Evaluation of in vitro antioxidant and apoptotic activities of Cyperus rotundus
Kilani-Jaziri Soumaya, Ghedira Zied, Nasr Nouha, Krifa Mounira, Ghedira Kamel, Franca Dijoux Marie Genviève, Ghedira Chekir Leila
1Unité de Substances Naturelles Bioactives et Biotechnologies UR12ES12, Université de Monastir, Faculté de Pharmacie de Monastir
2Laboratoire de Biologie Moléculaire et Cellulaire, Faculté de Médecine Dentaire de Monastir, Université de Monastir, Rue Avicenne, 5019 Monastir, Tunisie
3Laboratoire de Botanique, Pharmacognosie et Phytothérapie, UMR CNRS 5557 Ecologie Microbienne; Institut des Sciences Pharmaceutiques et Biologiques-Faculté de Pharmacie Université Claude Bernard, Bâtiment Nétien-8 Avenue Rockefeller-69373 Lyon cedex 08, France
Evaluation of in vitro antioxidant and apoptotic activities of Cyperus rotundus
Kilani-Jaziri Soumaya1,2, Ghedira Zied1,2, Nasr Nouha1,2, Krifa Mounira1,2, Ghedira Kamel1, Franca Dijoux Marie Genviève3, Ghedira Chekir Leila2*
1Unité de Substances Naturelles Bioactives et Biotechnologies UR12ES12, Université de Monastir, Faculté de Pharmacie de Monastir
2Laboratoire de Biologie Moléculaire et Cellulaire, Faculté de Médecine Dentaire de Monastir, Université de Monastir, Rue Avicenne, 5019 Monastir, Tunisie
3Laboratoire de Botanique, Pharmacognosie et Phytothérapie, UMR CNRS 5557 Ecologie Microbienne; Institut des Sciences Pharmaceutiques et Biologiques-Faculté de Pharmacie Université Claude Bernard, Bâtiment Nétien-8 Avenue Rockefeller-69373 Lyon cedex 08, France
Objective: To evaluate in vitro antioxidant and apoptotic activities of Cyperus rotundus (C. rotundus). Methods: The phytochemical study and the antioxidant activities of both methanol and aqueous extracts from C. rotundus aerial part were determined. In addition, these extracts were also investigated for their cytotoxic and apoptotic activities. The major compound of the methanol extract was isolated. Both methanol and aqueous extracts (300, 150, and 50 μg/mL) were evaluated for their antioxidant activity by the xanthine/xanthine oxidase assay system. However, 16, 8, and 4 mg/mL of each extract were tested to investigate their OH. formation scavenging potential. Aqueous extract (800, 400, and 200 μg/mL) and methanol extract (350, 175, and 88 μg/mL) were tested against lipid peroxidation, induced by 75 μM H2O2. The cytotoxicity (by MTT assay) and cell DNA fragmentation of both extracts were evaluated towards K562 and L1210 cell lines. The major compound was obtained from the butanol fraction of methanol extract and its structure was determined by RMN spectroscopic analysis. Results: The methanol and aqueous extracts showed respectively, 88% and 19% inhibition of xanthine oxidase activity. Yet, the same extracts inhibited lipid peroxidation by 61.5% and 42.0%, respectively. Both extracts inhibited OH. formation by 27.1% and 25.3%, respectively. Only methanol extract induced DNA degradation. Orientin was determined as the major compound isolated from the butanol fraction of methanol extract. Conclusions: It appears that C. rotundus extracts exhibit a potential use as a natural antioxidant and an apoptosis inducer.
ARTICLE INFO
Article history:
Received 10 July 2013
Received in revised form 15 August 2013
Accepted 15 September 2013
Available online 20 February 2014
Polyphenols
Orientin
Xanthine/Xanthine oxidase assay
Lipid peroxidation assay
Deoxyribose assay
DNA fragmentation
1. Introduction
Overproduction of reactive oxygen species is considered a major cause of molecular injury and is implicated in the pathogenesis of several human diseases and age-related degenerative processes[1]. Interest in the natural sources of antioxidant molecules in the food, beverage, and cosmetic industries has resulted in a large body of research in the recent years characterizing the mechanisms of action of well known lipid and water-soluble antioxidants such as tocopherols and ascorbic acid, respectively, as well as the antioxidants isolated from herbs, spices, oilseeds, green and black tea, citrus fruits, grapes, and alcoholic beverages[2]. Among these, the natural polyphenolic compounds, especially flavonoids, have been largely studied for their strong antioxidant capacity[3], and they have been demonstrated to possess a therapeutic potential in some human disorders, including cancer, atherosclerosis, and liver diseases[4]. Supporting this, the antioxidant andchemoprotective properties of individual food flavonoids or polyphenolic extracts have been widely reported in cultured cells[5], animal models[6] and humans[7].
Cyperus rotundus(C. rotundus) Linn, a sedge of the Cyperaceae family, is widely scattered in the Mediterranean basin areas. This plant, which grows naturally in tropical, sub-tropical and temperate regions, is widespread in the north-east, Center and south Tunisia[8].C. rotundusis a traditional medicinal plant appearing among Indian, Chinese and Japanese natural drugs used against spasms, stomach disorders, and inflammatory bowel diseases[9]. Although it has already been demonstrated that aerial parts ofC. rotunduscontain phenolic compounds, little is known about their antioxidant and antigenotoxic potentials[10,11]. We firstly investigated the effects of aqueous and methanol extracts from the aerial parts ofC. rotundus, on the genotoxicity induced by both Aflatoxin B1 (AFB1) and sodium azide, in a bacterial assay system, ie. the Ames test. The two extracts also exhibited an important free radical scavenging activity towards the 1,1-diphenyl-2-picrylhydrazyl (DPPH.) free radical[10]. Previous phytochemical studies onC. rotundusrevealed the presence of alkaloids, flavonoids, tannins, starch, glycosides and furochromones, and many novel sesquiterpenoids[12-14].
