The regulatory impact of immune inhibitors on T cells of SD rats
Chao-Hua Zhang, Yan Huang, Gang Han
1Uropoiesis Surgical Department, First Hospital of Baoding, Hebei, China
2Medicine Clinical, Hebei University College, Hebei, China
3The People's Liberation Army 252 Hospital, Baoding, Hebei, China
The regulatory impact of immune inhibitors on T cells of SD rats
Chao-Hua Zhang1,2*, Yan Huang1, Gang Han3
1Uropoiesis Surgical Department, First Hospital of Baoding, Hebei, China
2Medicine Clinical, Hebei University College, Hebei, China
3The People's Liberation Army 252 Hospital, Baoding, Hebei, China
Objective: To observe the regulatory impact of immune inhibitors on T cells in rats. Method: Forty SD rats were selected and randomly divided into experimental group and control group, Rapamycin (SRL) 0.4 mg/d to fill the stomach of the former one, saline lavage was used with the latter one for two weeks. Using flow cytometry to detect the two groups of rats with spleen and thymus level of CD4+ CD25+ T cells; and the spleen cells FoxP3 mRNA expression; Using ELISA method to detect TGF-β, IL-10 levels. Results: The peripheral blood, spleen and thymus of CD4+ CD25+ T cells accounted for the proportion of mononuclear cells were significantly higher than that of control group (P<0.05); FoxP3 mRNA expression quantity also significantly higher than the control group (P<0.05); Experimental TGF-β in rats, IL-10 levels are significantly higher than control group (P<0.05). Conclusions: Immune inhibitors can regulatory CD4+ CD25+ foxp3+ T cells in rats, a single nuclear cell proportion increase, shows that it can induce rat CD4+ CD25+ foxp3+ regulatory T cells proliferation.
ARTICLE INFO
Article history:
Received 10 September 2013
Received in revised form 15 October 2013
Accepted 15 December 2013
Available online 20 April 2014
Immune inhibitors
1. Introduction
Organ transplants were carried out gradually in recent years, but the postoperative autologous immune rejection become the most thorny problem[1-5]. Efficient immune inhibitor combined application can reduce the incidence of rejection after transplantation, obviously prolong survival time after transplantation[6-10]. The human body has the same reactive T cells of CD4+ CD25+ regulatory T cells (Tregs), it was showed that regulatory T cells content of the patients with immunosuppressant can be changed after transplantation[11-16]. Rapamycin (SRL) are one of macrolides families, studies have shown that SRL has stronger immune inhibition effect, which was widely used in the treatment of kidney transplant rejection[17-24]. The author aims to observe its influence on regulate T cell’s activation and function, SRL lavage was used on SD rats to observe its regulatory effect on CD4+, CD25+, FoxP3+ T cells, the results are reported as follows.
2. Materials and methods
2.1. Experimental animals
A total of 40 SD rats were selected, only our animal experiment center, clean level, male, weighting 210-220 g, aged 8 to 10 weeks, the rats were fed free with food and water, animal in the experimental were processed strictly according to the administration of experimental animals.
2.2. Instrument and preparation
Real time PCR instrument, FACS Aria TM flow cytometry instrument (BD co., United States); TGF-beta and IL-10 ELISA kit, RT-PCR kit (TaKaRa); Sirolimus (Wyeth Pharmaceutical co., LTD.)-the White House. Hemolysin, collagenase and hyaluronidase, Trizol reagent (Invitrogen Company). RPMI-1640 medium, PE-conjugated anti-CD25,APC-conjugated anti-CD4 (eBioscience co. US).
2.3. Methods
A total of 40 rats were randomly divided into experimental group and control group with 20 in each, the experimental group rats were given SRL lavage (0.4 mg/d) for 2 weeks; the control group given were given the same volume of saline lavage for 2 weeks. Pentobarbital was used for anesthesia (ip.), inferior vena venous blood was extracted for further use; Rats spleen and thymus were grinded using 200 mesh metal filter, for preparation of 106cells/mL suspension cells using RPMI-1640.
