Effect of VEGF, P53 and telomerase on angiogenesis of gastric carcinoma tissue
Yan-Fang Yu, Yong Zhang, Na Shen, Rui-Ying Zhang, Xin-Qing Lu
Affiliated Hospital of Hebei University of Engineering, Handan 056002, Hebei, China
Effect of VEGF, P53 and telomerase on angiogenesis of gastric carcinoma tissue
Yan-Fang Yu*, Yong Zhang, Na Shen, Rui-Ying Zhang, Xin-Qing Lu*
Affiliated Hospital of Hebei University of Engineering, Handan 056002, Hebei, China
Objective: To investigate the effect of vascular endothelial growth factor (VEGF), P53 and telomerase on angiogenesis in gastric carcinoma tissue. Methods: A total of 95 surgical resection samples of gastric cancer tissue after pathological diagnosis are collected to observe the VEGF, P53 and telomerase expression using immunohistochemical methods. Relationship between their expression and its influence on angiogenesis in gastric carcinoma tissue were analyzed. Results: Microvascular density (MVD) and the expression of VEGF, P53 and telomerase were positively correlated. Expression of VEGF and P53 protein were related to tumor type and lymph metastasis, and also a correlation was observed between P53 and VEGF. The telomerase expression had no correlation with VEGF, and P53. Conclusions: VEGF angiogenesis has a angiogenesis promoting effect on gastric cancer tissue development and plays an important role in tumor generation and metastasis. Mutant P53 promotes the tumor angiogenesis generation by adjusting VEGF. Telomerase has a certain role in promoting activity of angiogenesis through different way rather than P53.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
VEGF
P53
Telomerase
Angiogenesis
Gastric carcinoma
1. Introduction
Gastric cancer is a common malignant digestive system disease, with serious damages to patients’ health. Cancer cells transformation genes phenotypic change are closely related to angiogenesis. The growth and metastasis depends on the formation of new blood vessels, and tumor vascular endothelial growth factor (VEGF) plays a key role in the formation of new blood vessels[1-3]. P53 is an important tumor suppressor genes. It inhibits the growth of the transformed cells, and the cells in vivo tumorigenicity, which affect cell proliferation and prevent the happening of the carcinoma[4]. Studies have shown that[5], wild-type P53 can inhibit VEGF expression, tumor growth and metastasis. Mutant P53 can increase the expression of VEGF, promote tumor angiogenesis, growth and metastasis. Telomerase can regulate cell growth, prevent chromosomal integration end to end, restructuring and degradation, and thus can stabilize chromosome, ensuring a complete copy of chromosomes. Studies have shown that[6], the occurrence/development of malignant tumor is closely related to telomerase, which can be used as an effective marker for the diagnosis of malignant tumor. This study examined the 95 specimens of gastric cancer by immunohistochemical method to detect the relationship between of VEGF, P53 and telomerase, and their effects on tumor angiogenesis of gastric carcinoma.
2. Materials and methods
2.1. Reagent and instrument
Mouse anti human CD34 monoclonal antibody (dilution: 1:50), rabbit anti human VEGF polyclonal antibody, rabbitanti human TRT polyclonal antibody (dilution: 1:75) and rat P53 monoclonal antibody against human (dilution: 1:250) were provided by Santa Cruz co.; APES dropping pills, pancreatic enzyme digestive agent, second antibody, triple antibody, DAB chromogenic reagent kit were provided by the Golden Bridge Biotechnology co. LTD, China.
2.2. Specimen source
Surgical resection specimens were collected from gastric carcinoma tissue of 95 cases (55 male and female 40 cases, aged 27-78, an average of 60.23 years old) admitted in our hospital from January 2009 to January 2010. Histological type: 42 cases of tubular adenocarcinoma, 22 cases of papillary adenocarcinoma, 16 cases of mucous adenocarcinoma, 5 cases of gland scale cancer, 10 cases of Dijon cell carcinoma. Another 30 resection specimens of normal gastric tissue from distal carcinoma above were taken for contrast tests.