Accordingly, in the present study, the antioxidant properties of the aerial part extracts were evaluated through biochemical assaysie.; the xanthine/xanthine oxidase enzymatic assay system, deoxyribose assay and the inhibition of lipid peroxidation induced by H2O2in the Human chronic myelogenous leukemia cell line K562, through the formation of thiobarbituric acid-reactive substances. Furthermore, the effects ofC. rotunduson cell proliferation and apoptosis induction in murine and human leukemia cells were also examined. Besides, the main phenolic (orientin) compound in the methanol extract was isolated by chromatographic methods and was determined by spectroscopic data analysis and by a comparison with the literature.
2. Materials and methods
2.1. Plant material
C. rotundusaerial parts were collected in the region of Monastir in the Center of Tunisia in October 2007. The botanical identification was carried out by Pr. M. Chaieb (Department of Botany, Faculty of Sciences, University of Sfax, Tunisia), according to the flora of Tunisia[8]. A voucher specimen (Cp.10-07) was kept in the laboratory of Pharmacognosy, Faculty of Pharmacy of Monastir, for future reference.
2.2. Preparation of plant extracts
The fresh aerial parts ofC. rotundus, were dried at room temperature and reduced to coarse powder. In the present study, two extracts were investigated. The powdered leaves were extracted by boiling water for 15 to 20 min. The crude extract obtained was filtered and lyophilized (aqueous extract); Methanol extract was obtained by Soxhlet extraction (6 h). This extract was concentrated to dryness and the residue was kept at 4 ℃.
2.3. Quantitative analysis of extracts
2.3.1. Determination of total polyphenol and flavonoid content
The polyphenol content ofC. rotunduswas quantified by the Folin-Ciocalteau reagent[15].
The polyphenol content was expressed according to the following formula:
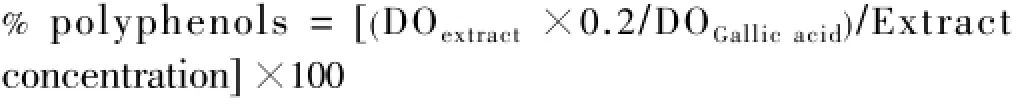
A known volume of each extract was placed in a 10 mL volumetric flask to estimate the flavonoid content according to the method of Zhishenet al[16].
The flavonoid content was expressed according to the following formula:
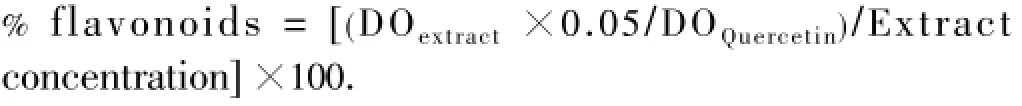
2.3.2. Determination of tannin content
The method described by Châabaneet al[17] was used for the determination of tannin content of the samples.
The tannin content (tannic acid equivalents) was calculated in triplicate, using the following formula:
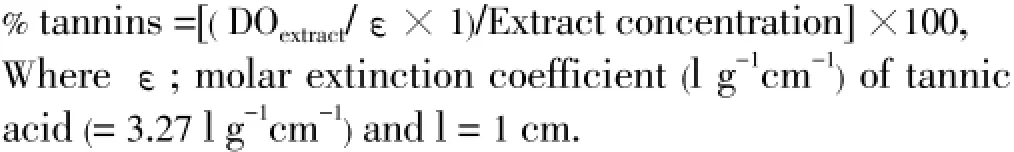
2.4. Extraction and isolation of orientin
The residue of the methanol extract (10.6 g) was suspended in warm water and incubated overnight at 4 ℃. The following day, the solution was filtered and successively portioned between water and chloroforme (v/v). The aqueous phase is then recuperated and treated with 1-butanol saturated in water (v/v). Each liquid-liquid extraction was carried out three times. The obtained sub-extracts were concentrated by evaporation under vacuum to dryness.
The butanol soluble fraction (0.9 g) was purified by passage through C-18 gel disposable extraction column eluted with a gradient H2O: MeOH (90:10 to 0:100) increasing MeOH contents and 15 sub-fractions were collected. Sub-fraction 9 was chromatographed over Sephadex LH-20 eluted with MeOH: H2O (90:10) to afford 3 mg of orientin. This compound was identified by comparaison of its NMR data with the literature[18]. Nuclear magnetic resonance spectra were obtained using a Bruker®Avance spectrometer, operating at 300 MHz for1H. Dimethyl sulfoxide (DMSO-d6) was used as a solvent.
2.5. Evaluation of xanthine oxidase inhibition effect
The inhibition of xanthine oxidase (XO) was measured according to the decrease in uric acid absorbance at 290 nm as proposed by Limemet al[19]. The tested concentration was 50, 150, and 300 μg/mL, for each extract.
2.6. Hydroxyl radical assay
The hydroxyl radical scavenging activity was determined according to the method of Halliwellet al[20]. The hydroxyl radical scavenging activity of the extracts was measured by the competition between deoxyribose and the extracts for the hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/ H2O2system (non site-specific assay) or Fe3+/ascorbate/ H2O2(site-specific assay). The hydroxyl radical scavenging activity was calculated using the following formula: Hydroxyl radical scavenging activity (%) = [(A0- A1/A0) ×100], where A0is the absorbance of the control (without extract), and A1is the absorbance in the presence of extract or the standard sample.
