Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells
Yan-Ni Xi, Xiao-Yan Xin, Hong-Mei Ye
1Department of Gynecology and Obstetrics, 2nd Hospital of Yulin, Yulin 719000, China
2Xijing Hospital of the Fourth Military Medical University, Xi’an 710032, China
3Department of Gynecology and Obstetrics, 2nd Hospital of Yulin, Yulin 719000, China
Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells
Yan-Ni Xi1, Xiao-Yan Xin2*, Hong-Mei Ye3
1Department of Gynecology and Obstetrics, 2nd Hospital of Yulin, Yulin 719000, China
2Xijing Hospital of the Fourth Military Medical University, Xi’an 710032, China
3Department of Gynecology and Obstetrics, 2nd Hospital of Yulin, Yulin 719000, China
Objective: To analyze effects of high mobility group AT-hook 2 (HMGA2) on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Methods: Three methods were applied to observe the effect on HMGA2 expression in ovarian cancer cells and ovarian epithelial cells. Results: After the application of siRNA-HMGA2, number of T29A2-cell clones was decreased, there was significant difference compared with the negative control Block-iT. After application of let-7c, number of T29A2+ cell clones was decreased significantly, however, after the application of Anti-let-7, the number of clones restored, and there was no significant difference compared with the negative control group. After interference, the number of T29A2- cells which passed through Matrigel polycarbonate membrane were significantly lower than the negative control group. After the treatment of siRNA-HMGA2, let-7c and sh-HMGA2 respectively, growth and proliferation of T29A2-, T29A2+ and SKOV3 were slower, and the phenomenon was most obvious in SKOV3. Stable interference of HMGA2 induced mesenchymalepithelial changes in the morphology of SKOV3-sh-HMGA2. Conclusions: HMGA2 can promote malignant transformation of ovarian cancer cells, enhance cell invasion and metastasis, and promote cell growth and proliferation of ovarian cancer cells, which can cause ovarian cancer to progress rapidly and affect the quality of life.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
High mobility group AT-hook 2
Ovarian cancer
invasion
Proliferation
Morphology
1. Introduction
Ovarian cancer is a malignant cancer which locates on ovary, most of ovarian cancers are primary and the morbidity is the third among female reproductive system malignant cancers which is just next to cervix cancer and urine uterine cancer[1,2]. The malignant degree of ovarian cancer is high, and affect life quality of patients significantly. Morbidity and progress mechanism is the key in clinical treatment. High mobility group AT-hook 2 (HMGA2) is involved in various biological processes such as the formation of the embryo and the development of malignant cancer[3]. We aimed to analyze its effects on the malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells.
2. Materials and methods
2.1. Materials
2.1.1. Cell lines
Stable high H-RAS protein expression cell line T29H mutated from ovarian cancer epithelial cell lines T29, T80 and T29 was selected. Ovarian cell lines SKOV3, HEY, OVCAR-3, Caov-3 and cells packed by Phonenix virus were selected as experimental cell lines in our research[4-6]. All above cell lines were purchased from American standard center culture collection (American Type Culture Collection).
2.1.2. Instruments and equipments
MTS kits were provided by Promega Corporation for cell proliferation and preserved at minus 20℃. 96-well plateswere purchased from American Scientific Corporation.
2.1.3. Cell culture media
The cell culture medias including dulbecco’s modified eagle medium, heat inactivated fetal bovine serum, M199 culture media, epithelial growth factor, complete medium, pancreatin-EDTA(0.05% and 0.25%), cell freezing medium, and Dulbecco’s phosphate buffered saline were obtained from Sigma-Aldrich Corporation, the preparation methods were referred to related references[7-11].
2.2. Interference methods
2.2.1. Production of transient transfection reagents
20 nmol siRNA-HMGA2 (20 μM) powder was obtained and dissolved in 1ml sterilized distilled water to be divided and preserved in refrigerator. let-7c and anti-let-7c: 5 nmol above powder was obtained and dissolved in sterilized distilled water without RNA enzyme to be divided and preserved in refrigerator. 20 μM control small RNA was obtained and divided to be preserved. Lipofectamine 2000 was directly preserved in refrigerator at constant 44℃[12,13].
2.2.2. Transfection methods
The cells were cultured and transfected when the confluence was up to 40%-50%[14-19]. to T29A2- and T80A2-cells was interfered by HMGA2-siRNA; T29A2+ cells was interfered by let-7c. Transfect HMGA2 expressed ovarian cancer SKOV3-sh-HMGA2 cell line was established by Lentivirus package. SKOV3-pGIPZ was used as control cell line. Fluorescent staining was used to verify the interference result after 48 h.
