Effect of dietary defatted diatom biomass on egg production and quality of laying hens
Xiangjun Leng,Kun-Nan Hsu,Richard E Austicand Xin’gen Lei*
Effect of dietary defatted diatom biomass on egg production and quality of laying hens
Xiangjun Leng1,2,Kun-Nan Hsu1,Richard E Austic1and Xin’gen Lei1*
Background:This study was to determine if feeding laying hens with defatted diatom microalgal biomass(DFA) from biofuel production affected their egg production and health status.
Defatted diatom algae,Egg,Health,Laying hen,Soybean
Background
Although fossil fuels are the major source of energy for heating,transportation,manufacturing,and the generation of electricity,these fuels are non-renewable.Therefore,the search for renewable energy sources has become a key challenge of this century.Microalgae are recognized as one of the oldest living micro-organisms,and are seen as a very attractive source of renewable biofuels,especially biodiesel.Many species of microalgae contain large amounts of lipids that are suitable for the production of biofuels[1].Microalgae are the natural food source for many important aquaculture species such as molluscs,shrimps and fish[2].Several species of microalgae have been reported to be acceptable for inclusion in diets for swine,rabbits,broiler chickens,laying hens,and ruminant animals[3,4].Soybean meal is a by-product of the extraction of oil from soybeans. With high protein content,it is rich in essential amino acids and serves as the main protein source for foodproducing animals.Due to the increased human demand for soybean products,soybean meal is becoming more expensive and limited in supply[5,6].Therefore, it is important to explore other high protein feedstuffs for the sustainable development of animal production.
Microalgae are a rich source of protein,essential fatty acids,vitamins and minerals[3].After lipid removal,the residual biomass contains even higher concentrations of protein and other nutrients.Microalgae are good sources of long chain polyunsaturated fatty acids(PUFA)and have been used to enrich diets with omega-3 PUFA [7-9].Diatoms are a class of microalgae that characteristically accumulate amorphous silicon in their membranes, resulting in distinct external features called frustules[10]. The defatted diatom biomass contains protein,residual fat,carbohydrates,silicon and a large mineral fraction that includes calcium,phosphorus,sodium,potassium,chloride,and several other nutritionally significant minerals[3,11-13].At the present time,a diatom species,Staurosira sp,is under investigation as a lipid source for biofuel production.Apparently,economics of that attempt would be remarkably enhanced if its defatted biomass is used as a feedstuff.However,no such study has been reported on feeding values of the defatted biomass of Staurosira sp or any diatom species in diets for poultry or swine.The frustules structure and high ash content in the defatted biomass could be a practical constraint for feed application. With a limited amount of defatted Staurosira sp microalgal biomass available for testing,we conducted the present laying hen experiment for 8 wk.Our objectives were to determine if the defatted biomass could partly replace soybean meal or a combination of corn and soybean meal in a practical diet;and how the replacements affected feed intake,health status,and egg production of the hens,and biochemical and biophysical properties of their eggs including pigmentation of yolk.
Materials and methods
Animals,dietary treatments,and management
ISA Babcock White Leghorn laying hens(n=100,47 wk old,Gallus gallus domesticus),with an initial body weight of 1.57±0.20 kg,were randomly assigned to 4 dietary treatments.There were 5 replicates for each treatment and each replicate consisted of 5 individually-caged hens. The cages with a size of 0.44 m high×0.30 m wide× 0.45 m deep were equipped with nipple drinkers and trough feeders.The hen-house was provided with 16 h light per day and the hens were given free access to feed and water.The duration of the experiment was 8 wk.The animal protocol was approved by the Institutional Animal Care and Use Committee at Cornell University.
