Expression of HSP90 and HIF-1α in human colorectal cancer tissue and its significance
*
1Department of Hepatobiliary Surgery, the First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an, Shaanxi Province 710061, China
2Department of Neurosurgery, the First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an, Shaanxi Province 710061, China
Expression of HSP90 and HIF-1α in human colorectal cancer tissue and its significance
Qiu-Ran Xu1, Xin Liu2, Ying-Min Yao1, Qing-Guang Liu1*
1Department of Hepatobiliary Surgery, the First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an, Shaanxi Province 710061, China
2Department of Neurosurgery, the First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an, Shaanxi Province 710061, China
Objective:To investigate the expression of HSP90 and HIF-1α in human colorectal cancer tissue, the influence of HSP90 and HIF-1α on human colorectal cancer biological behavior and their related factors.Methods:The expression of HSP90 and HIF-1α protein in human colorectal cancer as well as normal tissue were detected by immunohistochemical method.
Results:The positive expression rates of HSP90 and HIF-1α protein in normal human colorectal tissue as well as colorectal cancer tissue were 30%vs. 63.0%, 15.0%vs. 71.7%, respectively. There were significant difference (P=0.035 andP=0.005 respectively). The expression of HSP90 was significantly correlated with the differentiation, Dukes stages and lymph node metastasis (P<0.05), while the expression of HIF-1α was significantly correlated with the Dukes stages and lymph node metastasis (P<0.05). Association analysis showed that the expression of HSP90 protein was significantly correlated with that of HIF-1α protein(P<0.01).Conclusions:The expression of HSP90 and HIF-1α protein may be related to the development, metastasis and invasion of human colorectal cancer; and their synergistic effects may participate in the development of the colorectal carcinoma.
ARTICLE INFO
Article history:
Received 10 March 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 September 2014
Colorectal cancer
1. Introduction
Colorectal cancer includes large bowel cancer (colon cancer) and cancer of the back passage (rectal cancer or cancer of the rectum), with higher morbidity and mortality. In recent years, its prevalence shows an upward trend year by year and the ratio of male to female is about 1:1[1]. Heat shock protein 90 (Hsp90) plays an important role in vascularization, invasion and metastasis of tumors, proliferation of tumor cells, time course of cell cycles, as well as the conformation, stability and function maintenance of several carcinogenic proteins involving in the signal transduction pathway of cell apoptosis[2]; while Hypoxia-inducible factor (HIF)-1a could regulate tumor angiogenesis, glycolysis and invasiveness of tumors[3-5]. In our study, we detected the expression levels of HSP90 and HIF-1α by immunohistochemical SABC method in the colorectal cancer tissues, observed their relationships with the clinicopathologic features of colorectal cancer and explored their correlations, in order to investigate the effects of HSP90 and HIF-1α on the occurrence and development of colorectal cancer, as well as their roles in the invasion and metastasis process.
2. Materials and methods
2.1. General data
Paraffin-embedded tissue samples between October 2012and January 2004 and the fresh colorectal cancer tissues by surgical resection between October 2012 and January 2013 were selected. None had radio- and chemotherapy before and all cases were verified by pathological examination after operation. 46 cases of colorectal adenocarcinoma were enrolled in total, including 28 male and 18 female patients. The median age was 60.5 (36-81) years. These 46 cases included 25 cased with colon cancer and 21 cases with rectal cancer; 29 cases with high and medium differentiated adenocarcinoma and 17 cases with poorly differentiated adenocarcinoma; 16 cases with lymphatic metastasis and 30 cases without lymphatic metastasis; 30 cases at Dukes A+B and 16 case at Dukes C+D in accordance with the Dukes’stage. Meanwhile, 20 normal tissues were selected more than 10 cm away from the site of the tumor as the control group.
2.2. Primary reagents and methods
Mouse anti-human HSP90 monoclonal antibody (at a dilution of 1:100) and mouse anti-human HIF-1α monoclonal antibody (1:100) were purchased from Abcam. Secondary and third antibodies were obtained from Vector and Sigma respectively.
They were fixed by 4% paraformaldehyde, dehydrated, transparentized and paraffin embedded to obtain 4 µm serial paraffin sections. Then slices were baked using an electrothermostat, xylene dewaxed to water and membrane rupture. Slices were heated by two changes of medium temperature, heat-induced microwave in solution of citrate buffer (pH 6.0) for 5 min, with pause duration for 3 min. Thereafter, all the slices were washed with PBS and added with 3% H2O2to initiate the reaction away from light at room temperature for 30 min. They were washed again with PBS and the reaction started in the incubator at 37 ℃ for 20 min with the diluted enzyme k (1:2 000). They were washed again, reacted in the incubator at 37 ℃ for 30 min with 2% BSA; the slices were dried as much as possible to remove the liquid, then primary antibody was added. They were incubated for 36 h at 4 ℃ and equilibrated to room temperature for 2 h. They were washed again, added with secondary antibody and incubated at 37 ℃ for 1 h. Then they were washed with PBS, added withSABC solution to co-incubate at 37 ℃ for 1 h. Remaining antibody was washed off, then they were developed with DAB for 8-10 min, redyed with hematoxylin, then the slides were dehydrated routinely until they became transparent. They were sealed with neutral resin. In negative control group the primary antibody was replaced with PBS, while in the positive control group the available HSP90 and HIF-1α positive colorectal cancer tissue slices were used.