2.4. Observe project
A total of 100 μL spleen cells , thymus suspension and heparin anticoagulant was absorbed respectively, adding 1 μL APC-conjugatedanti-CD4 (0.25 μL), LPE-conjugated anti-CD2, incubated away from light for 15 min, hemolysin, centrifugal 5 min, abandon supernatant, PBS wash twice, plus 200 μL PBS blending, computer detection of CD4+ CD25+ T cells accounted for the proportion of mononuclear cells; with double antibody sandwich ELISA method to detect the rat TGF-beta and IL-10 in serum level; and the real-time PCR kits in the spleen cell SAP FoxP3 mRNA expression. The experiment was performed strictly in accordance with the above instructions.
2.5. Statistical processing
Using SPSS12.0 statistics software to process the experiment data, measurement data are expressed withgroup comparision was analysed byttest, P<0.05 for the difference was statistically significant.
3. Results
3.1. CD4+ and CD25+ T cells detection in the peripheral blood, thymus and spleen
The experimental group in the peripheral blood, spleen and thymus of CD4+ and CD25+ T cells accounted for the proportion of mononuclear cells are higher than the control group with statistically significant (P<0.05), the results are shown in Table 1.
3.2. Level of TGF-β, IL-10 in serum
Experimental rats serum TGF-β, IL-10 were significantly higher than that of control group (P<0.05), as shown in Figure 1.
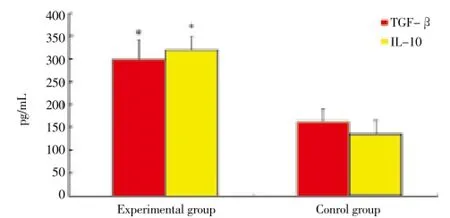
Figure 1. Level of TGF-β, IL-10 in serum. *P<0.05 compare with control group.
3.3. AP FoxP3 mRNA expression comparison in two groups
Experimental rats spleen FoxP3 mRNA was significantly higher than that of control group (P<0.05), as shown in Figure 2.
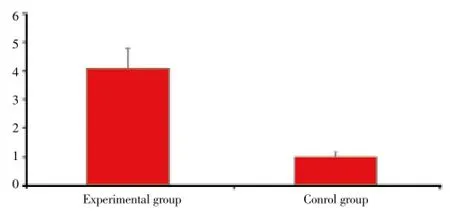
Figure 2. SAP FoxP3 mRNA expression comparison in two groups.
4. Discussion
CD4+ T cells were discovered in the thymus by Sakaguchiet al. in the 90 s, the new borne rats which accepted the thymus excision (d3Tx) injection of CD4+ CD25+ T cells can prevent autoimmune diseases[25-29]. The current universal view is that thymus is able to produce CD4+ CD25+ T in peripheral blood[30]. Studies have shown that[31-34], CD4+ andCD25+ T cells can prevent autoimmune reactions and tissue damage caused by rejection and inflammatory response after organ transplantation.

Table 1 CD4+ and CD25+ T cells detection in the peripheral blood, thymus and spleen (%).
Sirolimus is a potent immune inhibitor, has been widely applied in the immune rejection after kidney transplantation[35]. It works mainly by inhibiting the antigens and cytokines stimulation of T lymphocyte activation and proliferation of immune inhibition[36]. Sirolimus can selectively amplify CD4+ CD25+ and FoxP3+ T cells without blocking the CD4+ T cell expansion and activation to induce cell apoptosis[37]. CD4+ CD25+ and FoxP3+ regulatory T cells play an important role in maintaining immune tolerance. Studies have shown that[38-40], sirolimus can induce immune inhibition of regulatory T cells. In this study, the experimental group to sirolimus lavage (0.4 mg/d) after 2 weeks, the experimental group rats an increasing CD4+ CD25+ T cells proportion of mononuclear cells in peripheral blood, thymus and spleen significantly, much higher than that of control group (P<0.05); and the experimental group rats spleen foxp3 mRNA expression, TGF-beta, IL-10 levels were significantly higher than the control group (P<0.05), indicating that sirolimus can induce rats in vivo CD4+ CD25+ FoxP3+ regulatory T cell proliferation, leading to immune rejection tolerance.
