Dynamic Wnt5a expression in murine hair follicle cycle and its inhibitory effects on follicular in vivo
De-Ren Fang, Zhong-Fa Lv, Gang Qiao
1Department of Dermatology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China
2Department of Dermatology, the Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, China
3Department of Dermatology, the Third People’s Hospital of Hanghzhou, Hanghzhou, 310009 China
Dynamic Wnt5a expression in murine hair follicle cycle and its inhibitory effects on follicular in vivo
De-Ren Fang1*, Zhong-Fa Lv2, Gang Qiao3
1Department of Dermatology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China
2Department of Dermatology, the Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, China
3Department of Dermatology, the Third People’s Hospital of Hanghzhou, Hanghzhou, 310009 China
Objective: To analyze the dynamic expression of Wnt family member 5A (Wingless-type MMTV integration Wnt site family, member 5a) in murine hair cycle and its inhibitory effects on follicle in vivo. Methods: Situ hybridization in full-thickness skin was used to observe the change of mouse protein expression in different growth stages, and Ad-Wnt5a was injected after defeathering to observe the hair follicle growth in vivo. Results: The Wnt5a mRNA was expressed at birth, and was firstly increased then decreased along with the progress of the hair cycle. It reached the peak in advanced stage of growth cycle (P<0.05). Rhoa and β-catenin expression levels were significantly decreased in three groups. Rac2 expression was significantly up-regulated, and the expression level of Wnt5a, Shh and Frizzled2 was increased, but less significantly than group 2. Conclusions: The expression of Wnt5a mRNA is consistent with change of murine follicle cycle, and has obvious inhibitory effects on the growth of hair follicle in vivo, indicating that it is antagonistic to Wnts pathway and interferes the growth of follicle together.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 March 2014
Available online 20 April 2014
Wnt5a
1. Introduction
Wingless-type MMTV integration Wnt site family, member 5a is a non-classical Wnt signaling pathway which is closely related to the differentiation of skin tissue[1,2]. The theory that classical Wnt pathway can induce the morphological change of follicle and maintain the cycle has been verified universally[3-5]. However, report about the effects of non-classical Wnt pathway on follicle is lack. We analyzed the dynamic expression of Wnt 5a in murine hair follicle cycle and its inhibitory effects on folliclein vivoto explore the mechanism of Wnt signaling pathway in follicle cycle signal regulation network.
2. Materials and Methods
2.1. Experimental materials
115 C57BL/6J mice were purchased from Institute of Laboratory Animal, Chinese Academy of Medical Sciences, 15 mice were used for follicle cycle research and the rest 100 were used for the inhibitory effect analysis on folliclein vivo. Experimental reagents such as 40% paraformaldehyde solution, 0.01 M PBS solution, 0.75 mol/L sodium citrate, prehybridization solution, 2×SDS loading buffer, 30% (w/v) gel preservation liquid, Tris-HCl (1.5 mol/L and 1.0 mol/L), 11% electrophoresis separation gel, 4% electrohpresis condense gel, 5×Tris-Glycine electrophoresis buffer, 10% SDS, 10% APS, Electricity transfer buffer, 0.5M EDTA, Tris buffer, 80% glycerin, PBST, and sample loading buffer were prepared by ourselves according to related references[6-13].
2.2. Experimental methods
2.2.1. Wnt5a dynamic expression experiment
Five μL plasmid DNA was obtained for plasmidestablishment and probe preparation[14,15], 15 mice were divided into two groups according to body development: Group A (Advanced stage), Group B (early stage of growth), group C (advanced stage of growth), group D (regression stage) and group E (stationary stage) with 3 mice in each. Hybridization in situ in full-thickness skin, immunohistochemistry, Semi-quantitative RT-PCR, immunoblotting were applied on the middle of back skin according to methods in related references[16-18] to observe the difference of back follicles among different groups after treatment.
2.2.2. Inhibition of follicle in vivo
One hundred stationary stage mice were selected. Twenty were set as control, 80 were applied by mixture of rosin and beewax at back to defeather and induce the follicles to enter intermediate growth stage and establish hair follicle cycle synchronization model. The above 100 mice were divided into three groups, the control group as group 1 with 20 mice, group 2 and group 3 with 40 in either. Group 1 received no special treatment; group 2 had only defeathering treatment; group 3 had Ad-Wnt5a subcutaneous injection after defeathering. Skin phenotypic changes, HE staining, gene microarrays and immunohistochemistry were compared 7 days after treatment. The testing methods were referred to related references[19-21].