2.3. Evaluation criteria of the results
5 high-power fields were selected randomly in each slice, the percentages of positive tumor cells were: <10%, 0; 10%-25%, 1; 26%-50%, 2; 51%-75%, 3; >75%, 4. Staining intensity scores were: 0 was into colorless, 1 was divided into weak (pale yellow), 2 was divided into medium (brown), 3 was classified as strong (tan). Finally, the percentages of positive tumor cells were multiplied by the staining intensity scores:≤2 was divided into negative and ≥3 was divided into positive.
2.4. Statistical analysis
SPSS 12.0 software was used for statistical analysis of all the results, in which Spearman correlation test was adopted for correlation analysis andChi-square test for data comparison between both groups. P<0.05 was considered as statistical significant difference.
3. Results
3.1. Expression percentages of HSP90 and HIF-1α in colorectal cancer tissues and normal tissues
HSP90 was mainly expressed in the cytoplasm and nuclei of tumor cells as brown particles (Figure 1A), with the positive percentages in the normal tissues were significantly lower than those in the colorectal cancer tissues (30.0%vs. 63.0%) (Χ2=6.110,P=0.013) (Table 1). The amount of HIF-1α expressed in the cytoplasm and/or nuclei was highest, and appeared as brown particles (Figure 1B). The positive percentages of HIF-1α in the colorectal cancer tissues were 15.0% and 71.7% respectively, with statistical significant difference (Χ2=18.100,P<0.001) (Table 1).
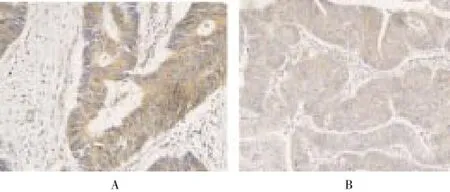
Figure 1. Expressions in colorectal cancer tissues (SABC method, magnification 400 times).A: HSP90; B: HIF-1α.
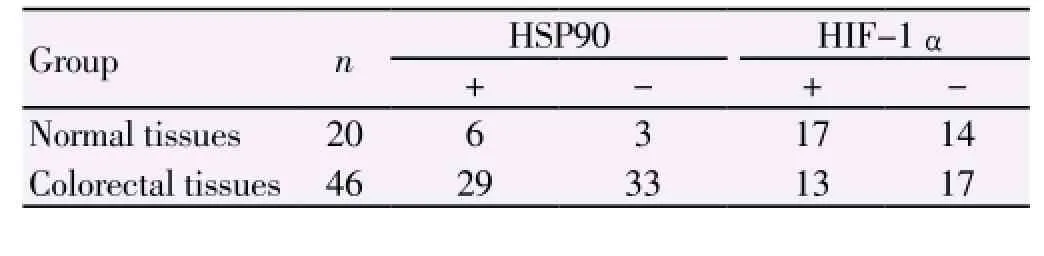
Table 1 Expression percentages of HSP90 in colorectal cancer tissues and normal tissues.
3.2. Expressions of HPS90 and HIF-1α in colorectal cancer and their respective correlations with the clinicopathologic features of colorectal cancer
There was no correlation of HSP90 expressions with patients’ sex, age, site of tumor without statistical significances (P>0.05). We found that HSP90 expressions were related with tumor differentiated degree, Dukes staging and lymph nodes metastasis with statistical significances (P<0.05), which were in line with the results from Dong Xinget al[6-8]. Also, there was no correlations of HIF-1α expressions with patients’ sex, age, site of tumor without statistical significances (P>0.05). We found that HSP90 expressions were related with Dukes staging and lymph nodes metastasis with statistical significances (P<0.05), which coincided with the results from Yoshimuraet al[1,9] (Table 2)

Table 2 Expressions of HSP90 and HIF-1α in colorectal cancer and their respective correlations with the clinicopathologic features of colorectal cancer.
3.3. Correlation of HSP90 and HIF-1α expressions in colorectal cancer tissues
There were 25 cases with positive HSP90 and HIF-1α, 9 cases with negative HSP90 and HIF-1α. Eight positive HIF-a cases showed negative HSP90 result; while 9 positive HSP90 cases showed negative HIF-1α result. Spearman correlation test showed that a positive correlation between HSP90 and HIF-1α in colorectal cancer tissues (γ=0.420,P<0.01).