Defatted Staurosira sp microalgal biomass(DFA)in the form of powder was generated from the research on algal cultures for biofuel production(Cellana,Kailua-Kona,HI). Proximate and mineral analyses of the defatted alga were done by Dairy One,Inc.(Ithaca,NY)and amino acid analyses were performed by Experiment Station Chemical Laboratories(University of Missouri,Columbia,MO).The composition of the defatted alga is presented in Table 1. The four experimental diets included a corn-soybean meal control diet,the diet with 7.5%DFA and the 5 most limiting amino acids(Lys,Met,Ile,Thr,Trp)substituting for 7.5%soybean meal(7.5%algae-SBM),and two diets with DFA substituting for 7.5%(7.5%algae-SBM/Corn)or 15% (15%algae-SBM/Corn)soybean meal and corn(about 1:3.1).The same source of algae was used for all diets.The DFA had a similar crude protein level and amino acids composition as the mixture of soybean meal and corn with a ratio of 1:3.1.All diets were formulated to be isocaloric(2.80 Mcal of ME/kg),and iso-Ca,and iso-P by adjusting vegetable oil,dicalcium phosphate and limestone.The DFA contained many other minerals,but thedietary levels were not adjusted because the objective of the experiment was to test the acceptability of the algae, with its high ash content,as a feed ingredient.The compositions and nutrient concentrations of the diets are presented in Table 2.

Table 1 Chemical composition of the defatted diatom microalgal biomass1
Sample collection and procedures
Body weights of the laying hens were recorded at the start and the end of the experiment.Eggs were collected daily and egg production was calculated on a hen-day basis.Feed intake and egg production was recorded weekly by replicates to calculate feed conversion ratio (FCR).Eggs produced on the last 3 d of the 4thwk and 8thwk were individually weighed and analyzed for their interior and exterior quality.Eggs were examined for shell quality by measuring egg shell thickness,breaking strength,and egg specific gravity.Shell thickness,without shell membrane,was measured by micrometer with a mean value of measurements at 3 locations on the eggs (near the equator).The strength of egg shells was determined as the compression pressure necessary to crack the shell when the egg was placed horizontally between the plates of an Instron model 5969(Instron,Norwood, MA).The specific gravity of eggs was determined by the buoyancy of eggs in salt solutions of varying density.Egg components,including albumen,yolk and shell,were weighed separately.Haugh units,a measure of the height of the albumen of eggs broken out on a flat surface were determined by the use of a micrometer[14].Yolk color, measured as L*-,a*-and b*-values,was determined with a Macbeth Color Eye(Macbeth Division of Kollmorgen Instruments Corp.Newburgh,NY).The L*value represents lightness(negative towards black,positive towards white),the a*value red-greenness(negative towardsgreen,positive towards red)and the b*value the blueyellow color scale(negative towards blue,positive towards yellow).

Table 2 Compositions and nutrient concentrations of experimental diets
Uric acid,alanine transaminase(ALT),cholesterol and glucose in plasma were determined with Uric acid liquid stable reagent(Infinity TM,Fisher Diagnostics,Fisher Scientific Company,LLC,Middletown,NY),ALT Liquid stable reagent(Thermo Electron Corporation,Pittsburgh, PA),Cholesterol reagent(Wako Pure Chemical Industries, Ltd,Osaka,Japan),and Glucose Assay Kit(Sigma,St Louis,MO),respectively.Alkaline phosphatase(AKP)was determined by the method of Bowers and McComb[15]. Yolk cholesterol was extracted by the method of Folch et al.[16]and measured by the HPLC method of Beyer and Jensen[17].
Statistical analysis
The experimental data were presented as mean±standard error,using SPSS17.0 for one-way analysis of variance,combined with Duncan’s method for multiple comparisons.The significance level for differences was P<0.05.
Results
Body weight,feed intake,egg production rate,and biochemical indices of hens
There were no significant differences in body weight among treatment groups at the beginning or the end of the experiment,despite an average loss of 20 to 70 g/ hen during the experiment across all groups(Table 3). In the first 4 wk,the inclusion of defatted algae tended to decrease feed intake,egg production rate,egg mass, and FCR.In the second 4 wk period,hens fed 15%DFA had lower(P<0.05)feed intake,egg production rate, egg mass,and higher(P<0.05)FCR than those of the control group.During the whole 8-wk period,hens fed 15%,but not 7.5%DFA had lower(P<0.05)egg production rate(-12%),daily feed intake(-9 g/hen/d)and higher(P<0.05)FCR(+0.09 g feed/g egg)than those fed the control diet(Table 3).There were no significant differences in plasma AKP,ALT,cholesterol,or glucose among dietary groups at the end of study(the 8thwk)(P>0.05) (Table 4).However,hens fed 15%DFA had lower(P<0.05)plasma uric acid level than that of control group.