2.7. Cytotoxicity studies in vitro
2.7.1. Culture of cells
K562 (human chronic myelogenous leukemia) and L1210 (murine leukemia) cell lines were obtained from American Type Culture Collection (Rockville, MD). The cells were cultivated in RPMI-1640 medium supplemented with 10% v/ v fetal calf serum, 1% gentamycin and 2 mM L-glutamine as a complete growth medium. The cells were incubated at 37 ℃ in an incubator with 5% CO2in humidified atmosphere.
2.7.2. Assay for cytotoxic activity
The cytotoxicity ofC. rotundusextracts against the two leukaemia cell lines was estimated by the MTT assay, based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells. The resulting blue formazan product can be measured spectrophotometrically. The cytotoxic effects of the extracts were estimated in terms of growth inhibition percentage and expressed as IC50[21].
2.8. Lipid peroxidation inhibitory activity
Lipid peroxidation inhibition activity was monitored by the measurement of malondialdehyde (MDA) according to the method of Ben Ammaret al[22]. The cells were exposed to various concentrations of each extract in the incubation during 2 h, followed by 75 μM H2O2treatment for 2 h. The inhibitory activity towards lipid peroxidation was expressed as IC50(concentration of extract giving 50% inhibition of MDA-TBA complex formation) compared to control solution, without the tested extract.
2.9. Electrophoretic analysis of DNA fragmentation
DNA fragmentation was analysed by agarose gel electrophoresis as described by Wanget al[23], with slight modifications. L1210 and K562 cells (1.5×106cells/mL) were exposed to the extract for 24 and 48 h and harvested by centrifugation. The cells were then treated to extract chromosomal DNA which was transferred to a 1.5% agarose gel. The gel was visualized under UV light after staining with ethidium bromide (1 μg/mL).
2.10. Statistical analysis
Data were collected and expressed as the mean±standard deviation of three independent experiments and IC50values, from thein vitrodata, were calculated by regression analysis. Statistical significance was determined using the Student’st-test. P<0.05 was considered as indicative of significance as compared to the control group.
3. Results
3.1. Total phenolics contents
The phytochemical study ofC. rotundusextracts showed the presence of various quantities of total polyphenolic, flavonoids compounds and tannins. In this work, the total polyphenol content of the extracts was expressed as gallic acid equivalents[24] following confirmation of linearity of the response of the assay using the extract. The total flavonoid content of theC. rotundusextracts was determined by the method of Zhishenet al[16].
The phytochemical study of both methanol and aqueous extracts revealed the presence of polyphenols [respectively, (290.0±14.5) and (200.0±3.0) μg equivalent of gallic acid/ mg of each extract], and falvonoids [respectively, (330.0± 11.0) and (260.0±2.5) μg equivalent of quercetin/mg of each extract). Yet, tannins were weaker with respectively (68.7± 2.5) and (59.6±8.5) μg/100 mg of the extract. The percent yield of the aqueous extract was 13.7% and the methanol extract was 13.2%. On the other hand, we were interested in fractionating the methanol extract not the aqueous extract in this work, because the methanol extract is the most active extract tested in the differentin vitromethods to evaluate the antioxidant and apoptotic activities compared to the aqueous extract.
3.2. Identification of orientin
The structural identification of orientin was established using spectroscopic analysis, especially, NMR spectra and a direct comparison with the published data. This predominant compound (Figure 1) was found to belong to the secondary metabolite class of flavonoids and it might influence the antioxidant and apoptotic activities of the methanol extract. This compound is one among other flavonoids, not yet identified in this extract, and which we discussed its potential role in the biological activities of the original extract.
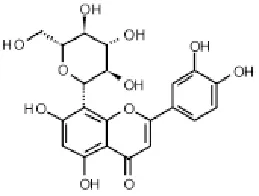
Figure 1. Orientin.
3.3. Evaluation of xanthine oxidase activity
We evaluated the influence of the aqueous and methanol extracts isolated fromC. rotundusaerial parts on the xanthine oxidase activity, in an enzymatic oxidation system of xanthine to uric acid and superoxide anion as final products. This system seems to be more related to the physiological conditions[25]. In Figure 2, we reported that increasing concentrations of both aqueous and methanol extracts lead to a progressively decreased formation of uric acid and therefore to the superoxide anion. It seems that methanol extract is more efficient to inhibit xanthine oxidase when compared to the aqueous extract with respectively 88% and 19% at the same concentration of 300 μg/mL.
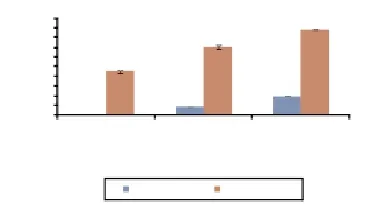
Figure 2. Inhibition of uric acid production by xanthine oxidase reaction in the presence of different concentrations of aqueous and methanol extracts from C. rotundus.*: P< 0.05 as compared with the control group.
3.4. Hydroxyl radical scavenging activity
In the present study, we examined the inhibitory action of aqueous and methanol extracts on deoxyribose assay which gives an indication of hydroxyl radical scavenging action and iron chelating activity[26]. Both aqueous and methanol extracts ofC. rotundusdisplayed hydroxyl radical scavenging activity. As shown in Figure 3, these extracts exhibited dose-dependent inhibitory effects on hydroxyl radical formation. The maximal inhibitory percentages were 25.3% and 27.1% in the presence of the aqueous and the methanol extracts (16 mg/mL), respectively. The methanol extract exhibited higher metal ion chelating activity than the aqueous extract with an inhibitory percentage of 62.5% at 16 mg/mL (Figure 4).
