Responses of Maximum Photosystem II Photochemical Efficiency of Phytoplankton Communities to Nutrient Limitation in the Coastal Sea of Qingdao,China
WANG Zhaoyu,WANG Jiangtao,and QI Hongju
Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education,Ocean University of China,Qingdao 266003,P. R.China
1 Introduction
The maximum photochemical efficiency of photosystem II (Fv /Fm) has been extensively used to evaluate the physiological status of phytoplankton communities in oceanographic studies (Geideret al.,1993a; Olaizolaet al.,1996; McMinn and Hegseth,2004; Martinet al.,2010;Painteret al.,2010).Laboratory studies on a range of algal taxa have suggested thatFv /Fmis high and constant under nutrient-replete conditions,and is depressed under nutrient-limited conditions.Fv /Fmrecovers rapidly following resupply of the limiting nutrient (Geideret al.,1993b; Lippemeieret al.,2001; Young and Beardall,2003; Liuet al.,2011; Zhouet al.,2011) and can be used as a basis for bioassays on natural phytoplankton assemblages (Beardallet al.,2001a).
In field studies,Fv /Fmmeasured in the nutrient enrichment experiments has been shown to be an effective diagnostic probe for iron (Fe) limitation (Greeneet al.,1994; Behrenfeldet al.,1996; Olsonet al.,2000; Boydet al.,2001; Gervaiset al.,2002; Mooreet al.,2006; Hopkinsonet al.,2007; Nielsdóttiret al.,2009).For example,following Fe addition,Kolberet al.(1994) reported a 70%increase inFv /Fmwithin 24 h in the nutrient-rich,irondeficient equatorial Pacific.In contrast,lack of sensitivity ofFv /Fmto relief of nitrogen (N) limitation has been found in some low-nitrogen,open-ocean regions (Grazianoet al.,1996; Almazán-Becerril and García-Mendoza,2008; Mooreet al.,2008),althoughFv /Fmhas have some successes in examining N limitation (Bergmannet al.,2002; Bergeset al.,2004; Gobleret al.,2006; Hassleret al.,2011).One potential explanation may be that the phytoplankton assemblages had been well acclimated to the stable environmental conditions of low N and maintained at near-maximal growth rates by efficient nutrient regeneration,despite the limitation of the yields of phytoplankton biomass by low nutrient supply (Mooreet al.,2008).Therefore,if the growth rate is at its maximum,lack ofFv /Fmresponse to N addition may be observed in low-nitrogen regions.In addition,only a few researches have focused on the validity ofFv /Fmas an index of phosphorus (P) limitation.Sylvanet al.(2007) provided the first field measurement usingFv /Fmcoupled with nutrient enrichment bioassays to study P limitation in marine phytoplankton.
Here we performed 7 nutrient enrichment experiments with N and P in the coastal sea of Qingdao,China,in the summer of 2009 and 2010.Fv /Fmwas measured to observe physiological responses of phytoplankton communities to N and P additions.Dissolved inorganic nutrient concentrations and chlorophylla(Chla) responses to added nutrients were also measured.The specific objectives are to determine whether N and P are limiting nutrients in the coastal sea of Qingdao during summer,and to further test the hypothesis thatFv /Fmvariations measured in the nutrient addition experiments can be used to detect N and P limitation of natural phytoplankton populations.
2 Materials and Methods
Qingdao,a city with a high population density and rapid economic development,is adjacent to the western Yellow Sea,China (Fig.1).Previously,relatively little has been reported on the nutrient limitation in the coastal waters of Qingdao.Here ambient nutrient ratios suggested that P was the major limiting nutrient during June−September 2003 (Chen,2005).Diatoms are prevalent during most time of the year,and dinoflagellates,chrysophytes or cyanobacteria are occasionally dominant during May−November (Liet al.,2004; Wen,2007; Yanget al.,2009).Fv/Fmranging from 0.123 to 0.598 throughout the year in Jiaozhou Bay,is highest in autumn and lowest in spring (Fu,2007).
Seven nutrient addition experiments were carried out at two stations (station A,36˚1´14´´N 120˚29´49´´E; station B,35˚58´1´N 120˚24´16´´E) in the coastal sea of Qingdao from late July to mid-September in 2009 and at the end of July 2010 (Fig.1; Table 1).In each experiment,surface water was collected at approximately 15:00 h local time and transported to the laboratory immediately.Then,the samples were prescreened through acid-washed 200 μm mesh to remove large zooplankton,and dispensed into acid-washed 5-liter transparent polyethylene bottles.The bottles were subsequently assigned in triplicate to one of the four treatments: control (no additions),+N(20 μmol L−1NO3−),+P (2 μmol L−1PO43−),and +NP (20 μmol L−1NO3−+ 2 μmol L−1PO43−).Incubations were conducted outdoors,in temperature-controlled water baths to approximatein situlight and temperature levels.Subsamples were collected at around 20:00 h local time on days 0,1 and 2 for measurements ofFv /Fm,Chla,dissolved inorganic nutrients and phytoplankton community compositions.
Fig.1 Map of the coastal sea of Qingdao,China,showing the sampling stations set up for the seven nutrient addition experiments.