2.3. Observation indicators
Soft AGAR cloning formation experiment, Matrigel invasion assay, growth curve method[20,21] were applied to observe malignant degree of cells, ability of invasion and metastasis, and proliferation ability, then the morphological change among cells was compared.
2.4. Statistical analysis
All date in our study were analyzed by SPSS13.0. Enumeration data was analyzed byChi-square test and measurement data was analyzed byttest. The test level was set as α=0.05. The difference was considered as statistically significant when P<0.05.
3. Results
3.1. Interference effects
Florescent staining method showed there was satisfactory interference effects in different cells, as shown in Figure 1.

Figure 1. Interference effects of siRNA-HMGA2 tested by florescent staining.
3.2. Malignant degree
After the application of siRNA-HMGA2, number of T29A2-cell clones was significantly decreased compared with the negative control Block-iT (t=13.509, 12.214, respectively, all
P<0.05). After the application of let-7c, number of T29A2+ cell clones was decreased significantly, however, after the application of anti-let-7, the number of clones restored, and there was no significant difference compared with the negative group, as shown in Figure 2.
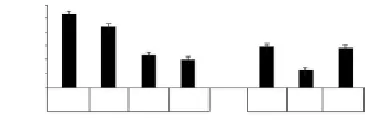
Figure 2. Results of cell line malignant degree by soft AGAR cloning formation experiment.
3.3. Ability of invasion and metastasis
After interference, the number of T29A2- cells which passed through Matrigel polycarbonate membrane was significantly lower than the negative control group, as shown in Figure 3.

Table 1 Comparison of 96 h cell growth condition(mean±sd).
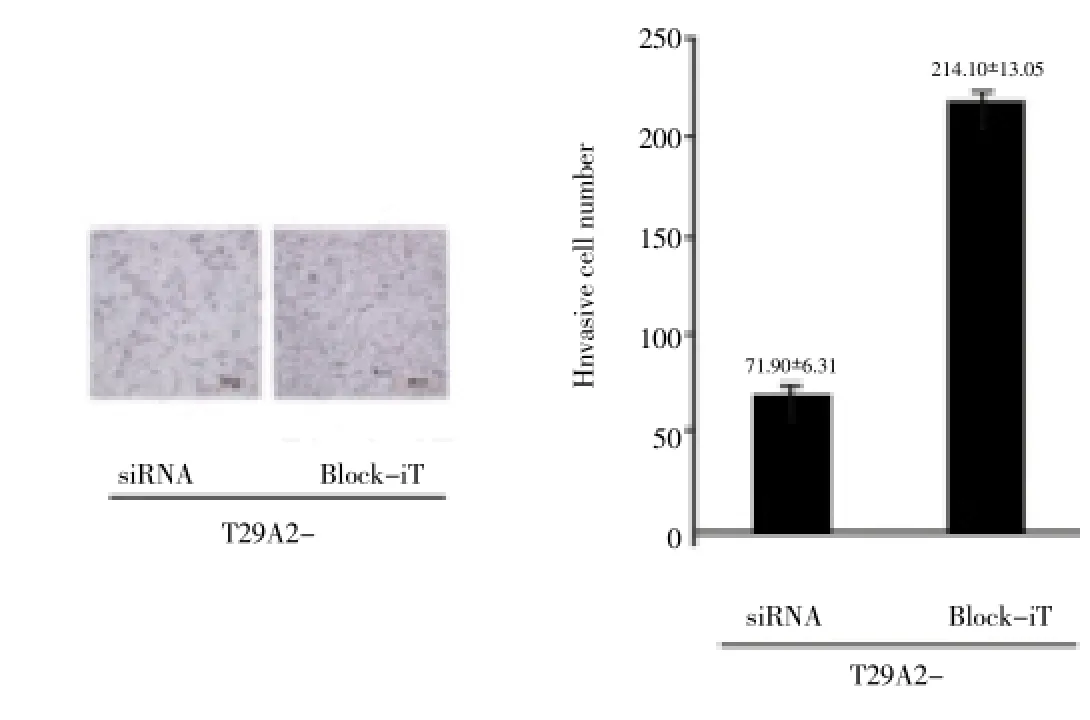
Figure 3. Matrigel invasion assay result of cell line invasion and metastasis ability.
3.4. Ability of proliferation
After the treatment of siRNA-HMGA2, let-7c and sh-HMGA2 respectively, proliferation of T29A2-, T29A2+ and SKOV3 was slower, and the phenomenon was most significant in SKOV3, as shown in Table 3 and Fig 4.