Egg quality and yolk color
At the end of 4thand 8thwk,there were no significant differences between the control and the DFA diets in egg quality indexes(Table 5),including egg weight, Haugh unit,yolk weight,shell weight,albumen weight, and shell thickness.However,the 15%Algae-SBM/Corn diet had higher(P<0.05)egg albumen weight and height than the 7.5%Algae-SBM and 7.5%Algae-SBM/ Corn diets at the 8thwk.Additionally,the DFA dietsproduced no difference from the control diet in egg specific gravity,egg shell breaking strength or yolk cholesterol at the end of study(the 8thwk).Including DFA in the diets affected(P<0.05)yolk color in a dose-dependent fashion (Table 6).The L*(lightness)value and+b*(yellowness) value of yolk decreased(P<0.05)with the increasing DFA of the diet.At the 8thwk,but not at the 4thwk,the values for lightness and yellowness were lower(P<0.05)for 7.5% algae-SBM/Corn than for 7.5%algae-SBM.The+a*(redness)values of yolk were elevated(P<0.05)by all three DFA diets at the end of 4thwk,but by only the two 7.5% DFA diets at the 8thwk.
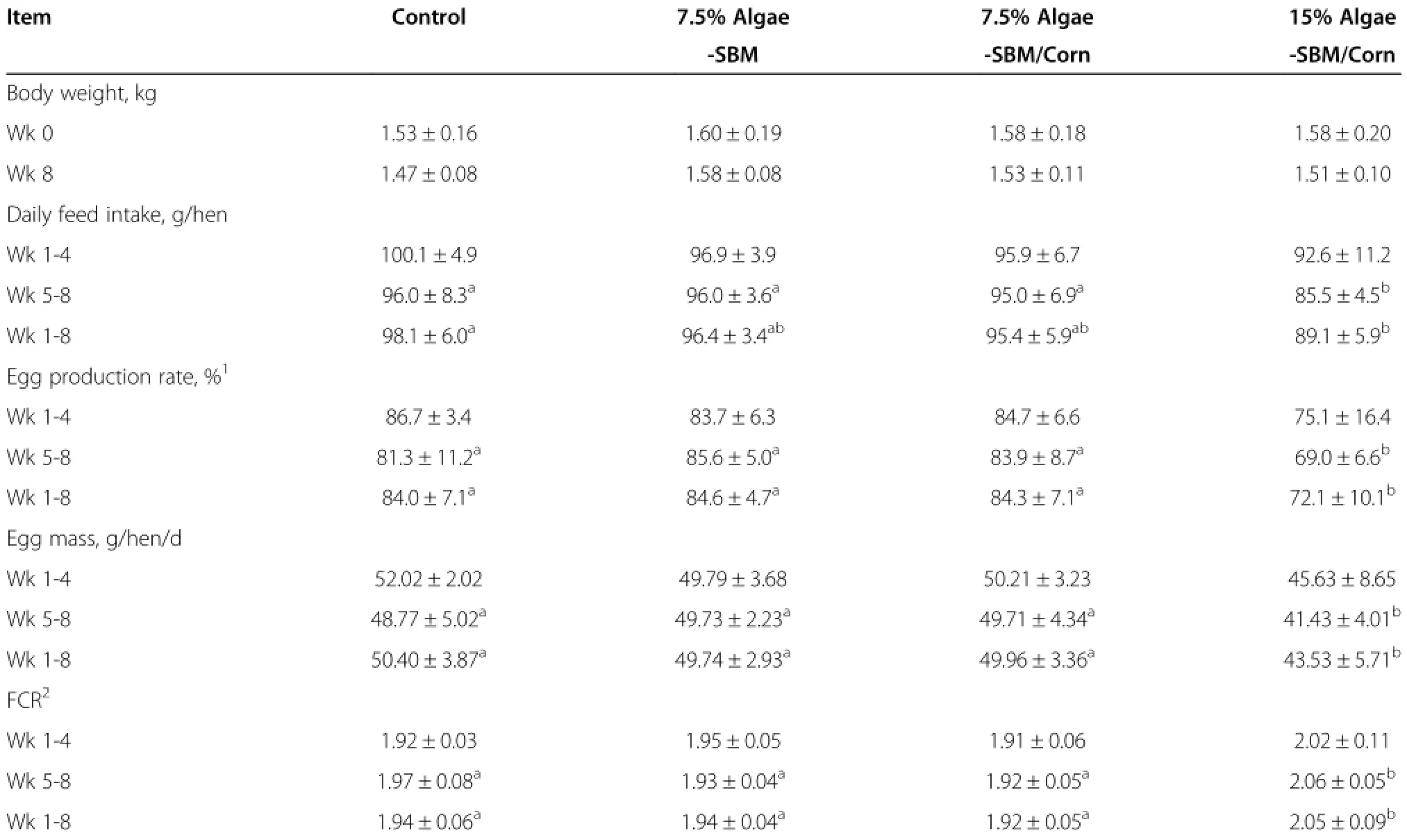
Table 3 Effect of defatted diatom microalgal biomass on body weight,feed intake,egg production rate,egg mass and FCR
Discussion
Results of the present study indicate that 7.5%DFA of Staurosira sp could replace 7.5%soybean meal or a combination of corn and soybean meal in diets for laying hens,without adverse effects on their body weights,feed intake,FCR,health status,or egg mass,egg production rate and quality.However,the inclusion of 15%DFA decreased hen feed intake by 11%and egg production rate by 12%.Past studies on inclusions of algae in diets for poultry have shown that algae can be used safely at dietary levels of 5%to 10%[18-24].Thus,our