, and are expressed at the early stages to form hyphae (Mortimer et al. 2009; Nagy et al. 2009). AfSM10, similar to genes located in chromosome 1 of Lotus japonicas genomic DNA, may have a very specific function at the establishment of AM symbiosis. AfSM11, similar to A. thaliana clone SINE9 transposon-insertion display band genomic sequence, has been found to be important in the evolution of AM (Nadimi et al. 2012; Bonfante and Genre 2009). AfSM12, similar to a photosynthesis gene, synthetically constructs 'Red/Green ancestor' ancestral fluorescent protein variant D07 gene, and is expressed at AM formation (Dumbrell et al. 2010; Kaschuk et al. 2010).
Many scientists concur that AM, which has existed for billions of years, has an important role in the evolution of land plants in enhancing plant adaptations to their environments. However, little is known regarding the mechanism of mycorrhizal estab-lishment and plant responses to adverse environments.
Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature, 435(7043): 824-827.
Antunes PM, Rajcan I, Goss MJ. 2006b. Specifc flavonoids as interconnecting signals in the tripartite symbiosis formed by arbuscular mycorrhizal fungi, Bradyrhizobium japonicum (Kirchner) Jordan and soybean (Glycine max (L.) Merr.). Soil Biology & Biochemistry, 38: 533-543.
Antunes PM, Varennes A, Rajcan I, Goss MJ. 2006a. Accumulation of specifc flavonoids in soybean (Glycine max (L.) Merr.) As a function of the early tripartite symbiosis with arbuscular mycorrhizal fungi and Bradyrhizobium japonicum (Kirchner) Jordan. Soil Biology & Biochemistry, 38: 1234-1242.
Barbour MM, Hanson DT. 2009. Stable carbon isotopes reveal dynamics of respiratory metabolism. New Phytol, 181(2):243-245.
Bauer D, Muuller H, Reich J, Ahrenkiel V, Warthoe P, Strauss M. 1993. Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR). Nucleic Acids Research, 21(18): 4272-4280.
Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N. 2006. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol, 4(7): 2-6.
Bonfante P, Genre A. 2008. Plants and arbuscular mycorrhizal fungi: an evolutionary- developmental perspective. Trends Plant Sci, 13(9): 492-498.
Bonfante P., Genre A. 2010. Mechanisms underlying beneficial plant fungus interactions in mycorrhizal symbiosis. Nat Commun, 1: 48.
Bozkurt O, Unver T, Akkaya MS. 2007. Genes associated with resistance to wheat yellow rust disease identifed by differential display analysis. Physiological and Molecular Plant Pathology, 71(4-6): 251-259.
Brechenmacher L, Weidmann S, van Tuinen D. 2004. Expression profiling of up-regulated plant and fungal genes in early and late stages of Medicago truncatula-Glomus mosseae interactions. Mycorrhiza, 14(4): 253-262.
Breuninger M, Requena N. 2004. Recognition events in AM symbiosis: analysis of fungal gene expression at the early appressorium stage. Fungal Genetics and Biology, 41(8): 794-804.
Brewer PB, Elizabeth AD, Ferguson BJ. 2009. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and arabidopsis. Plant Physiol, 150(1): 482-493.
Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytologist, 154(2): 275-304.
Bucher M, Wegmüller S, Drissner D. 2009. Chasing the structures of small molecules in arbuscular mycorrhizal signaling. Curr Opin Plant Biol, 12(4): 500-507.
Chen C, Fan C, Zhu H. 2009a. Antiquity and function of CASTOR and POLLUX, the twin ion channel-encoding genes key to the evolution of root symbioses in plants. Plant Physiol, 149(1): 306-317.
Chen CY, Huang DJ and Liu JQ. 2009b. Functions and toxicity of nickel in plants: recent advances and future prospects. Clean, 37(4-5): 304-313.
Corradi N, Sanders IR. 2006. Evolution of the P-type II ATPase gene family in the fungi and presence of structural genomic changes among isolates of Glomus intraradices. BMC Evol Biol, 6: 21-22.
Dumbrell AJ, Nelson M, Helgason T, Dytham C. 2010. A.H. Fitter Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: is there a role for stochastic processes. J Ecol, 98: 419–428.
Evelin H, Kapoor R, mGiri B. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of botany, 104(7): 1263-1280.
Fabi JP, Lajolo FM, do Nascimento JRO. 2009. Cloning and characterization of transcripts differentially expressed in the pulp of ripening papaya. Scientia Horticulturae, 121(2): 159-165.
Fitter A.H, Helgason T. 2011. A. Hodge Nutritional exchanges in the arbuscular mycorrhizal symbiosis: implications for sustainable agriculture. Fungal Biol Rev, 25: 68–72.
Gomez SK, Javot H, Deewatthanawong P. 2009. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol, 9: 10-11.