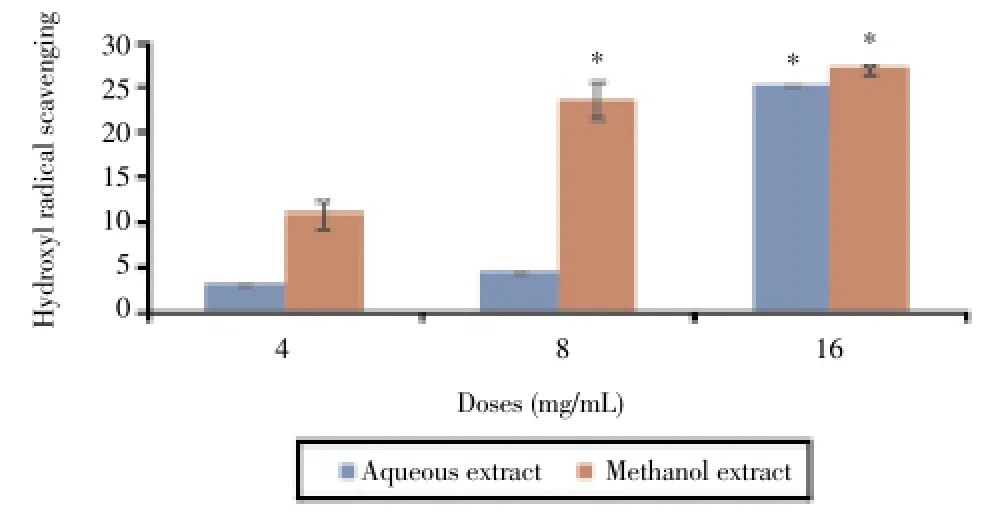
Figure 3. Hydroxyl radical scavenging effect of C. rotundus extracts.*: P< 0.05 as compared with the control group.
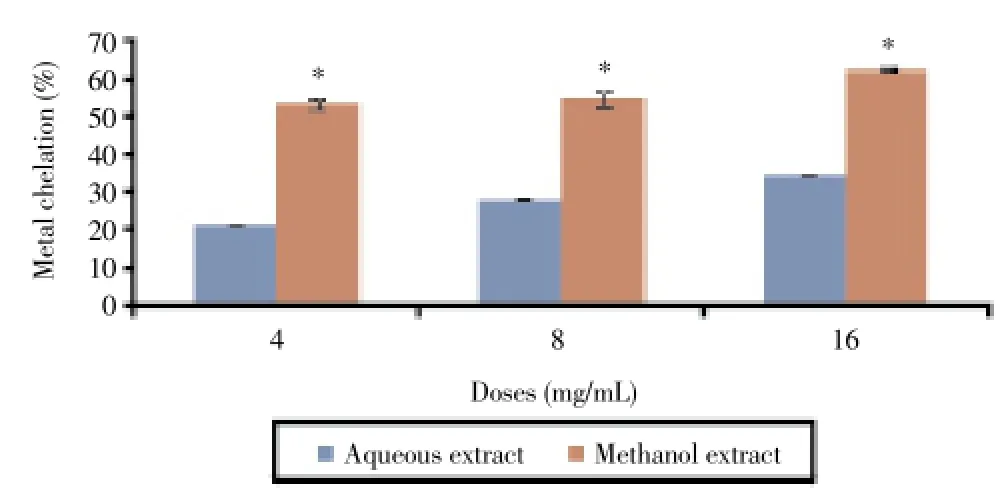
Figure 4. Metal ion chelating activity of C. rotundus extracts.*: P<0.05 as compared with the control group.
3.5. Effect of different extracts on the proliferation of L1210 and K562 leukemia cells
It is well known that the different cell lines might exhibit different sensitivities to a given compound, the use of more than one cell line is, therefore, considered necessary in the detection of the antiproliferative substances. Thus, the relationship between the concentration of the extracts and their cytotoxic effect on L1210 and K562 cells were investigated by MTT assay. The results of this experiment demonstrated that the methanol extract showed a higher cytotoxic effect on K562 cells [IC50= (175.0±1.2) μg/mL] compared to that observed on L1210 cells [IC50= (400.0±1.3) μg/mL]. No significant cytotoxic activity was shown towards L1210 and K562 cells after treatment with up to 800 μg/ mL aqueous extract. These results show that the methanol active extract fromC. rotundusinhibited the proliferation of both murine and human leukemia cells in a concentrationdependent manner.
3.6. Anti-lipid peroxidation activity
The inhibitory effects of the different concentrations ofC. rotundusextracts on MDA production, induced by H2O2in K562 cell line, are shown in Table 1. The inhibition of MDA formation increases with increasing concentrations of each of the tested extracts. The anti-lipid peroxidation activity was obtained at the concentration range of 88 to 350 μg/mL of methanol extract and of 200 to 800 μg/mL ofaqueous extract, and the inhibition rates were in the range of respectively, 57.3% to 61.5% and 24.7% to 42.0%. The methanol extract showed more significant protection effect than the aqueous extract at the same concentrations, against lipid peroxidation. The 50% MDA inhibitory concentration (IC50) value of the methanol extract was determined by the thiobarbituric acid test was 40 μg/mL, IC50of while aqueous extract was >800 μg/mL.