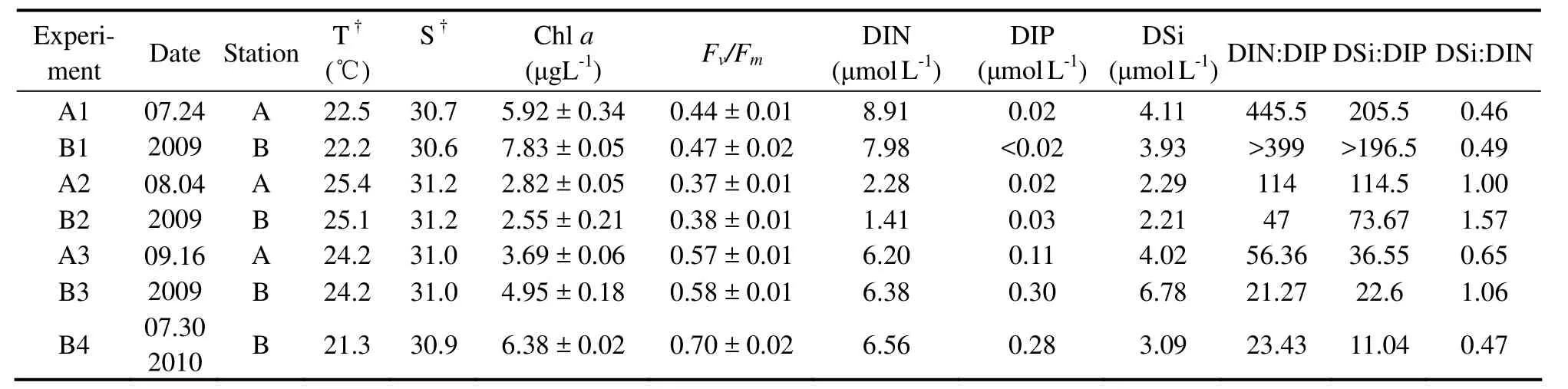
Table 1 Initial conditions for the seven nutrient addition experiments
2.1 Fv /Fm and in vivo Chlorophyll a
Fv /Fmandin vivoChlaconcentrations were obtained using a bbe Cuvette Fluorometer (bbe-Moldaenke GmbH,Kiel,Germany).The fluorometer detects Chlaconcentrations by measuring the chlorophyll fluorescence from LED-stimulation at five different wavelengths (450 nm,525 nm,570 nm,590 nm and 610 nm).The emission wavelength was fixed at 680 nm.The excitation spectrum obtained was compared to the so-called norm spectra stored in the fluorometer and Chlaconcentrations were calculated (Beutleret al.,2002).Fv /Fmwas calculated as(Fm−F0)/Fm,whereF0was the minimal fluorescence determined for 60 s at a very low light intensity below 1 μEm-2s-1,andFmrepresented the maximum fluorescence measured when all the reaction centers were closed by short (900 ms) laser light pulses (Vanselowet al.,1997).Samples were dark adapted for 30 min at ambient temperatures before measurement,and analyses were performed at the same time every night (about 20:00 h local time) to eliminate the effect of light onFv /Fm,making it possible to measure the maximum potential quantum yield.In addition,all fluorescence signals were corrected by subtracting the background fluorescence of 0.2 μm filtered samples.
2.2 Nutrients
Water samples were filtered through acid-washed 0.45 μm cellulose ester filters.Filtrates were frozen at −20℃and later analysed for dissolved inorganic nitrogen (DIN= NO3−+ NO2−+ NH4+),dissolved inorganic phosphorus(DIP = PO43−) and dissolved silicon (DSi = SiO32−) using an automated segmented flow analyzer (QuAAtor,Bran+Luebbe GmbH,Norderstedt,Germany) according to the colorimetric methods (JGOFS,1994).Detection limits were 0.01 μmol L−1for NO3−and NO2−,0.034 μmol L−1for NH4+,0.02 μmol L−1for PO43−,and 0.016 μmol L−1for SiO32−.
2.3 Phytoplankton Cell Counts
Phytoplankton samples were preserved with acid Lugol’s solution,then concentrated in Utermöhl chambers overnight,and enumerated under an Olympus inverted microscope at 200x−400x magnification.At least,300 individuals were counted in each sample.
2.4 Statistical Analyses
The data of ChlaandFv /Fmwere shown as means (n=3) ± standard deviation.One-way analysis of variance(ANOVA) followed by a Tukey post hoc test was employed to evaluate statistical relationships between different treatments.