Grunwald U, Nyamsuren O, Tamasloukht M. 2004. Identification of mycorrhiza-regulated genes with arbuscule development-related expression profile. Plant Mol Biol, 55(4):553-66.
Guether M, Balestrini R, Hannah M. 2008. Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytologist, 182(1): 200-212.
Halary S., Malik S.B., Lildhar L., Slamovits C.H. 2011. Conserved meiotic machinery in Glomus spp., a putatively ancient asexual fungal lineage. Genome Biol Evol, 3: 950–958.
Harrison MJ. 1998. Development of the arbuscular mycorrhizal symbiosis. Plant Biology, 1: 360-365.
Hausmann NT, Hawkes CV. 2009. Plant neighborhood control of arbuscular mycorrhizal community composition. New Phytol, 183(4): 1188-1200.
Helber N, Requena N. 2008. Expression of the fluorescence markers DsRed and GFP fused to a nuclear localization signal in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol, 177: 537-548.
Hua J, Lin X, Yin R. 2009. Effects of arbuscular mycorrhizal fungi inoculation on arsenic accumulation by tobacco (Nicotiana tabacum L.). J Environ Sci, 21(9): 1214-1220.
Karandashov V, Nagy R, Wegmuller S. 2004. Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA, 101(16): 6285-6290.
Kaschuk G, Leffelaar PA, Giller KE, Alberton O. 2010. Responses of legumes to rhizobia and arbuscular mycorrhizal fungi: A meta-analysis of potential photosynthate limitation of symbioses. Soil biology & biochemistry, 42: 125-127.
Kistner C, Winzer T, Pitzschke A, Mulder L. 2005. Seven Lotus japonicus Genes Required for Transcriptional Reprogramming of the Root during Fungal and Bacterial Symbiosis. Plant Cell, 17(8): 2217-2229.
Koch AM, Croll D, Sanders LR. 2006. Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett, 9(2): 103-110.
Kosuta S, Chabaud M, Dénarié J. 2003. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol, 131(3): 952-962.
Krüger C. M., Krüger, Walker, Stockinger. 2012. A. Schüßler Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol, 193: 970–984.
Küster H, Vieweg MF, Manthey K. 2007. Identification and expression regulation of symbiotically activated legume genes. Phytochemistry, 68(1): 8-18.
Lee J, Young JP. 2010. The mitochondrial genome sequence of the arbuscular mycorrhizal fungus Glomus intraradices isolate 494 and implications for the phylogenetic placement of Glomus. New Phytol, 183: 200–211.
Liu J, Blaylock LA, Endre G. 2003. Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell, 15(90): 2106-2123.
Liu YJ, Zhang AN, Jia JF and Li A. 2007. Cloning of salt stress responsive cDNA from wheat and resistant analysis of differential fragment SR07 in transgenic tobacco. J Genet Genomics, 34(9): 842-849.
Malloch DW, Pirozynski KA, Raven PH. 1980. Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants. Ecology, 77: 2113-2118.
Martin F, Gianinazzi-Pearson V. 2008. The long hard road to a completed Glomus intraradices genome. New Phytol, 180:747-750.
Miransari M, Bahrami HA, Rejali F. 2009. Effects of arbuscular mycorrhiza, soil sterilization, and soil compaction on wheat (Triticum aestivum L.) nutrients uptake. Soil & Tillage Research, 104(1): 48-55.
Mortimer PE, Perez-Fernandea MA, Hannahalentine AJ. 2009. Arbuscular mycorrhizae affect the N and C economy of nodulated Phaseolus vulgaris (L.) during NH4+nutrition. Soil Biology & Biochemistry, 41(10): 2115-2121.
Msiska Z, Morton JB. 2009. Isolation and sequence analysis of a-tubulin gene from arbuscular mycorrhizal fungi. Mycorrhiza, 19(7): 501-513.
Nadimi M, Beaudet D, Forget L. 2012. Group I intron–mediated trans-splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Mol Biol Evol http://dx.doi.org/10.1093/molbev/mss088 .
Nadimi M, Beaudet D, Forget L. 2012. Lang Group I intron–mediated trans-splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Mol Biol Evol: http://dx.doi.org/10.1093/molbev/mss088.
Nagy R, Drissner D, Amrhein N. 2009. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 181(4): 950-959.
Ohtomo R, Saito M. 2005. Polyphosphate dynamics in mycorrhizal roots during colonization of an arbuscular mycorrhizal fungus. New Phytol, 167(2): 571-578.
Olsson PA, Hansson MC, Burleigh SH. 2006. Effect of P availability on temporal dynamics of carbon allocation and Glomus intraradices high-affinity P transporter gene induction in arbuscular mycorrhiza. Appl Environ Microbiol, 72(6): 4115-4120.
Pandit SS, Kulkarni RS, Giri AP. 2010. Expression profiling of various genes during the fruit development and ripening of mango. Plant Physiol Biochem, 48(6): 426-433.