2.3. Statistical analysis
All date in our study were analyzed by SPSS13.0. Enumeration data was analyzed byChi-square test and measurement data was analyzed byttest. The test level was set as α=0.05. The difference was considered as statistically significant when P<0.05.
3. Results
3.1. Result of dynamic expression in different follicle cycle
3.1.1. Wnt5a mRNA expression in different cycles
Bluish violet color represents positive reaction. As shown in Figure 1, Wnt5a mRNA had high expression in group A follicle, especially condensed at part Mx (Figure 1A’). The part which migrates to corium layer; had significantly increased expression at part Mx in group B and decreased at part IRS of follicle (Figure 1B’). Expression was highest in group C. The positive signal trended to decrease in group D. There was only a little Wnt5a mRNA expression in group E.
3.1.2. Semi-quantitative RT-PCR results
The Wnt5a mRNA was expressed at birth. It was firstly increased then decreased along with the progress of the hair follicle cycle. It reached the peak at advanced stage of growth cycle (P<0.05) (Table 1 and Figure 2).
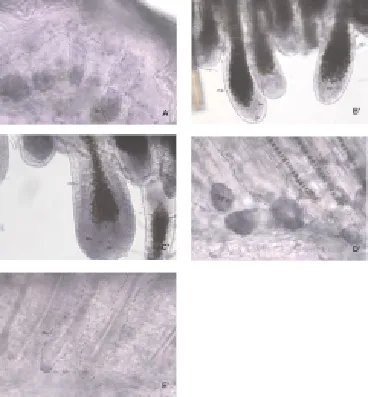
Figure 1. Wnt5a mRNA expression in different cycles.
Table 1 Comparison of Semi-quantitative RT-PCR results among different cycles
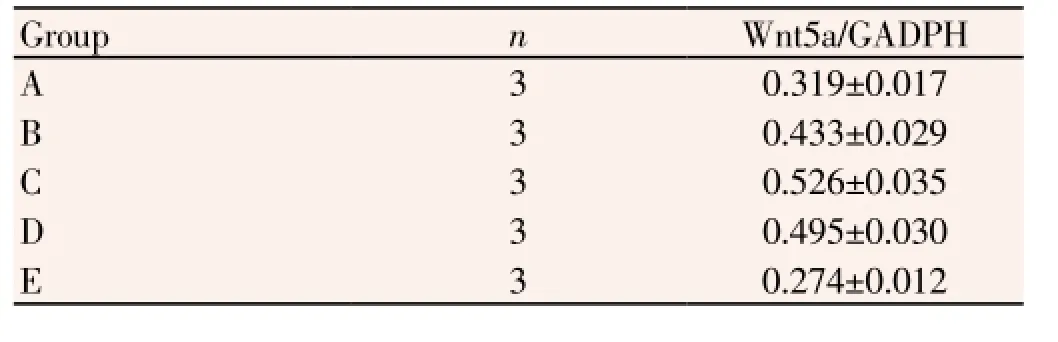
Table 1 Comparison of Semi-quantitative RT-PCR results among different cycles
?

Figure 2. Semi-quantitative RT-PCR results in different cycles.
3.1.3. Immunoblotting results
Immunoblotting results in different groups were in accordance with Semi-quantitative RT-PCR results, as shown in Figure 3.
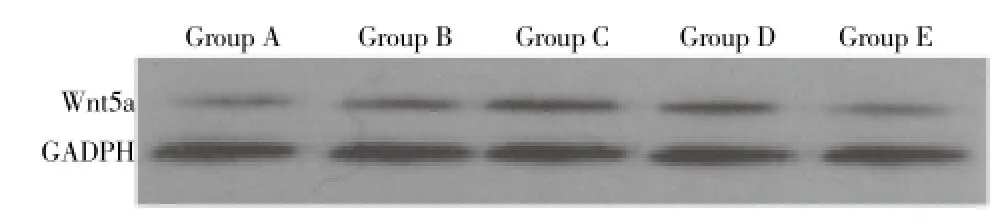
Figure 3. Immunoblotting results in different cycles.
3.2. Follicle growth in vivo
3.2.1. Model establishment.
Back skin of three groups showed pink color after defeathering, and turned to black after 7 days to effectively synchronize follicle cycles, as shown in Figure 4.