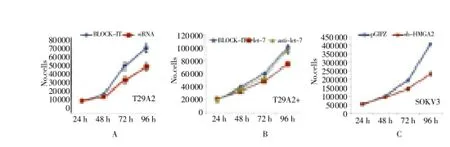
Figure 4. Growth curve analysis of cell line proliferation ability.
3.5. Cell morphology
Stable interference of HMGA2 induced mesenchymalepithelial changes in morphology of SKOV3-sh-HMGA2 (Figure 5).

Figure 5. Cell morphology results.
4. Discussio n
Ovarian cancer is divided in to typeⅠand type Ⅱ, malignant degree of type Ⅰincluding mucous carcinoma, clear cell carcinoma and endometrioid carcinoma is low, however, type Ⅱ has high morbidity and progresses rapidly, 5-year survival rate of which is less than 30% and there has been no effective therapy[22,23]. Thus, to analyze the biological behavior, mechanism of occurrence and development has become critical in guiding clinical treatment.
HMGA2 is a gene located at 12q13-15 which can code and construct transcriptional factor proteins. Dahiyaet al[24-27] found that HMGA2 showed significant effects on the nerve system development, pituitary adenoma development and DNA injury impairment in mouse, and was verified as a critical oncoprotein of many malignant cancers.
To explore the effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells, we applied siRNA, let-7c and antilet-7c to interfere the expression of HMGA2. After the application of siRNA-HMGA2, number of T29A2- cell clones decreased, there was significant difference compared with the negative control Block-iT; after the application of Let-7c, number of T29A2+ cell clones decreased significantly, however, after the application of anti-let-7, the number of clones restored, indicating that after interference of HMGA2 expression, the malignant degree of ovarian cancer significantly decreased; Matrigel invasion assay showed the ability of ovarian cell invasion and metastasis significantly decreased after interference of HMGA2 expression; after the treatment of siRNA-HMGA2, let-7c and sh-HMGA2 respectively, proliferation of T29A2-,T29A2+ and SKOV3 was slower, and the phenomenon was most obvious in SKOV3, indicating the cell growth and proliferation were affected significantly by interference of HMGA2, and the HMGA2 stably interfered cells were affected most[28]. In observation of cellular morphology, we found SKOV3-sh-HMGA2 cells had mesenchymal-epithelial change after HMGA2 interference, and epithelial-mesenchymal transition (EMT) plays important roles in development of various biological processes such as early embryo, invasion and metastasis of cancer and fibrosis of chronic inflammation[29,30]. We consider that the reason why HMGA2 affects the malignant degree, invasion, metastasis, proliferation ability of ovarian cancer is that it is involved in the formation of EMT to promote the malignant transformation, enhance the ability of invasion and metastasis and promote the proliferation of cells. Thus ovarian cancer progresses rapidly and affects the survival quality.
In conclusion, we can apply the HMGA2 expression level interference as the targeted therapy to inhibit the progress of cancer and improve the prognosis.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Van Jaarsveld M, Helleman J, Berns EMJJ, Wiemer EAC. MicroRNAs in ovarian cancer biology and therapy resistance. Intl J Biochem & Cell Biol 2010; 42(8): 1282-1290.
[2] Malek A, Tchernitsa O. Evaluation of targets for ovarian cancer gene silencing therapy: in vitro and in vivo approaches. Methods Mol Biol 2010; 623: 423--436.
[3] Mahajan A, Liu Z, Gellert L, Zou X, Yang G, Lee P, et al. HMGA2: a biomarker significantly overexpressed in high-grade ovarian serous carcinoma. Modern Pathol 2010; 23(5): 673-681.
[4] Mezzanzanica D, Bagnoli M, De Cecco L, Valeri B, Canevari S. Role of microRNAs in ovarian cancer pathogenesis and potential clinical implications. Int J Biochem & Cell Biol 2010; 42(8): 1262-1272.
[5] Boyerinas B, Park S M, Murmann A E, Gwin K, Montag AG, Zillhardt M, et al. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Intl J Cancer 2012; 130(8): 1787-1797.
[6] Hetland TE, Nymoen DA, Holth A, Brusegard K, Flørenes VA, Kærn J, et al. HMGA2 protein expression in ovarian serous carcinoma effusions, primary tumors, and solid metastases. Virchows Archiv 2012; 460(5): 505-513.
[7] Bayani J, Kuzmanov U, Saraon P, Fung WA, Soosaipillai A, Squire JA, et al. Copy number and expression alterations of miRNAs in the ovarian cancer cell line OVCAR-3: Impact on Kallikrein 6 protein expression. Clin Chem 2013; 59(1): 296-305.