Parniske M. 2004. Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol, 7(4):414-421.
Paszkowsk U. 2006. A journey through signaling in arbuscular mycorrhizal symbioses. New Phytol, 172(1): 35-46.
Paungfoo-Lonhienne C, Lonhienne TG. 2008. Plants can use protein as a nitrogen source without assistance from other organisms. Proc Natl Acad Sci USA, 105(11): 4524-4529.
Pitet M, Camprubí A, Calvet C, Estaún V. 2009. A modified staining technique for arbuscular mycorrhiza compatible with molecular probes. Mycorrhiza, 19(2): 125-131.
Ponce MA, Bompadre MJ, Scervino JM. 2009. Flavonoids, benzoic acids and cinnamic acids isolated from shoots and roots of Italian rye grass (Lolium multiflorum Lam.) with and without endophyte association and arbuscular mycorrhizal fungus. Biochemical Systematics and Ecology, 37: 245-253.
Rameau C. 2010. Strigolactones, a novel class of plant hormone controlling shoot branching. Comptes Rendus Biologies, 333(4): 344-349.
Rasmussen S, Parsons AJ, Fraser K. 2008. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol, 146(3): 1440-1453.
Reinhardt D. 2007. Programming good relations-development of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol, 10(1): 98-105.
Saito K, Yoshikawa M, Yano K. 2007. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell, 19(2): 610-624.
Sambrook J, Fritsch E F, Maniatis T. 1989. Molecular Cloning: A laboratory manual, 2nd edn. New York: Cold Spring Harbor Laboratory, p. 659.
Sanders IR, Croll D. 2010. Arbuscular mycorrhiza: the challenge to understand the genetics of the fungal partner. Annu Rev Genet, 44:271-292.
Scervino JM, Ponce MA, Erra-Bassells R. 2005. Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol Res, 109(7): 789-794.
Schenkluhn L, Hohnjec N, Niehaus K. 2010. Differential gel electrophoresis (DIGE) to quantitatively monitor early symbiosis- and pathogenesis-induced changes of the Medicago truncatula root proteome. J Proteomics, 73(4):753-768.
Schüßler A, Schwarzott D, Walker C. 2010. A new fungal phylum. The Glomeromycota: phylogeny and evolution. Mycol Res, 105: 1413–1421.
Sedzielewska KA, Fuchs J, Temsch EM. 2011. Estimation of the Glomus intraradices nuclear DNA content. New Phytol, 192: 794-797.
Smith S.E. 2011. Smith Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol, 62: 227–250.
Smith SE, Barker SJ, Zhu YG. 2006. Fast moves in arbuscular mycorrhizal symbiotic signalling. Trends Plant Sci, 11(8): 369-371.
Tisserant E, Kohler A, Dozolme-Seddas P.2012. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol, 193: 755-769.
Trouvelot A, Kough JL, Gianinazzi-Pearson V. 1986. Mesure dutaux de mycorhization VA d’un systme radiculaire ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Giani-nazzi S (eds), Les mycorhizes, physiologie et genetique, INRA, Paris: 217-221.
Uibopuu A, Moora M, Saks U. 2009. Differential effect of arbuscular mycorrhizal fungal communities from ecosystems along management gradient on the growth of forest understory plant species. Soil Biology & Biochemistry, 41: 2141-2146.
Vallino M, Greppi D, Novero M. 2009. Rice root colonisation by mycorrhizal and endophytic fungi in aerobic soil. Ann Appl Biol, 154(2): 195-204.
Wehner J, Antunes PM, Powell JR. 2010. Plant pathogen protection by arbuscular mycorrhizas: A role for fungal diversity? Pedobiologia, 53: 197-201.
Wu QS, Xia RX, Zou YN. 2006. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol, 163(11): 1101-1110.
Yoneyama K, Xie X, Sekimoto H. 2008. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol, 179(2): 484-494.
Yoshida S, Maruyama S, Nozaki H. 2010. Horizontal gene transfer by the parasitic plant Striga hermonthica. Science, 328:1128.
 Journal of Forestry Research2014年3期
Journal of Forestry Research2014年3期
- Journal of Forestry Research的其它文章
- Effects of shifting cultivation on biological and biochemical characteristics of soil microorganisms in Khagrachari hill district, Bangladesh
- An integrated method for matching forest machinery and a weight-value adjustment
- Finite element analysis of stress and strain distributions in mortise and loose tenon furniture joints
- The in fl uence of silane coupling agent and poplar particles on the wettability, surface roughness, and hardness of UF-bonded wheat straw (Triticum aestivum L.)/poplar wood particleboard
- A modified Murashige and Skoog media for efficient multipleshoot induction in G. arborea Roxb.
- Genetic variation and selection of introduced provenances of Siberian Pine (Pinus sibirica) in frigid regions of the Greater Xing’an Range, Northeast China