finding on 7.5%DFA as a tolerable level for hens just falls into themiddle of these studies.The decreases in feed intake and egg production rate in the 15%DFA group were likely attributed to the high ash(44.9%)and sodium chloride (10.3%)concentrations of the DFA used in the study.Although we removed salt and decreased limestone and dicalcium phosphate supplementation in the DFA diets, the 15%inclusion rate rendered the diet still salty and probably less palatable than the control diet.As expected,hens fed this diet were observed to drink more water and have bulky wet droppings.While the hens seemed to tolerate 7.5%DFA in the diet well for 8 wk, effects of long-term ingestion of high ash and salt on the health and egg production of these animals and on moisture and bulk of their excreta and the subsequent manure disposal should be evaluated in future research.

Table 4 Effect of defatted diatom microalgal biomass on plasma biochemical indices of hens at the 8thwk
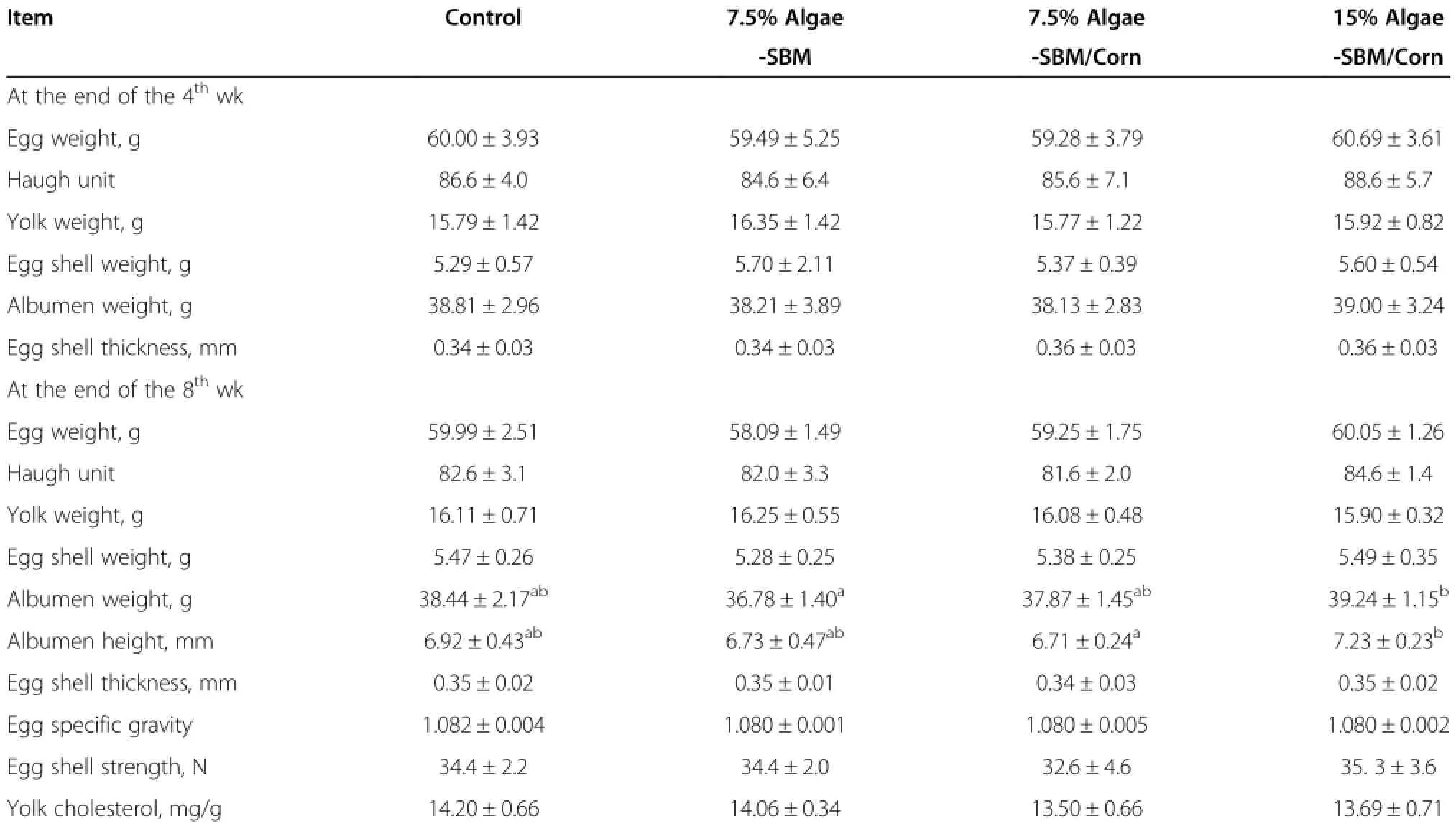
Table 5 Effect of defatted diatom microalgal biomass on egg quality at the 4thand 8thwk
Inclusion of 7.5 and 15%DFA resulted in dosedependent changes of egg yolk color,but did not significantly affect egg weights,egg shell weight,egg shell thickness,egg breaking strength,albumen firmness (Haugh units),or yolk cholesterol concentrations.While the latter outcome is consistent with other reports involving chickens[21,22,24-27]and Japanese quail[22], in which one or more of these traits were reported,the former is likely due to the presence of various carotenoids in DFA.Although algae powder appears as a bluishgreen color,it contains high levels of carotenoids such asβ-carotene,lutein and zeaxanthin[28].The red carotenoid astaxanthin