3 Results
3.1 Initial Conditions
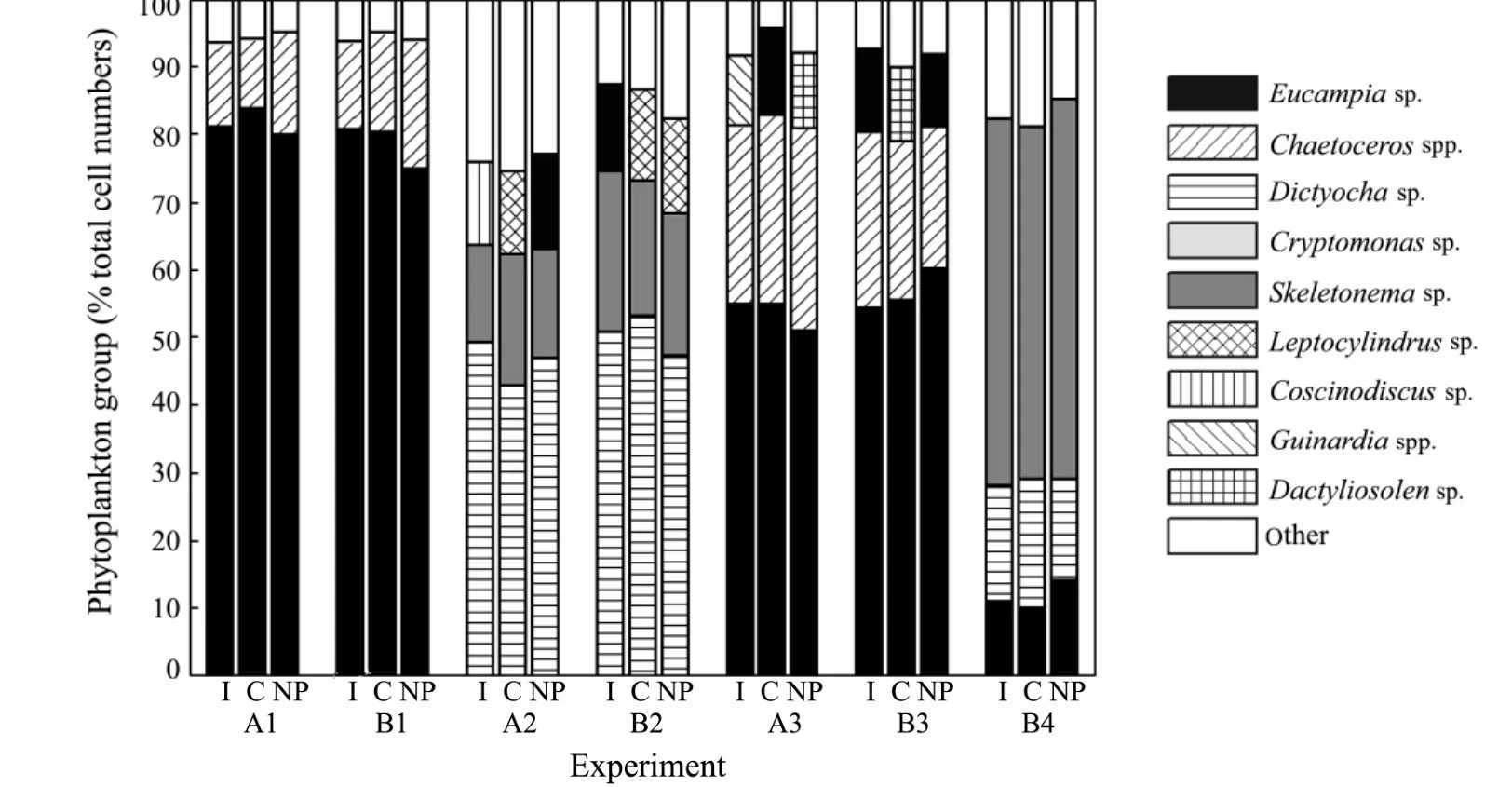
Fig.2 Phytoplankton community composition at the initiation of the seven experiments (I) and after 24 h incubation in the control (C) and nitrate plus phosphate addition treatments (NP).Data are percent contributions of various phytoplankton groups to total cell numbers.Major taxonomic groups are graphed and the ‘other’ category includes Ceratium spp.,Prorocentrum sp.,Rhizosolenia spp.,Nitzschia sp.,etc.,which rarely contributes substantially to total cell numbers individually.
The initial seawater characteristics from each experiment are shown in Table 1.In 4 out of 7 experiments (A1,B1,A2 and B2),DIP concentrations were close to the detection limit.The coefficientFv /Fmfor the initial samples ranged from 0.37 to 0.70.The phytoplankton community compositions were relatively diverse (Fig.2).Briefly,the chrysophyteDictyocha sp.was dominant in experiments A2 and B2; the cryptophyteCryptomonas sp.was prevalent in experiment B4; and diatoms primarilyEucampia sp.andChaetoceros spp.were the most abundant species in the remainder of the experiments.
3.2 Responses to Nutrient Enrichment
In experiments A1,B1 and A2,Fv /Fmof the phytoplankton populations in the P and NP treatments increased to the maximum values about 0.58,about 0.58 and about 0.52 at 24 h respectively,significantly higher than those in the control treatments (P< 0.05; Fig.3).In contrast,Fv /Fmdid not differ statistically between the N and control treatments (P> 0.05).In experiment B2,only simultaneous enrichments of N and P yielded observable increases ofFv /Fmover the control treatments (P< 0.05;Fig.3).Fv /Fmrose to the maximum value of 0.52 ± 0.01 at 24 h.In experiment A3,Fv /Fmin the P and NP treatments remained constant within 48 h,nearly equal to the initial ratios (0.57 ± 0.01),whileFv /Fmin the control and N treatments declined to 0.49 ± 0.01 and 0.48 ± 0.01 at 24 h respectively,dramatically lower than those in the P and NP treatments (P<0.05; Fig.3).In experiments B3 and B4,there was no marked change inFv /Fmin response to any of the nutrient addition within 48 h (P>0.05; Fig.3).The values were about 0.58 in experiment B3 and about 0.70 in experiment B4.

Fig.3 Effects of nutrient enrichments on the maximum photochemical efficiency of photosystem II (Fv /Fm) after 24 h incubation in the seven experiments.C,control; N,nitrate addition; P,phosphate addition; NP,nitrate plus phosphate additions.Error bars represent standard deviations of triplicate measurements.Arrows denote nutrient addition treatments that are significantly different from the control treatments (P < 0.05; ANOVA; Tukey).
In experiments A1,B1,A2 and A3,Chlaincreased by similar amounts in the P and NP treatments relative to all other treatments within 24 to 48 h (P< 0.05; Fig.4).In experiment B2,Chlaconcentrations were the highest in the NP treatments and higher in the P treatments than those in the control and N treatments (P< 0.05; Fig.4).In experiments B3 and B4,however,no significant differrences in Chlaconcentrations were observed between treatments within 48 h (P> 0.05; Fig.4).