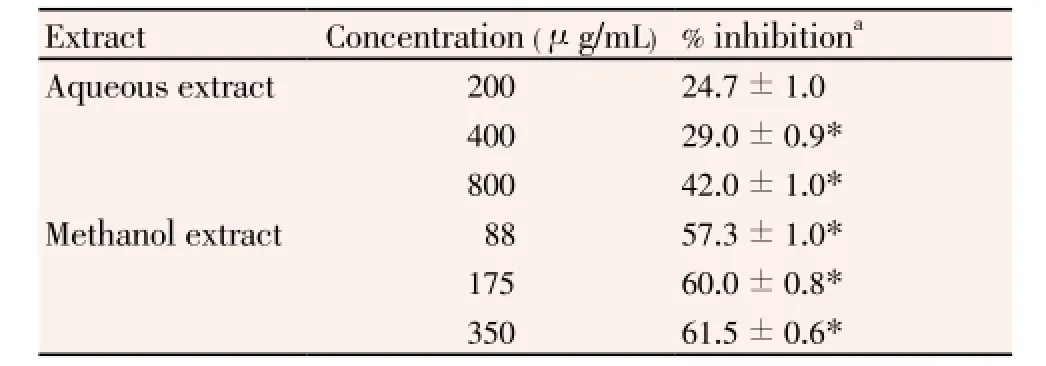
Table 1Protective effect of C. rotundus extracts against lipid peroxidation induced by H O 75 M in K562 cells.
3.7. Induction of apoptotic DNA fragmentation by extracts
The examination of electrophoretic profiles revealed a DNA ladder formation characteristic of apoptosis (Figure 5). L1210 cells exposed to 400 and 800 μg/mL of the methanol extract during 48 h, showed a clearly fragmented DNA, whereas the control cells did not provide this specific ladder DNA profile. However, the cells treated with the aqueous extract exhibited no ladder DNA profile with L1210 as well as with K562 cells. The higher sensitivity of L1210 cells to the methanol extractmediated inhibition of proliferation compared to aqueous extract, suggests a selective influence of methanol extract on L1210 cells. We deduce that the methanol extract fromC. rotundusinduce apoptosis of L1210 cells.
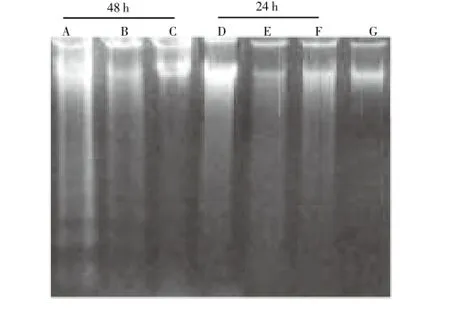
Figure 5. Agarose gel electrophoresis of DNA extracted from L1210 cells treated with methanol extract.(A) 800 μg/mL, (B) 400 μg/mL and (C) 200 μg/mL for 48 h, (D) 800 μg/mL, (E) 400 μg/mL and (F) 200 μg/mL for 24 h, (G) control (non treated cells).
3.8. Correlations of antioxidant activities in different in vitro model systems and cytotoxic effect of extracts with their total phenolic content
Polyphenols have been reported to be responsible for the antioxidant and apoptotic activities of the extracts[27]. We undertook to evaluate the correlation extent between the antioxidant activities and the phenolic content of the tested extracts by using a linear regression analysis. The correlation of the total phenolic content against the different activities was satisfactory (r>0.7). Our results proved that the phenolic content and the antioxidant activity induced by the aqueous extract correlate very well when testing its effect against uric acid and O2. formations using X/XO assay as well as its ability to chelate iron ions in deoxyribose assay (r=0.956 andr=0.954, respectively). We also detected a good correlation between the inhibition percentage of lipid peroxidation, hydroxyl radical scavenging percentage, and its chemical contents (r=0.872 andr=0.773, respectively). However, a higher correlation (r=0.997) was detected between the mean values of the total phenolic contents of methanol extract and its hydroxyl radical scavenging effect. This same extract revealed a significant inhibitory effect against lipid peroxidation and xanthine oxidase activity. These inhibitory effects are highly correlated to the total phenolic content of the extract respectively,r=0.976 andr=0.880.
Nevertheless, a less significant correlation was revealed between the hydroxyl radical scavenging effects of both aqueous extract and methanol extract, and their phenolic contents (respectivelyr=0.773 andr=0.734). These results could be partially explained by the fact that, the kinds of phenolic content should influence the biological activity of a given extract[28]. Besides, the cytotoxic effect towards K562 and L1210 cells, of both aqueous and methanol extracts, was strongly correlated with the total phenolic contents with respectively,r=0.982 andr=0.998 in the presence of aqueous extract andr=0.999 andr=0.897 in the presence of methanol extract.
4. Discussion
Increasing attention is given to the study of natural products, which may counteract the detrimental effects of environmental toxic compounds and prevent multiple human diseases such as neurodegenerative diseases, ageing, rheumatoid arthritis, metabolic diseases such as atherosclerosis, diabetes, hypertension, cancer, etc.. In this line, different medicinal plants have been re-evaluated and recognized as valuable sources of nutraceuticals[29]. Recently, several dietary supplements containing vitamins, polyphenols, or flavones also play a significant role in this matter. Phenolic compounds are very important plant constituents because they exhibit an antioxidant activity by inactivating lipid free radicals or preventing decompositionof hydroperoxides into free radicals[30].