is present in some algae[2].Because poultry and other animals cannot synthesize carotenoids de novo,the color of broiler skin and leg and egg yolk can be enhanced by including algae in feeds[3].In a study using a color meter to measure yolk pigmentation [29],2.4%or 4.8%of a commercial preparation of the marine algae,Schizochytrium,was fed to Leghorn hens over 4 wk.The yolk redness(+a*value)was increased, and the yellowness(+b*value)and lightness(+L*value) were decreased in a dose-responsive manner to the algae. In another study,10%and 20%algae(Nannochloropsis oculata)in diet increased the yolk+a*value,and decreased the+L*value[30].Consistently,the present study showed DFA dose-dependent increases in the+a value and decreases in the+b and+L*values of yolk. These color changes are supposed to be highly correlated with the Roche color fan that is used to measure the pigmentation in egg yolks and poultry skin by visual examination.Increases in the numerical color fan score have been observed in various studies on the use of algae in diets of laying hens[9,24,27,30,31].Depending on the amount of algae or lipid extract of algae incorporated into wheat-and sorghum-based feeds,scores ranged from about 7 to 9,which are considered acceptable for table eggs,to 12 to 15 which are in the range suitable for manufactured food products such as egg noodles and bakery items.Increases in yolk pigmentation were reported to occur within 24 h and to reach plateau at 7 to 8 d[9,31].In the present study,we found that 7.5 or 15%DFA shifted yolk pigmentation score from 7.9 in the control group to 9.3 to 9.5(data not shown).This shift implies that DFA may be used as a source of pigments for egg yolk formation to improve its consumers’acceptance.