With increase of Chlaconcentrations,nutrient concentrations decreased in all the experiments (Figs.5 and 6).For most experiments (A1,B1,A2,B2 and A3),greater drawdown of both DIN and DIP was obtained in the P and NP treatments relative to the control and N treatments.In contrast,for experiments B3 and B4,DIN and DIP drawdown did not differ between treatments.Nearly complete drawdown of DIN and DIP was measured in the P treatments of experiment B2 and in the control and N treatments of experiment A3 at 24 h,respectively.

Fig.4 Changes in chlorophyll a (Chl a) during 48 h incubation following nutrient enrichments in the seven experiments.Each point represents the average of triplicate measurements and error bars represent standard deviation of the means.Abbreviations are the same as those in Fig.3.
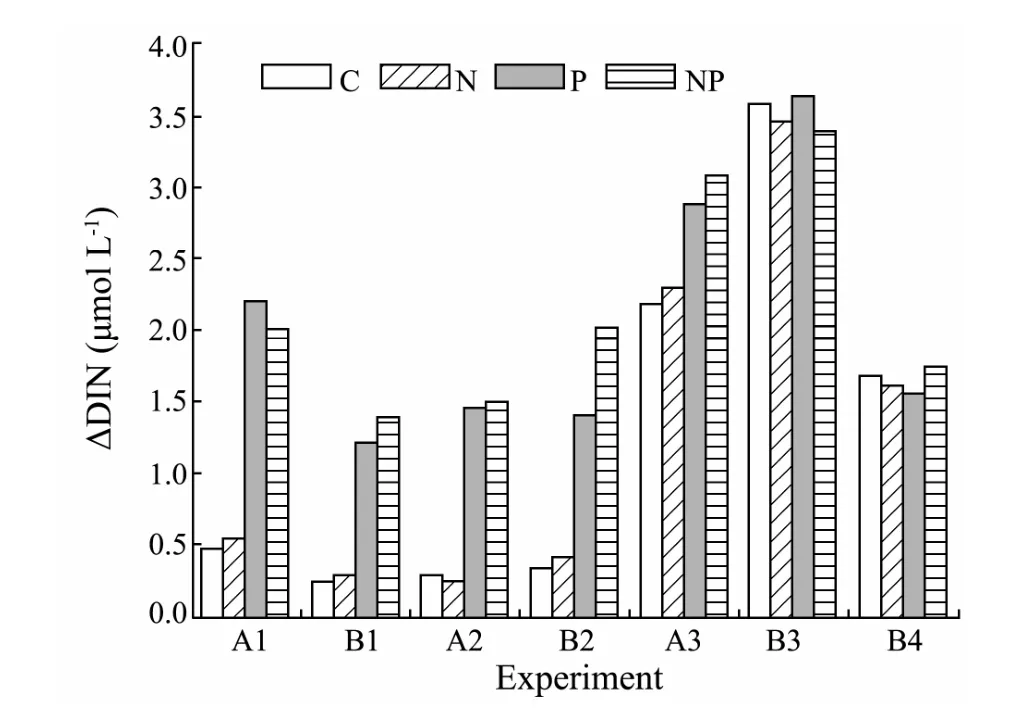
Fig.5 DIN drawdown (△DIN) at 24 h following nutrient enrichments in the seven experiments.Abbreviations are the same as those in Fig.3.

Fig.6 DIP drawdown (△DIP) at 24 h following nutrient enrichments in the seven experiments.Abbreviations are the same as those in Fig.3.
As indicated by microscopic cell counts,phytoplankton community structure for all the experiments was not strongly influenced by the nutrient additions within 24 h.The proportions of the major phytoplankton groups(chrysophytes,cryptophytes and diatoms) did not change by any great amount between the NP and control treatments (Fig.2).
4 Discussion
4.1 Limiting Nutrient in the Coastal Sea of Qingdao
In this study,Fv /Fmand two traditional methods,i.e.criteria of nutrient concentrations and ratios,such as those in Justićet al.(1995) and nutrient enrichment bioassays using chlorophyll response as indicators,were used to determine the nutrient status of phytoplankton communities in the coastal sea of Qingdao during summer.
In experiments A1,B1 and A2,lowFv /Fmat the start of experiments indicated physiological limitations of phytoplankton communities.Following P addition,these limitations were alleviated andFv /Fmwas elevated in the P and NP treatments relative to the control treatments,suggesting that P was the limiting factor.In contrast,in experiments B3 and B4,highFv /Fmin the initial samples and absence of significant change inFv /Fmin response to any of the nutrient addition indicated that N and P were replete and phytoplankton community was healthy.Also,results from the two traditional nutrient-status indicators conformed with theFv /Fmresults.In experiments A1,B1 and A2,low DIP concentrations in the initial samples coupled with Chlaresponses to P addition indicated that P was the limiting element.Conversely,in experiments B3 and B4,a lack of Chlaresponse following nutrient enrichments together with high nutrient concentrations at the time of sampling suggested that N and P were both non-limiting nutrients.