2.3. Dyeing method
Slides were soaked in acid pool for 24 h, they were rinsed and dried. APES was diluted with acetone 1:50 for 20-30 sec, APES uncombinated with pure acetone was removed, and boxed for later use. Gastric cancer tissue was sliced in 4 μm in oven at 80 ℃ for 3 h. Slice underwent conventional dewaxing, ethanol hydration, 3% hydrogen peroxide incubation for 10 min. It was rinsed with distilled water, PBS for 5 min, and underwent 1:50 EDTA antigen hot fix. Goat serum was added for incubation at room temperature for 15 min. The primary antibody was added, then they was washed by PBS, added with biotin secondary antibody drops for incubation at 37 ℃ for 15 min. They were washed with PBS, added with triple antibody drops, then were incubated at 37 ℃ for 15 min, washed by PBS. Telomerase dyeing methods: antigen retrieval was performed by enzyme digestion + hot retrieval method. Blank control was included in all dyeings above, with PBC instead of primary antibody.
2.3. Result judgement
Positive telomerase staining was defined with 10% tumor cell cytoplasm and cell membrane with tan color. Positive MVC dyeing was defined with obvious tumor cells, or other organization components had other a brown cells or cell cluster as a blood vessel, as long as the structure was not connected to a vascular branch structure. At low magnification, the highest microvascular density zones within the tumor tissue was looked up, them it was searched at high magnification. capillary number of 5 highest vascular density zones was counted, to calculate the average microvascular density (MVD). Positive VEGF staining was defined with over 10% per biopsy in the cytoplasm or cell membrane stained. P53 staining with nuclei in tan color was defined as positive.
2.4. statistical analysis
Data was analyzed bySPSS12.0 statistics software, measured data were represented with (mean±sd) usingttest, P<0.05 was considered as significant difference.
3. Results
3.1. VEGF expression results
VEGF, P53 and telomerase positive expression in normal gastric mucosa tissue was not found, VEGF positive expression 52 cases in gastric cancer group (56.52%).VEGF expression was mainly in the cytoplasm, a tan granular staining. More strong staining was found at tumor infiltration front, weak VEGF staining was found around the endothelial cells and tumor tissues, The expression rate of gastric cancer group was 42.11%, P53 was expressed in nucleus, tinting darker than in low differentiated adenocarcinoma well-differentiated adenocarcinomas. Telomerase positive expression positive was 74.74%, the positive staining for tan granule was mainly in the cytoplasm and cell membrane, patches were distributed in tumor tissues. (Figure 1).

Figure 1. Expression of VEGF, P53 and telomerase (×200).
3.2. Relationship between VEGF, P53, telomerase and MVD expression
On the basis of VEGF staining intensity, expression is divided into four grades, less than 10% tumor cells cytoplasm or membrane dyeing is identified for negative (-), 10%-20% for weakly positive (+), 20%-30% for positive (++), more than 30% for strong positive (+). VEGF (-) MVD was 32.5±9.3, (+) average MVD was 48.7±18.4, visible MVD increases with VEGF expression, difference between groups was statistically significant (P<0.05) (Figure 2a). There was positive correlation between P53 positive expression and MVD. P53 (-) MVD was 35.8±9.8, P53 (+) MVD was 46.1±16.7, which means MVD increases with P53 expression enhancement, difference between groups was statistically significant (P<0.05) (Figure 2b). MVD was 47.81±9.2 in telomerase positive tissue, 44.76 ±8.4 in the negative tissue, difference between groups was statistically significant (P<0.05) (Figure 2c).
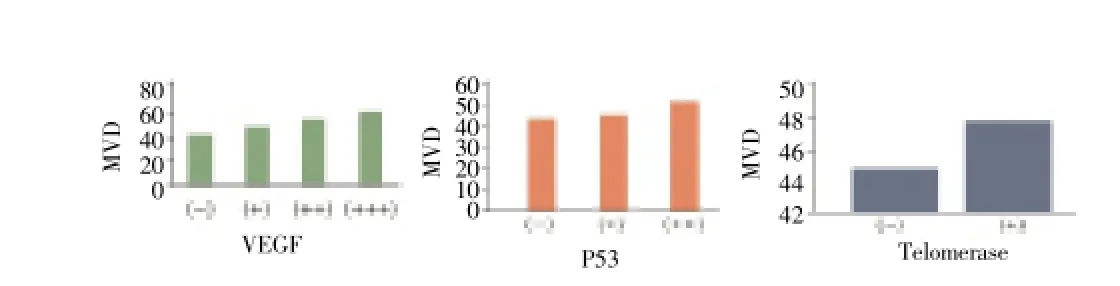
Figure 2. Relationship between VEGF, P53, telomerase and MVD expression.