Table 6 Effect of defatted algae on yolk color
Several genera of algae have been analyzed for protein quality based on protein digestibility,biological value, and net protein utilization[3,4].Although the results indicate that protein quality varies among algae,many species have relatively high protein content and excellent protein quality by these measures.In the present experiment,DFA was added to the diet in replacement of an equivalent weight of soybean meal or a mixture of soybean and corn.The DFA contained 19%crude protein in contrast to 48%crude protein in soybean meal.Substitution of one-third of the soybean meal with DFA necessarily means that the resulting diets had less protein than the control diet.Several amino acids were added to the 7.5%Algae-SBM diet in an attempt to meet essential amino acid requirements.This diet supported production responses that were similar to those of the control diet.Only uric acid,among the plasma biomarkers,was affected by the DFA treatments.The lower plasma uric acid concentration in the hens fed 15%defatted diatom suggests that less amino acid catabolism had occurred in this group of hens as compared to the control groups.A simple explanation for this observation would be that the 11%lower daily feed(and protein)consumption resulted in fewer amino acids available for catabolism.Alternatively or additionally,a low digestibility of the protein in the 15%DFA diet may have contributed to reduced absorption of amino acids,thereby resulting in lower amino acid catabolism.Additional research would be necessary to determine the actual cause of the reduction in plasma uric acid concentration.
Due to the limited amount of DFA available for the present study,our feeding experiment lasted for only 8 wk.However,all responses of feed intake,egg production,egg quality,and plasma biomarkers were consistent that DFA could serve at a dietary level of 7.5%over this period as a source of protein,energy,calcium,phosphorus,and sodium chloride for laying hens.The question is if the period of 8 wk was sufficient for the objectives of this experiment including determining effects of the DFA nutrients on egg production and composition.Physiologically,the formation of egg yolk occurs in a process that lasts about 9 d.Egg albumen, eggshell membranes,and eggshell are usually secreted within slightly more than 24 h.Experimentally,changes in yolk pigmentation occurred within 8 d after the addition of algae to the diet[9,31].Changes in egg production occurred within 2 to 3 mo of the feeding protein-or amino acid-deficient diets to laying hens[32,33].Concentrations of selenium or fat-soluble vitamins in eggs were affected by diet within days to a few weeks[34-36].Clearly,many nutritional effects on egg production and composition could be detected within 8 wk.In fact,we observed significant effects of DFA on egg yolk color measures after only 4-wk feeding.Metabolically,animals should be most responsive during the initial phase to negative impacts of DFA as longer time feeding may give them chance to adapt.Nevertheless,it is prudent that a feeding trial of several months or a full year would be necessary to evaluate long-term nutritional values of DFA during a full egg production cycle.
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
XGL and REA conceived the study,supervised the trial,and revised the manuscript.XL,K-NH,and REA raised the animals and carried out the laboratory analysis.XL and REA drafted the initial manuscript,and XGL helped revise and finalize the manuscript.All authors read and approved the final manuscript.
Acknowledgements
This study was supported in part by a USDA/DOE Biomass R&D Initiative grant.The defatted microalgal biomass was provided by Cellana,Kailua-Kona,HI,USA.We thank Dr.James Bartsch in the Department of Biological and Environmental Engineering and Dr.David Barbano in the Department of Food Science for instruction on the use,and access to the Instron andMacbeth COLOR EYE,respectively,for measurement of egg breaking strength and egg yolk color.
Author details
1Department of Animal Science,Cornell University,Ithaca,NY 14853,USA.
2College of Aquaculture and Life Science,Shanghai Ocean University, Shanghai 201306,China.
Received∶27 August 2013 Accepted∶2 January 2014
Published∶9 January 2014
1. Gouveia L,Oliveira AC:Microalgae as raw material for biofuels production.J Ind Microbiol Biotechnol 2009,36∶269-274.
2. Spolaore P,Joannis-Cassan C,Duran E,Isambert A:Commercial applications of microalgae.J Biosci Bioeng 2006,101(2):87-96.
3. Becker W:Microalgae in Human and Animal Nutrition.In Handbook of Microalgal Culture:Biotechnology and Applied Phycology.Edited by Richmond A.Oxford:Blackwell Science;2004:312-351.
4. Becker EW:Micro-algae as a source of protein.Biotechnol Adv 2007, 25∶207-210.
5. U.S.Department of Agriculture,Economic Research Service:Economics, Statistics,and Market Information System.Washington,DC:Oil Crops, Situation and Outlook Yearbook;2011.
6. Mielke T:Major challenges ahead∶world soybean supply and demand outlook for 2012/13.Int News on Fats,Oils and Related Materials(INFORM) 2012,23(7):468-470.