Figure 4. Model establishment in group 1.A. Right after defeathering; B.7 days after defeathering.
3.2.2. Phenotype change
Mice in group 1 and group 2 entered growth stage 14 days after defeathering, however, Ad-Wnt5a injection region in group 3 still stayed pink, as shown in Figure 5.

Figure 5. Phenotype change of nice in different groups 14 days after defeathering.
3.2.3. Differential gene analysis
Rhoa and β-catenin expression levels were significantly decreased in group 3, and Rac2 expression was significantly up-regulated. Wnt5a, Shh and Frizzled2 expression levels were increased, but less obvious than group 2, as shown in Table 2.
Table 2 Comparison of differential gene expression analysis among different groups after defeathering

Table 2 Comparison of differential gene expression analysis among different groups after defeathering
Note: group 1 was control group, its gene expression was set as standard.
?
4. Discussion
Skin of mammal is the primary protective barrier of body, and follicle is the main accessory of skin. The growth of follicle includes growth, regression and stationary stages to ensure the recycling growth and regression process under the regulation of complicated signaling network[22,23]. Research has proved that classical Wnts pathway promotes the follicle growth cycle, however, there has been less research about non-classical Wnts pathway on follicle cycle. Veraitchet al[24,25] found that Wnt5a showed high expression in psoriasis patients accomplished with the cornification differentiation disorder. Thus, research on mechanism of Wnt5s may provide explanation for variety of skin diseases and guide clinical treatment.
We observed the dynamic change of Wnt5a in mouse follicle cycle and found that Wnt5a mRNA expression was closely related to the murine follicle cycle. Wnt5a mRNA showed obvious high expression in cell matrix at follicle growing stage. Wnt5a mRNA first increased and then decreased along with the hair follicle cycle, then follicle epithelial cells stopped proliferation and started to apoptosis, which was consistent with the trend of Wnt5a mRNA. This indicated that Wnt5a had dynamic expression model in follicle cycle and may have critical effects in the process of follicle growth. In follicle inhibition experimentin vivowe found that overexpression of Wnt5a could inhibit the normal growth of follicle, and significant decrease of β -catenin in group 3 implied that follicle growth ability in mice which accepted Ad-Wnt5a greatly decreased, this was also verified in phenotype experiment. As the representive of non-classical pathways, the effects of Wnt5a is exactly opposite to Wnts pathway, indicating that the two pathways keep follicle growth balancein vivoantagonisticly[26-28]. For example, Wnt5a can activate JNK and inhibit Wnts pathway, but Wnts can inhibit Camk-Ⅱ and promote the β-catenin formation to promote follicle growth cycle[29]. Besides, Mardaryevet al[30] found that Wnt5a could active PCP pathway signaling chain with Rac2 involved to some extent. The mechanism may be related to PCP signaling, this also explained the reason of Rac2 upregulation and Rhoa downregulation.
In conclusion, Wnt5a mRNA expression is in accordance with murine hair follicle cycle, and can inhibit growth of folliclein vivosignificantly, indicating that it is antagonistic to Wnts pathway and affect the growth of follicle together.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The research was funded b by Science Research Foundation of Department of Education of Zhejiang Province, China(NO20061246 ) .
[1] Sugawara K, Schneider MR, Dahlhoff M, Kloepper JE, Paus R. Cutaneous consequences of inhibiting EGF receptor signaling in vivo: normal hair follicle development, but retarded hair cycle induction and inhibition of adipocyte growth in Egfr (Wa5) mice. J Dermatol Sci 2010; 57(3): 155.
[2] Lee J, Hoi CS, Lilja KC, White BS, Lee SE, Shalloway D, et al. Runx1 and p21 synergistically limit the extent of hair follicle stem cell quiescence in vivo. Proceed Nat Acad Sci 2013; 110(12): 4634-4639.
[3] Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011; 144(1): 92-105.
[4] Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012; 10(1): 63-75.
[5] Lyubimova A, Garber J J, Upadhyay G, Sharov A, Anastasoaie F, Yajnik V, et al. Neural Wiskott-Aldrich syndrome protein modulates Wnt signaling and is required for hair follicle cycling in mice. J Clin Inv 2010; 120(2): 446.