4. Discussion
The occurrence, development, metastasis and invasion of neoplams are result from interactions among polygenes, multiple factors and multi-stages.
HSPs, also known as molecular chaperon is essential for regulating intracellular protein balance with highly conserved amino acid sequence. Its main functions include direct protein folding within the cytoplasm, endoplasmic reticulum and mitochondria and its transmission and intracellular localization, repair and degrade denatured proteins and refold the misfolded proteins to prevent misparing and accumulation of proteome. In addition, HSPsinvolves in the final step of activations of several regulatory proteins in eukaryotic cells, the remolding of several macromolecular protein complexes which comprises the signal transduction, transcription, cell division and migrated and differentiated proteins. Furthermore, in the presence of stressors such as high temperature, heavy metal, hypoxia and acidosis, the content of HSPs will increase[10,11].
Based on the molecular weight of HSPs, it can be classed into several types such as HSP110, 90 and 70[12]. HSP90 which is mainly comprised of HSP90a and HSP90β, is a macromolecular protein with the molecular weight of 90KD, largest part of which exists in GRP94 of the endoplasmic reticulum (glucose regulated protein 94) and TRAP1 of the mitochondria (TNF receptor regulating protein)[13]. Under physiological status, HSP90 accounts for 1% to 2% total proteins in cells; while in a state of stress, its content increased by about 2 to 10 times[6,10,11]. It has been demonstrated in previous studies that, the expression levels of HSPs in several solid tumors (breast cancer[10], hepatocellular carcinoma[14], gastric cancer, nasopharynx cancer and ovarian cancer[15,16]) and hematological malignancies have been increased[10].
The target proteins of HSP90 include matrix metalloproteinase 2 and urokinase involving in the process of tumor cells invasion and metastasis, hypoxia-inducible factor (HIF) that maintains revascularization, epidermal growth factor receptor (rEFGR), steroid receptor coactivator andetc[11,13], which play a role by regulating the function of protein kinase B, tumor necrosis factor receptor, nuclear factor κB to inhibit the apoptosis of tumor cells. Researches have shown that the expression levels of HSP complex in colon cancer are correlated with proliferating cell nuclear antigen, considering HSP complex may contributes to the rapid proliferation of tumor cells[7,11]. In our study, the positive percentages of HSP90 in normal and colorectal adenocacinoma tissues were 30% and 63%, respectively, with statistical significance. Therefore, the apparently higher expression levels of HSP90 in colorectal cancer tissues than those in the normal tissues indicates that the existing stressors such as hypoxia and acidosis at the beginning of colorectal cancer formation may contribute to the statistically higher HSP90 expression level in colorectal cancer tissues than those in the normal tissues. Meanwhile, it is demonstrated that there are statistical differences between both groups in the expressions and differentiation degree of HSP90, Dukes staging and their relationship with lymph nodes metastasis (P<0.05), which indicate that HSP90 not only forms at the early stage of colorectal cancer, but also involves in several processes such as differentiation, invasion and metastasis.
HIF-1, a transcriptional activator of basic helix-loop-helix/ PER-ARNT-SIM, is comprised of HIF-1β and inducible expressed HIF-1α. The continuous expression of HIF-1β is not effected by the environment. As the major regulating component, the content of HIF-1α may be increased by several approaches like hypoxia and genetic variation[4,17-19]. Higher expressions of several types of tumors like colon, breast, gastric, lung, skin, ovarian, pancreatic, prostate and renal carcinoma can be observed[20].
As a transcriptional activator, HIF-1α involves in the gene coding procedures of 13 different glucose transporters, glycolytic enzymes and VEGF, as well as the coding of erythrogenin, transferrin, endothelin-1, inducible synthetase, hemeoxyganase 1, IGF-2, IGF binding protein 2 and 3, where most of the proteins are related to the occurrence and development of tumors[3,20]. In our study, the positive percentages of HIF-1α in normal and colorectal cancer tissues are 15.0% and 71.7%, respectively with statistical significance (P<0.05). Along with previous study observations that high expression levels of HIF-1α in noncancerous lesions like colonic adenoma and prostatic intraepithelial neoplasia[20-22], it can be manifested that the increased levels of HIF-1α expression may be the results of the higher expressions of several genes that facilitate the occurrence and development of tumors, thus provide advantageous environment for the occurrence of colorectal cancer. In addition, HIF-1α can regulate and encode genes that play an important role in the pathophysiological process of cell invasion which including cathepsin D, MMP2, urokinase-type plasminogen activator receptor, fibronectin 1, vimentin TGFa, autocrine motility factors, keratin 14, 18 and 19. It has been verified that even if tissues experience an instant of hypoxia, the invasiveness of tumor cells towards basilar membranein vitroalso increase. While in our study, the expression of HIF-1α correlates with Dukes staging and lymph nodes metastasis with statistical significance (P<0.05), indicating HIF-1α may also participate in the gene regulation of proteins related to tumor metastasis and invasion and is essential for the invasion and metastasis of colorectal cancer.