4. Discussion
The occurrence and development of tumor are complex biological process, malignant invasive tumor growth and metastasis are the main cause of treatment failure[7]. Its growth and metastasis depends on angiogenesis, new blood vessels not only provide nutrition for their growth, also provide channels for transfer. Studies have shown that[8-10], VEGF is widely expressed in gastric cancer cells, promoting angiogenesis, which is the important mechanism of the gastric cancer invasion and metastasis. Numerous studies have found that[11-13], VEGF expression is found in almost all malignant tumors, and not found or only low levels of expression in normal tissue, it also showed that VEGF expression is closely related to the occurrence, development and prognosis of gastric cancer. Other studies have suggested[14-17], P53 gene mutation is considered to be the common genetic events of all kinds of tumor and normal cells, wild-type P53 protein is difficult to measure by routine immunohistochemical method, while half-life prolong obviously as the gene mutation, and protein conformation change, therefore, the application of immunohistochemical method to detect of P53 are all mutant protein. Other studies have shown that[18-20], telomerase is closely related to the occurrence and development of malignant tumor, while the activity of telomerase on its own in the mammalian development showed no direct biological effect. The cells survival and organs balance requires enough telomerase structure to maintain in the cell division, and telomerase in most tumors telomerase activity can be detected.
This experiment showed that the expression of VEGF is associated with histological type, poorly differentiated tissue VEGF positive expression rate is higher; in gastric cancer tissues with VEGF positive expression, MVD count was significantly higher than that of VEGF negative (P<0.05), indicating that in the development process of gastric cancer, increased VEGF expression promotes the formation of new blood vessels in cancerous tissue, plays an important role in the process of the tumor occurrence and development. This group of materials, the expression of VEGF is increased along with the enhancement of P53 expression, it showed that P53 mutations can up-regulate VEGF expression and promote the tumor angiogenesis. This positive rate of telomerase expression in gastric cancer tissue was 74.74%, with higher MVD value in tumor tissue of telomerase (+) than that of (-). It indicates telomerase prompts angiogenesis by the way different from mutant P53, telomerase expression augmentation of the malignant tumors suggests an increase for the potential of occurrence, development and metastasiss its[21-23].
This study shows that the VEGF plays an important role in gastric cancer occurrence and development process, can be used as an index of diagnosis and predicting the prognosis of gastric cancer, by promoting the expression of VEGF. P53 mutation promotes tumor angiogenesis. As a key event in tumor angiogenesis formation, telomerase increases angiogenesis in gastric cancer tissue by other pathway different from P53.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Gao L, Cheng W, Zhu XG, Zhao H. VEGF somatostatin in gastric cancer SGC-7901 cells, the influence of the NF-kappa Bexpression. J Suzhou Univ (Medical Edition) 2010; (3): 483-486.
[2] Guo WH, Wang C, Hong TZ, Fu FM, Zhang J, Zheng MC, et al. Of somatostatin receptor subtype 2, 5 and the peptide expressed in gastric cancer cell lines in the inhibition of gastric cancer cells secrete VEGF. J Clin Lab Med 2010; 5: 321 -323.
[3] Shang SS, Zhang JQ. VEGF-C, VEGFR - 3 in the research progress in the role of tumor lymphatic vessel formation. J Modern Cancer Med 2011; 1: 185-188.
[4] Zhao B, Yang PM, Yang J, Cai D. A randomized trial of somatostatin to regulate the VEGF /VEGFs in patients with gastric cancer. Hepatogastroenterology 2011; 58(109): 1425-1430.
[5] Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12): 2893-2917.
[6] Lin M, Lin HZ, Ma SP, Ji P, Xie D, Yu JX. Vascular endothelial growth factor-A and -C: expression and correlations with lymphatic metastasis and prognosis in colorectal cancer. Med Oncol 2011; 28(1): 151-158.
[7] Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A, et al. Predictive significance of preoperative serum VEGF-C and VEGF-D, independently and combined with Cal9-9, for the presence of malignancy and lymph node metastasis in patients with gastric cancer. J Surg Oncol 2010; 102(6): 699-703.
[8] Du B, Yang ZY, Zhong XY, Fang M, Yan YR, Qi GL, et al. Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World J Gastroenterol 2011; 17(9): 1219-1226.