According to the results of this study, immune inhibitors can increase the rat proportion of CD4+ CD25+ and FoxP3+ regulatory T cells in the mononuclear cells in vivo, shows that it can induce proliferation of CD4+ CD25+ and FoxP3+ regulatory T cells in rats, so it can play an important role in maintaining immune tolerance.
Conflict of interest statement
We declare that we have no conflict of interest
[1] Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production ofretinoic acid. J Immunol 2013; 190(5): 2009-2016.
[2] Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol 2012; 188(8): 3667-3677.
[3] Yoshizawa K, Abe H, Kubo Y, Kitahara T, Aizawa R, Matsuoka M, et al. Expansion of CD4+ CD25+ Foxp3+ regulatory T cells in hepatitis C virus-related chronic hepatitis, cirrhosis and hepatocellular carcinoma. Hepatol Res 2010; 40(2): 179-187.
[4] Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol 2010; 52(3): 315-321.
[5] Xu HT, Xing TJ, Li H, Ye J. Association of T regulatory cells with natural course and response to treatment with interferon-α in patients with chronic hepatitis B infection. Chin Med J (Engl) 2012; 125(8): 1465-1468.
[6] Cheng ST, Feng L, Liu LN. Regulatory effects of A- interferon on Foxp3+ T cells in liver fibrosis in mice liver tissue. J Chin Med Univ 2013; 42(5): 402-403.
[7] Shen S, Liu D, Li C. Changing proportion of peripheral blood CD4+ and CD25+ high regulatory T cells in transplanted immune surveillance after kidney transplant. Chin J Clin Rehabil 2009; 13(18): 3425-3426.
[8] Tawara I, Sun Y, Liu C, Toubai T, Nieves E, Evers R, et al. Donor-but not host-derived interleukin-10 contributes to the regulation of experimental graft-versus-host disease. J Leukoc Biol 2012; 91(4): 667-675.
[9] Hippen KL, Bucher C, Schirm DK, Bearl AM, Brender T, Mink KA, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood 2012; 119(2): 619-628.
[10] Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4(+)T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+)T regulatory cells. Blood 2009; 114(24): 5003-5006.
[11] Fan H, Yang J, Hao J, Ren Y, Chen L, Li G, et al. Comparative study of regulatory T cells expanded ex vivo from cord blood and adult peripheral blood. Immunology 2012; 136(2): 218-230.
[12] Sun XH, He LY, ZhangY. Distribution and clinical significance of hyperplasia of prostate tissue in T lymphocyte subsets. Chin J Androl 2012; 26(11): 20-24.
[13] Zhang W, Han RF. Expression of CD8, CD20, IL-6 and IL-23 in benign prostatic hyperplasia tissues. Tianjin Med 2011; 39(8): 711-713.
[14] Zhang SW, Li W, Zhang YZ. Expression and significance of peripheral regulatory T cells in non-small cell lung cancer patients. J Chin Pract Diagn Ther 2012; 26(7): 645-647.
[15] Luo L, Luo DM. Regulatory T cells and transforming growth factor β and early rheumatoid arthritis correlation studies. J Chin Pract Diagn Ther 2011; 25(7): 680-683.
[16] Gao HY, Li LX. The expression and meaning CD4 and CD25 regulatory T cells in patients with prostate hyperplasia. J Chin Prac Diagn Ther 2013; 27(8): 781-782.
[17] Feng X, Li B, Ye H, Long D. Increased frequency of CD4+ CD25 (high) FoxP3+ regulatory T cells in patients with hepatocellular carcinoma. Arch Immunol Ther Exp (Warsz) 2011; 59(4): 309-314.
[18] Xu T, Duan Q, Wang G, Hu B. CD4+ CD25 high regulatory T cell numbers and FOXP3 mRNA expression in patients with advanced esophagealcancer before and after chemotherapy. Cell Bio-chem Biophys 2011; 61(2): 389-392.
[19] Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with Glioblastoma. Clin Dev Immunol 2010.
[20] Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versushost disease. Blood 2009; 114(4): 891-900.