According to the analysis of our results, there is a positive correlation between HSP90 and HIF-1α (r=0.420), with statistical significance (P<0.01), showing they may exert a synergistic effect on the occurrence, development, invasionand metastasis of colorectal cancer. Therefore, it reminds us the combination of HSP90 and HIF-1α inhibitor may further improve the treatment efficacy of colorectal cancer.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Li C, Lu HJ, Na FF, Deng L, Xue JX, Wang JW, et al. Prognostic role of hypoxic inducible factor expression in non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev 2013; 14(6): 3607-3612.
[2] Milicevic Z, Bogojevic D, Mihailovic M, Petrovic M, KrivokapicZ. Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int J Oncol 2012, 32(6): 1169-1178.
[3] Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 2011; 63(5): 1138-1143.
[4] Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC.. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon caner. Cancer Res 2011; 64(5): 1765-1772.
[5] Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of αlpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. Bio Med Central Cancer 2009; 9: 432
[6] Dong X, Lang L, Yu WJ. HSP90a and HSP70 expression in colorectal cancer and its biological behavior relationship. Chin J General Surg 2011; 20(10): 1120-1122.
[7] Chen Y, Ran ZH, Chen X, Xiao SD. Expression of heat shock protein 70 and 90 and their relationships with biological behaviors of colon cancer. J World Chin Digest Mag 2012; 14(33): 3201-3205.
[8] Zhu Q, Hu Y, Gong LS, Zhang YD. Expression of HSP90 beta in colon cancer tissues and cells and its relationship with chemotherapy drug resistance. Chin J General Surg 2011; 18(4): 353-357.
[9] Ding Z, Yang L, Xie X, Xie F, Pan F, Li J, et al. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/ P-glycoprotein in human colon carcinoma tissue and cell. J Cancer Res Clin Oncol 2010; 136(11):1687-1707.
[10] Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nature 2013; 5: 761-772.
[11] Trotta AP, Need EF, Selth LA, et al. Knockdown of the cochaperone SGTA results in the suppression of androgen and PI3K/Akt signaling and inhibition of prostate cancer cell proliferation. Int J Cancer 2013; 133(12): 2812-2823.
[12] Bagatell R, Whitesell L. Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Caner Ther 2012; 3(8): 1021-1030.
[13] Geotz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol 2013; 14(8):1169-1176.
[14] Workman P, Burrows F, Neckers L, Rosen N.. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addition and tumor stress. Anna New York Academy Sci 2012; 1113: 202-216.
[15] Qi XL, Bo AH, Yue SC, Wang CY, Liu HB. Heat shock protein 90 mrna expressed in adenocarcinoma and adenoma of significance. Proceed Military Academy Med Sci 2011; 30(4): 351-353.
[16] Bo AH, Dai J, Li SG, Zuo DS, Chen XL, Yan YJ, et al. HSP90mRNA expressed in stomach and large intestine cancer research. Chin J Histochem Cytochem 2011; 14(2): 213-216.
[17] Zhu Q, Zhang YD, Hu Y, Liu H, Lu Y, Gong LS, et al. Expression of heat shock protein 90 beta in colorectal tissues and its implications . Modern Biomed Progr 2012; 7(7): 1042-1044.
[18] Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression,which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 2012; 277(41): 38205-38211.
[19] Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, et al. HIF-1 α and HIF-2 alpha have divergent roles in colon cancer. Int J Cancer 2011; 124(4): 763-771.
[20] Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2012; 3(10): 721-732.
[21] Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1a in common human cancers and their metastases. Cancer Res 2012; 59(22): 5830-5835.
[22] Donderski R, Szczepanek J, Domagalski K, Tretyn A, Korenkiewicz J, Marszałek A, et al. Analysis of relative expression level of VEGF, HIF-1α and CTGF genes in chronic glomerulonephritis patients. Kidney Blood Press Res 2014; (1): 83-91.
ment heading
10.1016/S1995-7645(14)60123-1
*Corresponding author: Qing-Guang Liu, MD, Department of Hepatobiliary Surgery, the First Affiliated Hospital of Medical College of Xi’an Jiaotong University, No. 277, West Yanta Road, Xi’an, Shaanxi Province 710061, China.
Tel: 029-85323989
E-mail: liuqingguang@vip.sina.com
Foundation project: It is supported by Shaanxi province health department key funds: sx201227273.
Heat shock protein 90
Hypoxia inducible-factor 1-α Immunohistochemical method
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Sonic Hedgehog signaling pathway in primary liver cancer cells