This study is designed to evaluate the antioxidant and apoptotic activities ofC. rotundusextracts employing a variety ofin vitromethods. The antioxidant activity was tested using differentin vitrotestsie.; the xanthine/xanthine oxidase enzymatic assay system and the deoxyribose assay. The xanthine/xanthine oxidase assay demonstrated that the methanol extract is an effective inhibitor of xanthine oxidase. In fact, the preliminary chemical study showed that flavonoid, tannin and polyphenol contents in the methanol extract are higher than those detected in the aqueous extract[10]. Thus, we can ascribe the higher antioxidant activity of the methanol extract compared to that of the aqueous extract to its high flavonoids, a group of natural products exhibiting many biological and pharmacological activities. The inhibition of several enzymes by flavonoids has been demonstrated[31]. Besides, it has been reported that flavonoids inhibit xanthine oxidase and have superoxide scavenging activities[17]. As orientin is the predominant compound in methanol extract, we hypothesized that it might influence the antioxidant activity of this extract. In fact, orientin is reported by Makioet al[32] to have anti-DPPH activity and superoxide radical scavenging effect. However, we cannot exclude other minor phenolic[11,33] or non-phenolic compounds that could participate in the antioxidant activity of this extract. The antioxidant activity is generally the result of the combined activities of a wide range of compounds, including phenolics (eg.orientin), peptides, organic acids and other components[34].
Likewise, the antioxidant capacity ofC. rotundusextracts was evaluated by deoxyribose assay which gives an indication of hydroxyl radical scavenging action and iron chelating activity. The hydroxyl radical is an extremely reactive free radical formed in biological systems and is implicated as a highly damaging species in free radical pathology, capable of damaging the biomolecules of the living cells. In addition, this radical species has the capacity to cause DNA strand breakage, which contributes to carcinogenesis, mutagenesis and cytotoxicity[35]. On the other hand, iron is known to generate free radicals through the Fenton and Haber-Weiss reaction. The metal ion chelating activity of an antioxidant component prevents oxyradical generation and the resulting oxidative damages. Metal ion chelating capacity also plays a significant role in the antioxidant mechanism since it reduces the concentration of the catalysing transition metal in lipid peroxidation[36]. In fact, the methanolic extract exhibited higher metal ion chelating activity than aqueous extract and displayed hydroxyl radical scavenging activity.
We conclude that, the antioxidant activity of the tested extracts evaluated by the different methods may be correlated with their phenolic contents. Plant phenolic compounds constitute one of the major groups of compounds acting as primary antioxidants and free radical terminators by donating hydrogen from the phenolic hydroxyl groups[17,37]. Flavones C-glycosides are of particular interest because of their limited occurrence in plants and their important therapeutic properties, including the prevention of cardiovascular disorders related to oxidative stress. In tissue cultures ofPassiflora quadrangularis, several C-glycosylated flavones such as isoorientin, orientin, vitexin and isovitexin were induced in varied amounts, and their antioxidant activity was determined by DPPH assay[38,39]. However, orientin was predominant inC. rotundusmethanol extract, and might influence the antioxidant activity in the different testedin vitromodel systems.
In order to screen the cytotoxic effects of all extracts, we performed a preliminary study on the cytotoxicity of the extracts in K562 and L1210 cell lines exposed to various extract concentrations. Our results indicate that methanol extract was the most active extract against both of the two cell-lines which differ by their origin and morphology. It is suggested that polyphenolic compounds have inhibitory effects on mutagenesis and carcinogenesis in humans[10,37]. Some studies have shown that flavonoids are able to influence a variety of cell function by modulating cell signalling, altering proliferation and inducing cytotoxicity in cancer cell lines[40]. Moreover, flavonoids showed cytotoxic effects on various human cell lines, such as leukaemia cells and ovarian cancer cells[41]. Orientin is one of the main flavonoids identified in the methanol extract ofC. rotundus. This compound is described as reducing by half the number of cancer-associated changes in the cells of human blood exposed to radiation[42]. However, minor components could also contribute to the cytotoxic activity of this extracts. It is also possible that the components act synergistically to allow the extract to be active.
On the other hand, the low cytotoxicity exhibited by the aqueous extract indicates that the compounds present in this extract are weakly cytotoxic. The difference in cytotoxicity of both extracts may be attributed to the differences in the ratio of compounds involved in the cytotoxicity of each extract. However, we cannot exclude the role of other compounds apart from polyphenols, tannins or flavonoids in this cytotoxic effect. In fact, as reactive oxygen radicals play an important role in carcinogenesis[43], it is possible to suggest that the presence of antioxidants in theC. rotundusextracts may play some roles in reducing cancerous cell number.
Lipid peroxidation has been broadly defined as the oxidative deterioration of polyunsaturated lipids. MDA is usually taken as a marker of lipid peroxidation and oxidative stress[44]. The data obtained showed that the inhibition percentage of MDA formation induced by H2O2in the leukemia cell line K562 increases with increasing concentrations of each of the tested extracts. In our previous study, we showed that the tested extracts contained higher flavonoids and tannins in the methanol extract than in the aqueous extract. These findings suggest that there can be a correlation between the antioxidant activity and phenolic content of each extract. On the other hand, the aqueousextract showed the lowest antioxidant activity compared to the methanol extract. This may be due to variation in the quality and quantity of the different compounds present in the aqueous extracts.
In fact, flavonoids have been shown to exhibit antioxidative, antiviral, antimicrobial, antiplatelet and antitoxic activities[45]. The biological activities of these polyphenols in the different systems are believed to be due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygens, or decomposing peroxides[46,47]. Markhamet al[48] reported that 3',4'-dihydroxyflavonoids (eg.orientin, and quercetin glycoside) are capable of free radical scavenging and this might be a very important response to UV-damage in plants.