7. Herber SM,van Elswyk ME:Dietary marine algae promotes efficient deposition of n-3 fatty acids for the production of enriched shell eggs. Poult Sci 1996,75∶1501-1507.
8. Barclay W,Abril R,Abril P,Weaver C,Ashford A:Production of docosahexaenoic acid from microalgae and its benefits for use in animal feeds.In The return of ω3 fatty acids into the food supply.I.Land-based animal food products and their health effects.Edited by Simopoulos AP.Basel, Switzerland:Karger:World Rev Nutr Diet;1988,83∶61-76.
9. Nitsan Z,Mokady S,Sukenik A:Enrichment of poultry products with ω3 fatty acids by dietary supplementation with the alga Nannochloropsis and mantur oil.J Agric Food Chem 1999,47∶5127-5132.
10.Martin-Jézéquel V,Hildebrand M,Brezinski MA:Silicon metabolism in diatoms∶implications for growth.J Phycol 2000,36∶821-840.
11.Harrison G,Morel FMM:Response of the marine diatom,Thalassiosira weissflogii,to iron stress.Limnol Oceanogr 1986,3(5):989-997.
12.Taraldsvik M,Myklestad SM:The effect of pH on growth rate,biochemical composition and extracellular carbohydrate production of the marine diatom,Skeletonema costatum.Eur J Phycol 2000,35∶189-194.
13.Pahl SL,Lewis DM,Chen F,King KD:Growth dynamics and the proximate biochemical composition and fatty acid profile of the heterotrophically grown diatom Cyclotella cryptica.J Appl Phychol 2010,22∶165-171.
14.USDA:U.S.Department of Agriculture,Consumer and Marketing Service. Washington,DC:Egg grading manual.Agriculture Handbook No.75;1969.
15.Bowers GN Jr,McComb RB:A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase.Clin Chem 1966, 12∶70-89.
16.Folch J,Lees M,Stanley GHS:A simple method for the isolation and purification of total lipides from animal tissues.J Biol Chem 1957, 226∶497-509.
17.Beyer RS,Jensen LS:Overestimation of the cholesterol content of eggs. J Agric Food Chem 1989,37∶917-920.
18.Combs GF:Algae(Chlorella)as a source of nutrients for the chick.Science 1952,116∶453-454.
19.Grau CR,Klein NW:Sewage-grown algae as a feedstuff for chicks.Poult Sci 1957,36∶1046-1051.
20.Yoshida M,Hoshii H:Nutritive value of new type of Chlorella for poultry feed.Jpn Poult Sci 1982,19∶56-58.
21.Lipstein B,Hurwitz S:The nutritional value of sewage-grown samples of Chlorella and Micractinium in broiler diets.Poult Sci 1983,62∶1254-1260.
22.Ross E,Dominy W:The nutritional value of dehydrated,blue-green algae (Spirulina platensis)for poultry.Poult Sci 1990,69∶794-800.
23.Venkataraman LV,Somasekaran T,Becker EW:Replacement value of blue-green alga(Spirulina platensis)for fishmeal and a vitamin-mineral premix for broiler chicks.Br Poult Sci 1994,35∶373-381.
24.Halle I,Janczyk P,Freyer G,Souffrant WB:Effect of microalgae Chlorella vulgaris on laying hen performance.Arch Zootech 2009,12(2):5-13.
25.Lipstein B,Hurwitz S,Bornstein S:The nutritional value of algae for poultry.Dried Chlorella in layer diets.Br Poult Sci 1980,21∶23-27.
26.Lipstein B,Hurwitz S:The nutritional value of sewage-grown,alum flocculated Micractinium algae in broiler and layer diets.Poult Sci 1981,60∶2628-2638.
27.Sauveur B,Zybko A,Colas B:Protéines alimentaires et qualité de l’oeuf.I. Effet de quelques protéines sur la qualité interne de l’oeuf et les propriétes fonctionnelles.Ann Zootech 1979,28∶271-295.
28.Miki W,Yamaguchi K,Konosu S:Carotenoid composition of Spirulina maxima.Bull Jpn Soc Sci Fish 1986,52∶1225-1227.
29.Herber-McNeill SM,van Elswyk ME:Dietary marine algae maintains egg consumer acceptability while enhancing yolk color.Poult Sci 1998, 77∶493-496.