In experiment A3,the initialFv /Fmand phytoplankton community structure were similar to those in experiment B3 (Table 1; Fig.2).Furthermore,Fv /Fmdid not increase after N and P additions and maintained the original values.Therefore,phytoplankton community was near its maximal photosynthetic capacity and in a healthy state on the day of collection.During the incubation,Fv /Fmin treatments without P addition decreased,significantly lower than those with P addition at 24 h,indicating the onset of P limitation.Further supporting evidence was revealed by the DIP concentrations in treatments lacking added P,which declined from 0.11 μmol L-1to below detection after 24 h,suggesting that P was exhausted over the course of incubation.Thus,phytoplankton population was in a healthy statein situand P would first become limiting as growth proceeded.In addition,results from the two traditional nutrient status indicators were consistent with theFv /Fmresults.High DIN/DIP and DSi/DIP ratios in the initial samples and greater Chlaconcentrations at 48 h in treatments with added P than those without supported potentially P limitation.Compared with the two conventional methods,the results based on the efficiencyFv /Fmcan provide more detailed information about the physiological states of phytoplankton populations.Changes inFv /Fmfollowing nutrient supply were observed at 24 h,and preceding Chlaresponses to nutrient additions occurred at 48 h,suggesting that the use ofFv /Fmcoupled with nutrient enrichment experiments can offer more rapid results than bioassays using chlorophyll response as an indicator.
In experiment B2,relative increases inFv/Fmwere only observed in NP treatments,indicating N-P co-limitation.However,if we applied the nutrient criteria to the experiments,the low DIP concentrations and high DIN/DIP and DSi/DIP ratios in the initial samples indicated that P might be the sole limiting nutrient for phytoplankton growth.Based on the results from Chlaresponses,the increases of Chlain treatments with added P compared with those without added P were suggestive of P limitation.Further increases of Chlain the NP treatments over the P treatments indicated that N also limited the phytoplankton growth.Additional evidence for N limitation was provided by the observations of DIN concentrations in the P and NP treatments.At 24 h,considerable DIN concentrations were still available in the NP treatments due to N addition.In contrast,DIN concentrations in the P treatments were close to the detection limit,limiting the growth of phytoplankton communities,resulting in lower Chlaconcentrations andFv /Fmvalues in the P treatments than those in the NP treatments.Therefore,the phytoplankton populations were indeed limited by N.As already mentioned,the responses ofFv /Fmand Chlato nutrient additions here provided clear evidences for N/P co-limitation,similar to those reported by Suggettet al.(2009a) in the Gulf of Aqaba.A low DIP concentration and high DIN/DIP and DSi/DIP ratios do not necessarily suggest only P limitation.The use of nutrient criteria to diagnose co-limitation is problematic.Fv /Fmand Chlacan be more accurate indicators of co-limitation.
As discussed above,in late July of 2009,DIP concentrations were close to the detection limit and the phytoplankton communities were P limited at the two sampling stations in the coastal waters of Qingdao.In early August,the low DIP levels still limited the growth of phytoplankton communities.DIN concentrations were drawn down to low levels.At station B,N was also the limiting nutrient.In mid-September,nutrient concentrations were elevated,which alleviated the nutrient limitation.The phytoplankton community was in a healthy state.N and P were replete at station B.P was the potential limiting nutrient at station A,and would be exhausted firstly as growth proceeded.At the end of July 2010,neither N nor P was limited at station B,which was different from the results in the same period of 2009.
4.2 Application of Fv/Fm As an Indicator of Nutrient Limitation
Our study suggests thatFv/Fmcoupled with 24-h-long nutrient enrichment bioassays could be used to detect P limitation and N/P co-limitation of natural phytoplankton communities.Fv/Fmshows a positive response to P addition in P limited sites and to additions of N and P together in N/P co-limited sites,with no response in nutrient-replete sites,which is consistent with previous laboratory and field results (Geideret al.,1993b; Wykoffet al.,1998; Beardallet al.,2001b; Lippemeieret al.,2001;Sylvanet al.,2007; Suggettet al.,2009a).Moreover,this method can be more accurate for assessing co-limitation than the use of nutrient criteria as indicators,and can provide more rapid results than bioassays using chlorophyll response as indicators,when phytoplankton community is potentially limited.Compared with the two conventional indicators,the results based on theFv /Fmcan also provide more detailed information about the physiological states of the phytoplankton populations.
Kolberet al.(1988) found thatFv /Fmreaches a maximum value of about 0.65 under nutrient-replete conditions,independent of algal taxa.Our study indicates that the maximal values ofFv /Fmfor diatom-dominated communities (about 0.58) and chrysophyte-dominated communities (about 0.52),obtained by nutrient enrichment experiments,do not exceed 0.60,whereasFv /Fmvalues for cryptophyte-dominated communities could be as high as 0.70.Contrary to Kolberet al.(1988),phytoplankton community structure may have an important influence on maximumFv /Fmvalues.Similarly,Suggeettet al.(2009b)pointed out that in nutrient-replete laboratory cultures,Fv/Fmvalues vary among algal taxa,ranging from 0.10 to 0.70.Mooreet al.(2005) showed that diatom-dominated communities have higherFv /Fmthan flagellate-dominated communities during the North Atlantic spring bloom.
As mentioned above,a common maximum value of 0.65 forFv /Fmcannot be assigned to all algae.Algal taxa can exhibit different maximum values.In many cases,Fv/Fmvalues lower than 0.65 are not necessarily interpretable in terms of physiological stress of algal populations.Algae groups with lowFv /Fmunder nutrient-replete conditions may be a contributing factor for the low fluorescence signal,such as cyanobacteria,whoseFv /Fmvalues can be as low as 0.10 (Suggettet al.,2009b).Thus,the use of absolute values ofFv /Fmin situas a tool for ascertaining physiological state of natural populations requires the accurate knowledge ofFv /Fmvalues for nutrient-replete populations in the survey area.