[8] Weirauch U, Gutsch D, Höbel S, Aigner A. Polymer-based delivery of rna-based therapeutics in ovarian cancer. Methods Mol Biol 2013; 1049: 443-465.
[9] Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocrine-related Cancer 2010; 17(1): F19-F36.
[10] Kim TH, Kim YK, Kwon Y, Heo JH, Kang H, Kim G, et al. Deregulation of miR-519a, 153, and 485-5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology 2010; 57(5): 734-743.
[11] Lu L, Schwartz P, Scarampi L, Rutherford T, Canuto EM, Yu H, et al. MicroRNA let-7a: a potential marker for selection of paclitaxel in ovarian cancer management. Gynecologic Oncol 2011; 122(2): 366-371.
[12] McCluggage WG, Connolly LE, McBride HA, Kalloger S, Gilks CB. HMGA2 is commonly expressed in uterine serous carcinomas and is a useful adjunct to diagnosis. Histopathology 2012; 60(4): 547-553.
[13] Kwon MJ, Shin YK. Epigenetic regulation of cancer-associated genes in ovarian cancer. Int J Molecular Sci 2011; 12(2): 983-1008.
[14] Harter P, Hilpert F, Mahner S, Heitz F, Pfisterer J, du Bois A. Systemic therapy in recurrent ovarian cancer: current treatment options and new drugs. Expert Rev Anticancer Ther 2010; 10(1): 81-88.
[15] Bose CK. Anomalous endocrine feedback of peri-menopause in the etiology of type II ovarian cancer. Future Oncol 2013; 9(9): 1257-1261.
[16] Davidson B, Smith Y, Nesland JM, Kærn J, Reich R, Tropè CG. Defining a prognostic marker panel for patients with ovarian serous carcinoma effusion. Human Pathol 2013; 44(11): 2449-2460.
[17] Mezzanzanica D, Canevari S, Cecco LD, Bagnoli M. miRNA control of apoptotic programs: focus on ovarian cancer. Expert Rev Mol Diagnostics 2011; 11(3): 277-286.
[18] Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 2009; 8(6): 843-852.
[19] Verhaak RGW, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clinical Invest 2013; 123(1): 517.
[20] Zaman MS, Maher DM, Khan S, Jaggi M, Chauhan SC. Current status and implications of microRNAs in ovarian cancer diagnosis and therapy. J Ovarian Res 2012; 5(1): 44.
[21] Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. American J Surgical Pathol 2012; 36(6): 799-807.
[22] Dutta S, Wang F, Phalen A, Fishman DA. Biomarkers for ovarian cancer detection and therapy. Cancer Biol & Ther 2010; 9(9): 668-677.
[23] Winter N, Nimzyk R, Bösche C, Meyer A, Bullerdiek J. Chromatin immunoprecipitation to analyze DNA binding sites of HMGA2. PloS One 2011; 6(4): e18837.
[24] Dahiya N, Morin PJ. MicroRNAs in ovarian carcinomas. Endocrine-related Cancer 2010; 17(1): F77-F89.
[25] Kobayashi E, Ueda Y, Matsuzaki S, Yokoyama T, Kimura T, Yoshino K, et al. Biomarkers for screening, diagnosis, and monitoring of ovarian cancer. Cancer Epidemiol Biomarkers & Prev 2012; 21(11): 1902-1912.
[26] Chung YW, Bae HS, Song JY, Lee JK, Lee NW, Kim T, et al. Detection of microrna as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. Intl J Gynecol Cancer 2013; 23(4): 673-679.
[27] Karve TM, Li X, Saha T. BRCA1-mediated signaling pathways in ovarian carcinogenesis. Functional & Integrative Genomics 2012; 12(1): 63-79.
[28] Wan SM, Lv F, Guan T. Identification of genes and microRNAs involved in ovarian carcinogenesis. Asian Pac J Cancer Prev 2012; 13: 3997-4000.
[29] Natarajan S, Hombach-Klonisch S, Dröge P, Klonisch T. HMGA2 inhibits apoptosis through interaction with ATR-CHK1 signaling complex in human cancer cells. Neoplasia (NY) 2013; 15(3): 263.
[30] Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S,et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol 2010; 11(2): 136-146.
ment heading
10.1016/S1995-7645(14)60040-7
*Corresponding author: Xiao-Yan Xin, Xijing Hospital of the Fourth Military Medical University, Xi’an 710032, China.
Tel: 15929989224
E-mail: 584332674 @qq.com
Foundation project: It is supported by National Natural Science Foundation of China (6821183728).
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