[21] Ma Xuehua, Zhang Xuan, Zhang Na, et al. Peripheral blood lymphocytes in patients with ankylosing spondylitis subsets and its clinical significance. Chin J Lab Diagn 2011; 15(10): 1765-1766.
[22] Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone marrowderived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc 2009; 41(10): 4352-4356.
[23] Hippen KL, Bucher C, Schirm DK, Bearl AM, Brender T, Mink KA, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood 2012; 119(2): 619-628.
[24] Ahern D, LloydC M, Robinson DS. Chemokine responsiveness of CD4 CD25 regulatory and CD4 CD25 T cells from atopic and nonatopic donors. Allergy 2009; 64(8): 1121-1129.
[25] Fan H, Yang J, Hao J, Ren Y, Chen L, Li G, et al. Comparative study of regulatory T cells expanded ex vivo from cord blood and adult peripheral blood. Immunology 2012, 136(2): 218-230.
[26] Delisle JS, Giroux M, Boucher G, Landry JR, Hardy MP, Lemieux S, et al. The TGF-β-Smad3 pathway inhibits CD28-dependent cell growth and proliferation of CD4 T cells. Genes Immun 2013.
[27] Centuori SM, Trad M, LaCasse CJ, Alizadeh D, Larmonier CB, Hanke NT, et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-β-induced differentiation of CD4+CD2+FoxP3+ Tregs from CD4+CD25+FoxP3-T cells. J Leukoc Biol 2012; 92(5): 987-997.
[28] Rutegard M, Charonis K, Lu Y, Lagergren P, Lagergren J, Rouvelas I. Population-based esophageal cancer survival after resection without neoadjuvant therapy: an update. Surgery 2012; 152(5): 903-910.
[29] Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69-90.
[30] Wei WQ, Yang J, Zhang SW, Chen WQ, Qiao YL. Esophageal cancer mortality trends during the last 30 years in high risk areas in China: comparison results from national death surveys conducted in the 1970,s,1990,s and 2004-2005. Asian Pac J Cancer Prev 2011; 12(7): 1821-1826.
[31] Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer 2010; 127(4): 759-767.
[32] Triulzi T, Tagliabue E, Balsari A, Casalini P. Foxp3 expression in tumor cells and implications for cancer progression. J Cell Physiol 2013; 228(1); 30-35.
[33] Wang G, Liu G,Liu Y, Li X, Su Z. FOXP3 expression in esophageal cancer cells is associated with poor prognosis in esophageal cancer. Hepatogastroenterology 2012; 59(119): 2186-2191.
[34] Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, et al. Distribution of Thl7 cells and Foxp3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumordraining lymphnodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 2010; 101(9): 1947-1954.
[35] Cheng G, Yu A, Dee MJ, Malek TR. IL-2R Signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol 2013, 190(4): 1567-1675.
[36] Vela-Ojeda J, Montiel-Cervantes L, Granados-Lara P, Reyes-Maldonado E, Garcia-Latorre E, Garcia-Chavez J, et al. Role of CD4+CD25+(high) Foxp3+CD62L+ regulatory T cells and invariant NKT cells in human allogeneic hematopoietic stem cell transplantation. Stem Cells Dev 2010; 19(3): 333-340.
[37] Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10(7): 490-500.
[38] Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother 2010; 59(9): 1367-1377.
[39] Kim JI, Sonawane SB, Lee MK, Lee SH, Duff PE, Moore DJ, et al. Blockade of GITR-GITRL interaction maintains Treg function to prolong allograft survival. Eur J Immunol 2010; 40(5): 1369-1374.
[40] Xue L,Lu HQ, He J, Zhao XW, Zhong L, Zhang ZZ, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus 2010; 23(4): 340-346.
ment heading
10.1016/S1995-7645(14)60044-4
*Corresponding author: Chao-Hua Zhang, Associate Professor, Deputy Pirector, M.M., Uropoiesis Surgical Department, First Hospital of Baoding, Hebei, China.
Tel: 13831296111
E-mail: 13831296111zch@sina.com
Funding project: It is supported by Hebei Province Baoding City Science and Technology Project (No: 13ZF074).
Regulatory T cells
Influence
Research
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