Furthermore,interpretation ofFv /Fmvariations in nutrient addition experiments must be treated with caution,because changes inFv /Fmcan be driven by changes in both nutrient status and phytoplankton community structure.If such experiments are of short duration and thus relatively free of major changes in community composition,the increases inFv /Fmcan only be induced by the changes in nutrient status andFv /Fmcoupled with nutrient enrichment experiments can be valid for determining which nutrient is limiting.However,if such experiments are conducted over long periods and community composition changes very much during incubations,the methods may be problematic,because shifts in community structure may also contribute to the variations inFv /Fm,potentially masking signals caused by the changes in nutrient status.In our 24-h-long nutrient enrichment experiments,the proportions of the major phytoplankton groups did not change by any great amount and the increases inFv/Fmwere only induced by the changes in nutrient status.Therefore,Fv /Fmcoupled with 24-h-long nutrient enrichment experiments can be valid for determining P limitation and N/P co-limitation.
In conclusion,our experiments show thatFv /Fmcoupled with 24-h nutrient enrichment bioassays can be used to detect P limitation and N/P co-limitation of natural phytoplankton communities.In late July of 2009,lowFv/Fmin the initial samples combined withFv /Fmresponses to P addition indicated P limitation at the two stations in the coastal waters of Qingdao.In early August,P was still the limiting nutrient at station A.Relative increases inFv/Fmwere only observed in the NP treatments at station B,suggesting an N/P co-limitation.In mid-September,lack ofFv /Fmresponses following nutrient enrichments together with highFv /Fmat the start of experiment indicated N and P repletion and healthy phytoplankton communities at station B.At station A,the initialFv /Fmand phytoplankton community structure were similar to those at station B andFv /Fmdid not increase after N and P additions,suggesting that communities were healthyin situ.During the incubation,Fv/Fmin treatments without P ad-dition decreased below the levels in the treatments with P addition,suggesting that P is the potential limiting nutrient and would be exhausted first as growth proceeds.At the end of July 2010,neither N nor P was limited at station B.In addition,our study indicates thatFv /Fmmethod can be more accurate for assessing co-limitation than the use of nutrient criteria as indicators,and can provide more rapid results than bioassays using chlorophyll response as an indicator,when phytoplankton community is potentially limited.Compared with the two conventional indicators,the results based on the efficiencyFv /Fmcan also provide more detailed information about the physiological states of the phytoplankton population.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.41076065) and the National Basic Research Program of China (973 Program)(No.2010CB428701).We thank Prof.Ruixiang Li from the First Institute of Oceanography,State Oceanic Administration,for her help in taxonomic identification and enumeration.
Almazán-Becerril,A.,and García-Mendoza,E.,2008.Maximum efficiency of charge separation of photosystem Ⅱ of the phytoplankton community in the Eastern Tropical North Pacific off Mexico: A nutrient stress diagnostic tool?Ciencias Marinas,34: 29-43.
Beardall,J.,Young,E.,and Roberts,S.,2001a.Approaches for determining phytoplankton nutrient limitation.Aquatic sciences,63: 44-69,DOI: 10.1007/PL00001344.
Beardall,J.,Berman,T.,Heraud,P.,Kadiri,M.O.,Light,B.R.,Patterson,G.,Roberts,S.,Sulzberger,B.,Sahan,E.,Uehlinger,U.,and Wood,B.,2001b.A comparison of methods for detection of phosphate limitation in microalgae.Aquatic Sciences,63: 107-121,DOI: 10.1007/PL00001342.
Behrenfeld,M.J.,Bale,A.J.,Kolber,Z.S.,Aiken,J.,and Falkowski,P.G.,1996.Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean.Nature,383: 508-511,DOI: 10.1038/383508a0.
Berges,J.A.,Gibson,C.E.,and Stewart,B.M.,2004.Physiological responses of phytoplankton communities in the Irish Sea to simulated upwelling.Hydrobiologia,517: 121-132,DOI: 10.1023/B:HYDR.0000027338.38899.d2.
Bergmann,T.,Richardson,T.L.,Paerl,H.W.,Pinckney,J.L.,and Schofield,O.,2002.Synergy of light and nutrients on the photosynthetic efficiency of phytoplankton populations from the Neuse River Estuary,North Carolina.Journal of Plankton Research,24: 923-933,DOI: 10.1093/plankt/24.9.923.
Beutler,M.,Wiltshire,K.H.,Meyer,B.,Moldaenke,C.,Lüring,C.,Meyerhöfer,M.,Hansen,U.-P.,and Dau,H.,2002.A fluorometric method for the differentiation of algal populationsin vivoandin situ.Photosynthesis Research, 72: 39-53,DOI: 10.1023/A:1016026607048.
Boyd,P.W.,and Abraham,E.R.,2001.Iron-mediated changes in phytoplankton photosynthetic competence during SOIREE.Deep-Sea ResearchII,48: 2529-2550,DOI: 10.1016/S 0967-0645(01)00007-8.
Chen,L.,2005.Preliminary analysis of the environmental quality and estimated calculation of environmental capacities in the 2008 Olympics boat sailing field of Qingdao.Master thesis,Ocean University of China,Qingdao,111pp (in Chinese with English abstract).
Fu,X.,2007.Studies on photosynthesis of phytoplankton in China sea: modeling of primary production andin vivochlorophyll fluorescence measurement.Ph.D.thesis,Institute of Oceanology,Chinese Academy of Sciences,Qingdao,144pp(in Chinese with English abstract).