[6] Lefever T, Pedersen E, Basse A, Paus R, Quondamatteo F, Stanley AC, et al. N-WASP is a novel regulator of hair-follicle cycling that controls antiproliferative TGFβ pathways. J Cell Sci 2010; 123(1): 128-140.
[7] Mardaryev A N, Ahmed MI, Vlahov NV, Fessing MY, Gill JH, Sharov AA, et al. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. FASEB J 2010; 24(10): 3869-3881.
[8] Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci 2010; 57(1): 2-11.
[9] Kloepper JE, Sugawara K, Al-Nuaimi Y, Gáspár E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol 2010; 19(3): 305-312.
[10] Brownell I, Guevara E, Bai CB, Loomis CA., Joyner, A.L .Nervederived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011; 8(5): 552-565.
[11] Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol 2009; 19(3): R132-R142.
[12] Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010; 327(5965): 542-545.
[13] Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes & Dev 2011; 25(5): 485-498.
[14] Huh SH, Närhi K, Lindfors PH, Haara O, Yang L, Ornitz DM, et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes & Dev 2013; 27(4): 450-458.
[15] Samuelov L, Sprecher E, Tsuruta D, Bíró T, Kloepper JE, Paus RSamuelov L, et al. P-Cadherin regulates human hair growth and cycling via canonical WNT signaling and transforming growth factor-β2. J Inv Dermatol 2012; 132(10): 2332-2341.
[16] Philpott MP. Hair follicle culture. TRP channels in drug discovery. Humana Press; 2012, p. 287-299.
[17] Botchkarev VA, Fessing MY, Botchkareva NV, Westgate G, Tobin DJ. First international symposium “epigenetic control of skin development and regeneration”: How chromatin regulators orchestrate skin functions. J Inv Dermatol 2013; 133(8): 1918-1921.
[18] Genander M, Holmberg J, Frisén J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells 2010; 28(7): 1196-1205.
[19] Coulombe PA, Caterina MJ. The incidental pore: CaV1. 2 and stem cell activation in quiescent hair follicles. Genes & Dev 2013; 27(12): 1315-1317.
[20] Folgueras AR, Guo X, Pasolli HA, Stokes N, Polak L, Zheng D, et al. Architectural niche organization by LHX2 is linked to hair follicle stem cell function. Cell Stem Cell 2013; 13(3): 314-327.
[21] Chueh SC, Lin SJ, Chen CC, Lei M, Wang LM, Widelitz R, et al. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther 2013; 13(3): 377-391.
[22] Kimura-Ueki M, Oda Y, Oki J, Komi-Kuramochi A, Honda E, Asada M, et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J Inv Dermatol 2012; 132(5): 1338-1345.
[23] Yucel G, Altindag B, Gomez-Ospina N, Rana A, Panagiotakos G, Lara MF, et al. State-dependent signaling by Cav1. 2 regulates hair follicle stem cell function. Genes & Dev 2013; 27(11): 1217-1222.
[24] Qi J, Kim H, Scortegagna M, Ronai ZA. Regulators and effectors of Siah ubiquitin ligases. Cell Biochem Biophy 2013: 1-10.
[25] Plikus MV, Baker R E, Chen CC, Fare C, de la Cruz D, Andl T, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 2011; 332(6029): 586-589.
[26] Takeda N, Jain R, LeBoeuf MR, Li D, Li L, Lu MM, et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 2013; 140(8): 1655-1664.
[27] Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity. Cell Biochem Biophys 2009; 55(2): 55-79.
[28] Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011; 8(2): 177-187.
[29] Sengupta A, Lichti UF, Carlson BA, Cataisson C, Ryscavage AO, Mikulec C, et al. Targeted disruption of glutathione peroxidase 4 in mouse skin epithelial cells impairs postnatal hair follicle morphogenesis that is partially rescued through inhibition of COX-2. J Inv Dermatol 2013; 133(7): 1731-1741.
[30] Mardaryev A N, Meier N, Poterlowicz K, Sharov AA, Sharova TY, Ahmed MI, et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 2011; 138(22): 4843-4852.
ment heading
10.1016/S1995-7645(14)60039-0
*Corresponding author: De-Ren Fang, Department of Dermatology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China.
Tel: 86-571-87236385
E-mail: fangdr@gmail.com
Foundation project: It is supported by Zhejiang University Medical Key Funds (No 201028382).
Hair follicle cycle
Iinhibition
Proliferation
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