[9] Zhang C, Hao L, Wang L, Xiao Y, Ge H, Zhu Z, et al. Elevated IGFIR expression regulating VEGF and VEGF-C predicts lymph node metastasis in human colorectal cancer. BMC Cancer 2010; 10: 184.
[10] Schoppmann SF, Tamandl D,Roberts L, Jomrich G, Schoppmann A, Zwrtek R, et al. HER2/neu expression correlates with vascular endothelial growth factor-C and lymphangiogenesis in lymph node-positive breast cancer. Ann Oncol 2010; 21(5): 955-960.
[11] Tanaka T, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Katada T, et al. Vascular endothelial growth factor C (VEGF-C) in esophageal cancer correlates with lymph node metastasis and poor patient prognosis. J Exp Clin Cancer Res 2010; 29: 83.
[12] Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, et al. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. AnatRec (Hoboken) 2010; 293(11): 1838-1846.
[13] Wang TB, Wang J, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C combined with multi-detector CT in the preoperative diagnosis of lymph node metastasis of gastric cancer. Asia Pac J Clin Oncol 2012; 8(2): 180-186.
[14] Moreira LR, Schenka AA, Latuf-Filho P, Penná AL, Lima CS, Soares FA, et al. Immunohistochemical analysis of vascular density and area in colorectal carcinoma using different markers and comparison with clinicopathologic prognostic factors. Tumour Biol 2011; 32(3): 527-534.
[15] Chen H, Zhao GP, Xiao NX, Xia Q, Lai JS, Zheng DS, et al, Correlation of the expressions of VEGF-C and VEGFR-3 to the pathological grade of prostate cancer. Nan Fang Yi Ke Da Xue Xue Bao 2011; 31(1): 155-159.
[16] Dobrzycka B, Tcrlikowski SJ, Kowalczuk O, Kulikowski M, Nikliriski J. Serum levels of VEGF and VEGF-C in patients with endometrial canccr. Cur Cytokine Nctw 2011; 22(1): 45-51.
[17] Xie LX, Zhai TT, Yang LP, Yang E, Zhang XH, Chen JY, et al. Lymphangiogenesis and prognostic significance of vascular endothelial growth factor C in gastro-oesophageal junction adenocarcinoma. Int J Exp Pathol 2013; 94(1): 39-46.
[18] Han FH, Li HM, Zheng DH, He YL, Zhan WH. The effect of the expression of vascular endothelial growth factor (VEGF)-C and VEGF receptor-3 on the clinical outcome in patients with gastric carcinoma. Eur J Surg Oncol 2010; 36(12): 1172-1179.
[19] Pang B, Wei PK, Li YJ. Effect of xiaotan sanjie recipe on expressions of VEGF-C and VEGFR-3 in nude mice with transplanted human gastric adenocarcinoma cell MKN-45. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011; 31(2): 204-208.
[20] Fukui R, Tanabe E, Kitayoshi M, Yoshikawa K, Fukushima N, Tsujiuchi T. Negative regulation of cell motile and invasive activities by lysophosphatidic acid receptor-3 in colon cancer HCTl 16 cells. Tumour Biol 2012; 33(6): 1899-1905.
[21] Raica M, Cimpean AM,Ceausu R, Ribatti D. Lymphatic microvessel density,VEGF-C, and VEGFR-3 expression in different molecular types of breast cancer. Anticancer Res 2011; 31(5): 1757-1764.
[22] Deeb G, Vaughan MM. Mclnnis L, Ford LA, Sait SN, Starostik P. Hypoxia-inducible factor-1 alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res 2011; 35(5): 579-584.
[23] Yan A, Avraham T, Zampell JC, Aschen SZ, Mehrara BJ. Mechanisms of lymphatic regeneration after tissue transfer. PLOS ONE. 2011. 6(2): el 720.
ment heading
10.1016/S1995-7645(14)60041-9
*Corresponding author: Yan-Tang Yu, M.M., Affiliated Hospital of Hebei University of Engineering, Handan 056002, Hebei, China.
Tel: 13513106510
E-mail: yyfangtg@163.com
Xin-Qing Lu, Ph.D., Chief Physician, Affiliated Hospital of Hebei University of Engineering, Handan 056002, Hebei, China.
Foundation project: It is supported by Handan City Technology Bureau Scientific Research Project (No 1023108101-8).
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