Geider,R.J.,Greene,R.M.,Kolber,Z.,Macintyre,H.L.,and Palkowski,P.G.,1993a.Fluorescence assessment of the maximum quantum yield efficiency of photosynthesis in the western North Atlantic.Deep-Sea ResearchI,40: 1205- 1224,DOI: 10.1016/0967- 0637(93)90134-o.
Geider,R.J.,Roche,J.L.,Greene,R.M.,and Olaizola,M.,1993b.Response of the photosynthetic apparatus ofPhaeodactylum tricornutum(bacillariophyceae) to nitrate,phosphate,or iron starvation.Journal of Phycology,29: 755-766,DOI: 10.1111/j.0022-3646.1993.00755.x.
Gervais,F.,Riebesell,U.,and Gorbunov,M.Y.,2002.Changes in primary productivity and chlorophyllain response to iron fertilization in the Southern Polar Frontal Zone.Limnology and Oceanography,47: 1324-1335,DOI: 10.4319/lo.2002.47.5.1324.
Gobler,C.J.,Buck,N.J.,Sieracki,M.E.,and Sañudo-Wilhelmy,S.A.,2006.Nitrogen and silicon limitation of phytoplankton communities across an urban estuary: The East River-Long Island Sound system.Estuarine,Coastal and Shelf Science,68: 127-138,DOI: 10.1016/j.ecss.2006.02.001.
Graziano,L.M.,Geider,R.J.,Li,W.K.W.,and Olaizola,M.,1996.Nitrogen limitation of North Atlantic phytoplankton:analysis of physiological condition in nutrient enrichment experiments.Aquatic Microbial Ecologu,11: 53-64,DOI:10.3354/ame011053.
Greene,R.M.,Kolber,Z.S.,Swift,D.G.,Tindale,N.W.,and Falkowski,P.G.,1994.Physiological limitation of phytoplankton photosynthesis in the eastern equatorial Pacific determined from variability in the quantum yield of fluorescence.Limnology and Oceanography,39: 1061-1074,DOI:10.4319/lo.1994.39.5.1061.
Hassler,C.S.,Djajadikarta,J.R.,Doblin,M.A.,Everett,J.D.,and Thompson,P.A.,2011.Characterisation of water masses and phytoplankton nutrient limitation in the East Australian Current separation zone during spring 2008.Deep-Sea Research Ⅱ,58: 664-677,DOI: 10.1016/j.dsr2.2010.06.008.
Hopkinson,B.M.,Mitchell,B.G.,Reynlods,R.A.,Wang,H.,Selph,K.E.,Measures,C.I.,Hewes,C.D.,Holm-Hansen,O.,and Barbeau,K.A.,2007.Iron limitation across chlorophyll gradients in the southern Drake Passage: Phytoplankton responses to iron addition and photosynthetic indicators of iron stress.Limnology and Oceanography,52: 2540-2554.
JGOFS,1994.Protocols for the Joint Global Ocean Flux Study core measurements.International JGOFS Report Series,19,174pp.
Justić,D.,Rabalais,N.N.,Turner,R.E.,and Dortch,Q.,1995.Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences.Estuarine,Coastal and Shelf Science,40: 339-356,DOI:10.1016/S0272-7714(05)80014-9.
Kolber,Z.,Zehr,J.,and Falkowski,P.,1988.Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II.Plant Physiology,88: 923-929,DOI: 10.1104/pp.88.3.923.
Kolber,Z.S.,Barber,R.T.,Coale,K.H.,Fitzwater,S.E.,Greene,R.M.,Johnson,K.S.,Lindley,S.,and Falkowski,P.G.,1994.Iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean.Nature,371: 145-148,DOI:10.1038/371145a0.
Li,R.,Wang,B.,Wang,Z.,Wu,R.,and Feng,M.,2004.The status of chemical and biological factors and mesocosm experiment of eutrophication to cause bloom in the area for the Olympic sailing games in Qingdao.Acta Ecologica Sinica,24:837-842 (in Chinese with English abstract).
Lippemeier,S.,Hintze,R.,Vanselow,K.H.,Hartig,P.,and Colijn,F.,2001.In-line recording of PAM fluorescence of phytoplankton cultures as a new tool for studying effects of fluctuating nutrient supply on photosynthesis.European Journal of Phycology,36: 89-100,DOI: 10.1017/S0967026201003031.
Liu,S.,Guo,Z.,Li,T.,Huang,H.,and Lin,S.,2011.Photosynthetic efficiency,cell volume,and elemental stoichiometric ratios inThalassirosira weissflogiiunder phosphorus limitation.Chinese Journal of Oceanology and Limnology,29:1048-1056,DOI: 10.1007/ s00343-011-0224-2.
Martin,J.,Tremblay,J-É.,Gagnon,J.,Tremblay,G.,Lapoussière,A.,Jose,C.,Poulin,M.,Gosselin,M.,Gratton,Y.,and Michel,C.,2010.Prevalence,structure and properties of subsurface chlorophyll maxima in Canadian Arctic waters.Marine Ecology Progress Series,412: 69-84,DOI: 10.3354/meps08666.
McMinn,A.,and Hegseth,E.N.,2004.Quantum yield and photosynthetic parameters of marine microalgae from the southern Arctic Ocean,Svalbard.Journal of the Marine Biological Association of the United Kingdom,84: 865-871,DOI:10.1017/S0025315404010112h.
Moore,C.M.,Lucas,M.I.,Sanders,R.,and Davidson,R.,2005.Basin-scale variability of phytoplankton bio-optical characteristics in relation to bloom state and community structure in the Northeast Atlantic.Deep-Sea Research I,52: 401-419,DOI: 10.1016/j.dsr.2004.09.003.