30.Fredriksson S,Elwinger K,Pickova J:Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens.Food Chem 2006,99∶530-537.
31.Zahroojian N,Morave H,Shivazad M:Comparison of marine algae (Spirulina platensis)and synthetic pigment in enhancing egg yolk colour of laying hens.Br Poult Sci 2011,52(5):584-588.
32.Summers JD,Atkinson JL,Spratt D:Supplementation of a low protein diet in an attempt to optimize egg mass output.Can J Anim Sci 1991,71∶211-220.
33.Harms RH,Russell GB:A re-evaluation of the methionine requirement of the commercial layer.J Appl Anim Res 1996,9∶141-151.
34.Latshaw JD,Osman M:Distribution of selenium in egg white and yolk after feeding natural and synthetic selenium compounds.Poult Sci 1975, 54∶1244-1252.
35.Suzuki Y,Okamoto M:Production of hen’s eggs rich in vitamin K.Nutr Res 1997,17(10):1607-1615.
36.Mattila P,Rokka T,Konko K,Valaja J,Rossow L,Ryhanen EL:Effect of cholecalciferol enriched hen feed on egg quality.J Agric Food Chem 2003, 51∶283-287.
doi∶10.1186/2049-1891-5-3
Cite this article as:Leng et al.:Effect of dietary defatted diatom biomass on egg production and quality of laying hens.Journal of Animal Science and Biotechnology 2014 5:3.
Submit your next manuscript to BioMed Central and take full advantage of:
· Convenient online submission
· Thorough peer review
· No space constraints or color fi gure charges
· Immediate publication on acceptance
· Inclusion in PubMed, CAS, Scopus and Google Scholar
· Research which is freely available for redistribution
Submit your manuscript at www.biomedcentral.com/submit
*Correspondence:XL20@cornell.edu
1Department of Animal Science,Cornell University,Ithaca,NY 14853,USA Full list of author information is available at the end of the article
©2014 Leng et al.;licensee BioMed Central Ltd.This is an open access article distributed under the terms of the Creative Commons Attribution License(http://creativecommons.org/licenses/by/2.0),which permits unrestricted use,distribution,and reproduction in any medium,provided the original work is properly cited.
Methods:Five replicates of 5 individually caged ISA Babcock White leghorn hens were fed 4 diets,including a corn-soybean meal control diet,a diet containing 7.5%DFA substituting for soybean meal,and diets containing 7.5%or 15%DFA substituting for corn and soybean meal.Body weights,feed intake,feed conversion ratio(FCR), rate of egg production,egg size,egg mass,and several characteristics of eggs were determined at 4 and 8 wk. Venous blood was sampled at 4 and 8 wk for measurement of 5 biomarkers of health.
Results:The 15%DFA diet decreased(P<0.05)feed intake,egg production,and plasma uric acid concentrations as compared with the control diet,but increased(P<0.05)egg albumen weight and height compared with the 7.5%DFA diets.The two levels of DFA produced dose-dependent(P<0.05)changes in three color measures of egg yolk,without affecting four hen plasma biochemical indicators of health.
Conclusions:Feeding laying hens with 7.5%DFA in the corn-soybean meal diet for 8 wk had no adverse effect on their health,egg production,or egg quality,but 15%inclusion reduced feed intake,egg production,and efficiency of feed utilization.
 Journal of Animal Science and Biotechnology2014年3期
Journal of Animal Science and Biotechnology2014年3期
- Journal of Animal Science and Biotechnology的其它文章
- Free-radical scavenging properties of low molecular weight peptide(s)isolated from S1 cultivar of mulberry leaves and their impact on Bombyx mori(L.)(Bombycidae)
- Effects of Chinese herbal medicine on plasma glucose,protein and energy metabolism in sheep
- Effect of dietary nonphytate phosphorus on laying performance and small intestinal epithelial phosphate transporter expression in Dwarf pink-shell laying hens
- The correlationship between the metabolizable energy content,chemical composition and color score in different sources of corn DDGS
- Comparison of acid-detergent lignin, alkaline-peroxide lignin,and acid-detergent insoluble ash as internal markers for predicting fecal output and digestibility by cattle offered bermudagrass hays of varying nutrient composition
- Predicting corn digestible and metabolizable energy content from its chemical composition in growing pigs