To investigate whether the anti-proliferative effect ofC. rotundusextracts is due to induction of apoptosis, an electrophoretic analysis of DNA fragmentation was used. Methanol extract exhibited a ladder DNA profile only in L1210 cells. The apoptotic effect ofC. rotunduson leukemia L1210 cells reflects its potential therapeutic value in cancer treatment and we hypothesised that the regulation of apoptotic genes may be controlled by their phenolic content. As far asC. rotundusextracts tested in the present study were in crude form and probably contained many compounds which may well act synergistically, it is not possible to say which compounds are responsible for the observed effects. However, our data suggest that the biological effects exhibited by this plant, under these experimental conditions, could be related to an overall effect of the tannins and flavonoids in these extracts. However, as for asorientin, one of the major compound detected in the methanol extract, it is reported to have significant protection to the human lymphocytes against the clastogenic effect of radiation, radiation lethality and chromosomal aberrationsin vivo[49]. We believe that orientin largely contribute to the apoptotic effect of the methanol extract on L1210 cells.
The correlation coefficients exhibited a high relationship between total phenolic content in the methanol extract and its cytotoxic effect on one hand and its antioxidant activity on the other hand compared to the aqueous extract.
The present study suggests that the extracts ofC. rotundusplant are potential sources of natural antioxidants and cytotoxicant. However, thein vivosafety of these extracts needs to be thoroughly investigated in animal experiments prior to its possible application as an antioxidant ingredient, either in animal feeds or in human healthy foods. Further studies are needed on the isolation and characterization of individual compounds to elucidate their different antioxidant mechanisms and the existence of possible synergism, if any, among the compounds.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are grateful to the “Ministère Français des Affaires Etrangères” (Action Intégrée de Coopération Inter universitaire Franco-Tunisienne, PHC UTIQUE 07 G0836 PAR), to the Ministry of Higher Education, Scientific Research and Technology in Tunisia for the financial assistance of this study and also thank Mr. Samir Boukattaya (Pr. of English at the Faculty of Dental Medicine, Tunisia) for English editing.
[1] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84.
[2] Shahidi F. Natural antioxidants: An overview. In: Shahidi F. (editor). Natural antioxidants, chemistry, health effects and applications. Champaign: AOCS Press; 1997, p. 1-11.
[3] Rice-Evans C. Flavonoid antioxidants. Curr Med Chem 2001; 8: 797-807.
[4] Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Mol Nutr Food Res 2001; 52: 79-104.
[5] Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 2008; 52: 507-526.
[6] Mukhatar H, Ahmad N. Tea polyphenols: Prevention of cancer and optimizing health. Am J Clin Nutr 2000; 71: 698S-702S.
[7] Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev 2003; 12: 383-390.
[8] Cuénod A. Flore de la Tunisie: Cryptogames vasculaires, gymnospermes et monocotylédones. Office de l'expérimentation et de la vulgarisation agricoles de Tunisie, Tunis ; 1954, p. 149.
[9] Gupta MB, Palit TK, Singh N, Bhargava KP. Pharmacological studies to isolate the active constituents from Cyperus rotundus possessing anti-inflammatory, anti-pyretic and analgesic activities. Indian J Med Res 1971; 59: 76-82.
[10] Kilani S, Ben Ammar R, Bouhlel I, Abdelwahed A, Hayder N, Ghedira-Chekir L. Investigation of extracts from (Tunisian) Cyperus rotundus as antimutagens and radical scavengers. Environ Toxicol Pharmacol 2005; 20: 478-484.
[11] Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Ben Sghair M, et al. Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact 2009; 181: 85-94.
[12] Dhillon RS, Singh S, Kundra S, Basra AS. Studies on the chemical composition and biological activity of essential oil from Cyperus rotundus Linn. Plant Growth Regul 1993; 13: 89-93.
[13] Sayed HM, Mohamed MH, Farag SF, Mohamed GA, Proksch P. A new steroid glycoside and furochromones from Cyperus rotundus L. Nat Prod Res 2007; 21: 343-350.
[14] Jeong SJ, Miyamoto T, Inagaki M, Kim YC, Higuchi R. Rotundines A-C, three novel sesquiterpene alkaloids from Cyperus rotundus. J Nat Prod 2000; 63: 673-675.
[15] Yuan VY, Bone DE, Carrington F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem 2005; 9: 485-494.
[16] Jia ZS, Tang MC, Wu JM. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555-559.
[17] Chaabane F, Boubaker J, Loussaif A, Neffati A, Kilani-Jaziri S, Ghedira K, et al. Antioxidant, genotoxic and antigenotoxic activities of Daphne gnidium leaf extracts. BMC Complement Altern Med 2012; 12: 153.
[18] Toshihiro K, Takayuki K, Shigeru M, Shingo S, Jun-ichi O. Synthesis of 8-C-glucosylflavones. Carbohydrate Res 2001; 334: 183-193.
[19] Limem I, Bouhlel I, Bouchemi M, Kilani S, Boubaker J, Ben-Sghaier M, et al. Phlomis mauritanica extracts reduce the xanthine oxidase activity, scavenge the superoxide anions, and inhibit the aflatoxin B1-, sodium azide-, and 4-nitrophenyldiamine-induced mutagenicity in bacteria. J Med Food 2010; 13(3): 717-724.
[20] Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: A simple assay for determination of rate constants for reaction of hydroxyl radicals. Anal Biochem 1987; 165: 215-219.
[21] Uliasz TF, Hewett SJ. A microtiter trypan blue absorbance assay for the quantitative determination of excytotoxic neuronal injury in cell culture. J Neurosc Meth 2000; 100: 157-163.