Moore,C.M.,Mills,M.M.,Milne,A.,Langlois,R.,Achterberg,E.,Lochte,K.,Geider,R.J.,and Roche,J.L.,2006.Iron supply limits primary productivity during spring bloom development in the central North Atlantic.Global Change Biology,12: 626-634,DOI: 10.1111/j.1365-2486.2006.01122.x.
Moore,C.M.,Mills,M.M.,Langlois,R.,Milne,A.,Achterberg,E.P.,Roche,J.L.,and Geider,R.J.,2008.Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean.Limnology and Oceanography,53: 291-305.
Nielsdóttir,M.C.,Moore,C.M.,Sanders,R.,Hinz,D.J.,and Achterberg,E.P.,2009.Iron limitation of the postbloom phytoplankton communities in the Iceland Basin.Global Biogeochemical Cycles,23: GB3001,DOI: 10.1029/2008GB 003410.
Olaizola,M.,Geider,R.J.,Harrison,W.G.,Graziano,L.M.,Ferrari,G.M.,and Schlittenhardt,P.M.,1996.Synoptic study of variations in the fluorescence-based maximum quantum efficiency of photosynthesis across the North Atlantic Ocean.Limnology and Oceanography,41: 755-765.
Olson,R.J.,Sosik,H.M.,Chekalyuk,A.M.,and Shalapyonok,A.,2000.Effects of iron enrichment on phytoplankton in the Southern Ocean during late summer: Active fluorescence and flow cytometric analyses.Deep-Sea Research II,47: 3181-3200,DOI: 10.1016/ S0967-0645(00)00064-3.
Painter,S.C.,Lucas,M.I.,Stinchcombe,M.C.,Bibby,T.S.,and Poulton,A.J.,2010.Summertime trends in pelagic biogeochemistry at theP orcupine Abyssal Plain study site in the northeast Atlantic.Deep-Sea Research II,57: 1313-1323,DOI: 10.1016/j.dsr2.2010.01.008.
Suggett,D.J.,Stambler,N.,Prášil,O.,Kolber,Z.,Quigg,A.,Vázquez-Domínguez,E.,Zohary,T.,Berman,T.,Iluz,D.,Levitan,O.,Lawson,T.,Meeder,E.,Lazar,B.,Bar-Zeev,E.,Medova,H.,and Berman-Frank,I.,2009a.Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba.Aquatic Microbial Ecology,56:227-239,DOI: 10.3354/ame01357.
Suggett,D.J.,Moore,C.M.,Hickman,A.E.,and Geider,R.G.,2009b.Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state.Marine Ecology Progress Series,376:1-19,DOI: 10.3354/meps07830.
Sylvan,J.B.,Quigg,A.,Tozzi,S.,and Ammerman,J.W.,2007.Eutrophication-induced phosphorus limitation in the Mississippi River plume: Evidence from fast repetition rate fluorometry.Limnology and Oceanography,52: 2679-2685.
Vanselow,K.H.,Hintze,R.,Hartig,P.,and Colijn,F.,1997.Comparison of different fluorometers (PAM,1-Hz,BackScat and Turner) measuring either chlorophyll concentration or photosynthetic activity.German Journal of Hydrography,49:375-383.DOI: 10.1007/ BF02764046.
Wen,D.,2007.Study on annual variation of phytoplankton assemblage in the Xiaoqingdao,China.Master thesis,Ocean University of China,Qingdao,45pp (in Chinese with English abstract).
Wykoff,D.D.,Davis,J.P.,Melis,A.,and Grossman,A.R.,1998.The regulation of photosynthetic electron transport during nutrient deprivation inChlamydomonasreinhardtii.Plant Physiology,117: 129-139,DOI: 10.1104/pp.117.1.129.
Yang,S.,Dong,S.,Dou,M.,Liu,C.,and Wu,Y.,2009.Features of phytoplankton communigy weekly observed at a station south of Qingdao from 2004 to 2005.Advances in Marine Science,27: 523-536 (in Chinese with English abstract).
Young,E.B.,and Beardall,J.,2003.Photosynthetic function inDunaliella tertiolecta(chlorophyta) during a nitrogen starvation and recovery cycle.Journal of Phycology,39: 897-905.
Zhou,J.,Gal,C.,Li,Y.,Jiang,M.,Zhang,Y.,and Wang,Z.,2011.Effects of different supplement ways of phosphate on the growth and toxins production ofAlexandrium tamarense.Advances in Marine Science,29: 487-497 (in Chinese with English abstract).
 Journal of Ocean University of China2014年1期
Journal of Ocean University of China2014年1期
- Journal of Ocean University of China的其它文章
- Estimating the Budgets of Nutrients for Phytoplankton Bloom in the Central Yellow Sea Using a Modified Lower Tropic Ecosystem Model
- Phytoplankton Assemblage Structure Shaped by Key Environmental Variables in the Pearl River Estuary,South China
- Purification and Characterization of 2-Haloacid Dehalogenase from Marine Bacterium Paracoccus sp.DEH99,Isolated from Marine Sponge Hymeniacidon perlevis
- Molecular Phylogeny of Parapenaeopsis Alcock,1901(Decapoda: Penaeidae) Based on Chinese Materials and 16S rDNA and COI Sequence
- Cytogenetic Mechanism for the Aneuploidy and Mosaicism Found in Tetraploid Pacific Oyster Crassostrea gigas (Thunberg)
- Effect of Hydraulic Loading Rate on the Efficiency of Effluent Treatment in a Recirculating Puffer Aquaculture System Coupled with Constructed Wetlands