[22] Ben Ammar R, Neffati A, Skandrani I, Ben Sghaier M, Bhouri W, Ghedira K, et al. Anti-lipid peroxidation and induction of apoptosis in the erythroleukaemic cell line K562 by extracts from (Tunisian) Rhamnus alaternus L. (Rhamnaceae). Nat Prod Res 2011; 11(1): 1047-1058.
[23] Wang CC, Chen LG, Yang LL. Cytotoxic activity of sesquiterpenoids from Atractylodes ovata on leukaemia cell lines. Planta Med 2002; 68: 204-208.
[24] Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 1999; 47: 3954-3962.
[25] Nam SH, Choi SP, Kang MY, Kozukue N, Friedman MA. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J Agric Food Chem 2005; 53: 816-822.
[26] Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ion. Biochim Bioph Acta 1999; 1472: 142-152.
[27] Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum products. J Agricultural Food Chem 2003; 51: 6657-6662.
[28] Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agr Food Chem 2001; 49(11): 5165-5170.
[29] Feugang JM, Konarski P, Zou D, Stintzing FC, Zou C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front Biosci 2006; 1(11): 2574-2589.
[30] Norra I. Free radical scavenging activity and phenolic content of Ficus deltoidea accessions MFD4 and MFD6 leaves (Aktiviti pemusnahan sisa radikal bebas dan kandungan fenol daun Ficus deltoidea aksesi MFD4 dan MFD6). J Trop Agric Fd Sc 2011; 39 (1): 1-8.
[31] Devi R, Arumughan CO. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem Toxicol 2007; 45: 2014-2021.
[32] Makio S, Koji K, Masahiko T, Masahide Y, Kimiye B. Antioxidant constituents in the dayflower (Commelina communis L.) and their glucosidase-inhibitory activity. J Nat Med 2008; 62: 349-353.
[33] Bouhlel Chatti I, Limem I, Boubaker J, Skandrani I, Kilani S, Bhouri W, et al. Phytochemical, antibacterial, antiproliferative, and antioxidant. Potentials and DNA damage-protecting activity of Acacia salicina extracts. J Med Food 2009; 12: 675-683.
[34] Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK. Radiation protection by the Ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat Res 2000; 154: 455-460.
[35] Farhat B, Syed MS, Rocha JBT, Asad HS, Zafar SS, Syed DA. Evaluation of antioxidant and free radical scavenging activities of fruit extract from Zanthoxylum alatum: a commonly used spice from Pakistan. Pak J Bot 2010; 42(6): 4299-4311.
[36] Beyhan O, Elmastas M, Gedikli F. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). J Med Plants Res 2010; 4(11): 1065-1072.
[37] Ben Sghaier M, Skandrani I, Nasr N, Franca MGD, Chekir-Ghedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: A structure-activity relationship study. Environ Toxicol Pharmacol 2011; 32(3): 13.
[38] Antogononi F, Zheng S, Pagnucco C, Baraldi R, Poli F, Biondis S. Induction of flavonoid production by UVB-radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007; 78: 345-352.
[39] Wu N, Fu K, Fu YJ, Zu YG, Chang FR, Chen YH, et al, Cheng-Bo G. Antioxidant activities of extracts and main components of Pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules 2009; 14: 1032-1043.
[40] Kuntz S, Wenzel U, Daniel H. Comparative analysis of effects of flavonoids on proliferation, cytotoxicity and apoptosis in human colon cancer cell lines. European J Nutr 1999; 38: 133-142.
[41] Boubaker J, Bhouri W, Ben Sghaier M, Bouhlel I, Skandrani I, Ghedira K, et al. Leaf extracts from Nitraria retusa promote cell population growth of human cancer cells by inducing apoptosis. Cancer Cell Int 2011; 11(1): 37.
[42] Vrinda B, Uma-Devi P. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res 2001; 498(1-2): 39-46.
[43] Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P, et al. Study of antimutagenic and antioxidant activities of gallic acid and 1, 2, 3, 4, 6-pentagalloylglucose from Pistacia lentiscus: Confirmation by microarray expression profiling. Chem Biol Interact 2007; 165: 1-13.
[44] Janero D. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 1990; 9: 515-540.
[45] Ben Ammar R, Kilani S, Bouhlel I, Ezzi L, Skandrani I, Boubaker J, et al. Antiproliferative, antioxidant, and antimutagenic activities of flavonoid-enriched extracts from (Tunisian) Rhamnus alaternus L.: Combination with the phytochemical composition. Drug Chem Toxicol 2008; 31(1): 61-80.
[46] Fabri RL, Nogueira MS, Braga FG, Coimbra ES, Scio E. Mitracarpus frigidus aerial parts exhibited potent antimicrobial, antileishmanial, and antioxidant effects. Biores Technol 2009; 100(1): 428-433.
[47] Kumbhare MR, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in-vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis 2012; 2(2): 144-150.
[48] Markham KR, Tanner GJ, Caasi-Lit M, Whitecross MF, Nayudu M, Mitchell KA. Protective role for 3',4'-dihydroxyflavones induced by UV-B in a tolerant rice cultivar. Phytochemistry 1998; 49: 1913-1919.
[49] Vrinda B, Uma-Devi P. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res 2001; 498(1-2): 39-46.
*Corresponding authors: Leila G. Chekir, Faculty of Dental Medicine, Rue Avicenne, 5019 Monastir, Tunisia.
Fax: 00216 73 461 150
E-mail: leila.chekir@laposte.net
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Hepatic effect of NAC on sevear acute pancteatise of rats
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
