A comparative study between Embosphere®and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST
Guang Cao, Xu Zhu, Jian Li, Lin Shen, Renjie Yang, Hui Chen, Xiaodong Wang, Song Gao, Haifeng Xu, Linzhong Zhu, Peng Liu, Jianhai Guo
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Interventional Therapy,2Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Corresponding to: Xu Zhu. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, Beijing 100142, China. Email: drzhuxu@163.com.
A comparative study between Embosphere®and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST
Guang Cao1, Xu Zhu1, Jian Li2, Lin Shen2, Renjie Yang1, Hui Chen1, Xiaodong Wang1, Song Gao1, Haifeng Xu1, Linzhong Zhu1, Peng Liu1, Jianhai Guo1
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Interventional Therapy,2Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Corresponding to: Xu Zhu. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, Beijing 100142, China. Email: drzhuxu@163.com.
Objective:Transcatheter arterial chemoembolization (TACE) is a standard treatment for hepatocellular carcinoma (HCC) and/or some unresectable liver metastasis tumors. Hypervascular liver metastatic lesions such as metastasis from gastrointestinal stromal tumor (GIST) are an indication for transcatheter arterial embolization (TAE). The purpose of this study was to evaluate the effcacy and safety of Embosphere®-TAE (Embo-TAE) in comparison with conventional TACE (cTACE) for the treatment of liver metastasis from GIST.
Methods:A total of 45 patients who underwent TACE between Aug 2008 and Feb 2013 were enrolled. Patients with GIST who underwent TAE with Embosphere®(n=19) were compared with controls who received cTACE (n=26). The primary end points were treatment response and treatment-related adverse events. The secondary end points were progression-free survival (PFS) and overall survival (OS).
Results:The treatment response of Embo-TAE group was signifcantly higher than that of the cTACE group (P<0.001). The PFS was signifcantly better in the Embosphere®-group than in the cTACE group (56.6 and 42.1 weeks, respectively; P=0.003). However, there was no statistically signifcant difference in liver toxicity between the two groups (P>0.05). The median OS in the Embo-TAE group was longer than that in the cTACE group (74.0 weeks, 95% CI: 68.2-79.8 vs. 61.7 weeks, 95% CI: 56.2-67.2 weeks) (unadjusted P=0.045). The use of Embo-TAE signifcantly reduced the risk of death in patients with GIST with liver metastases according to the Cox proportional hazards regression model [hazard ratio (HR): 0.149; 95% CI: 0.064-0.475].
Conclusions:TAE with Embosphere®showed better treatment response and delayed tumor progression compared with cTACE. There was no signifcant difference in treatment-related hepatic toxicities. Embo-TAE thus appears to be a feasible and promising approach in the treatment of liver metastasis from GIST.
Transcatheter arterial chemoembolization (TACE); gastrointestinal stromal tumor (GIST); embolization
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/3352/4185
我渐渐有点明白了。我说,这么说,你是在为我找上工作之后才与季经理发生了关系,后来又通过传播这个段子让季经理怕你,不敢再来缠你,并且引发得连李老板也不待见你了。而你这么做,都是为了我……
高校作为科技和人才的重要结合点,在积累智力资本、提高科技水平、建设创新型国家中具有重要地位,而创新能力的提升已经成为高等教育发展的迫切需要。
Introduction
Gastrointestinal stromal tumors (GISTs) are one of the most common mesenchymal tumors, and account for approximately 2% of gastrointestinal tract tumors (1,2). The liver is the most common site for metastasis from GIST, with a reported incidence of 55-72% in patients with tumor recurrence, and metastatic liver disease is a major determinant of patient survival (3,4). Some studies (5-7) have shown favorable results of transcatheter arterial chemoembolization (TACE) for GIST with liver metastases. At present, there is no standard treatment for metastatic GIST after imatinib and/or sunitinib failure. Buta few studies (8,9) have confrmed the role of TACE in the treatment of patients with GIST after failure of tyrosine kinase inhibitors (TKIs).
TACE is primarily used to treat patients with GIST hepatic metastasis who are not suitable candidates for curative treatment (10,11). The rationale for TACE is that intraarterial chemotherapy with lipiodol and chemotherapeutic agents, followed by selective vascular embolization, will result in a strong cytotoxic effect combined with ischemia [conventional TACE (cTACE)] (12). Recently, another new embolization material Embosphere®(Embospheres, Biosphere Medical, Rockland, MA, USA) has provided a new option for transcatheter embolization. Embosphere®consists of nonabsorbable hydrophilic particles that are calibrated precisely by size. It has the ability to actively reach the microcirculation more effectively compared with conventional, lipiodol-based regimens. Researchers from western countries have also suggested that embolization with these particles offers a superior effect as compared with bland embolization or cTACE for tumors with liver metastasis (13-15).
Furthermore, all the cases enrolled in this study were patients with TKI therapy failure. Almost all cases had already entered into a late stage with a poor liver function reserve. As per experience from this study, a better early treatment response and control of disease progression following Embo-TAE may play a role in the survival beneft by delaying the deleterious effects of tumor progression. Previous studies have shown that the TACE/TAE was significantly better than the BSC control group. The difference in OS rates between the Embo-TAE and cTACE groups was statistically signifcant only in early-stage GIST (Child-Pugh class A or liver involvement within 50%) compared with late-stage GIST with hepatic metastasis (Child-Pugh class B and C, or liver involvement more than 50%) (P=0.003 and 0.002, respectively). Therefore, patients in early stage survived longer after treatment with Embo-TAE than with cTACE. At the same time, it can be assumed that patients with GIST liver metastasis should receive TAE or TACE as early as possible combined with TKI (imatinib/ sunitinib) therapy, just like the combination of sorafenib with TACE to treat the advanced HCC nowadays. Although I may have raised more questions than I've answered, I hope that I have at least provided a window into the "when to perform TACE" and "how to choose a TACE protocol (particles TAE vs. conventional bland embolization" debate. Would a randomized controlled trial of the "best" chemoembolization protocol be implementedin the future?
Materials and methods
Study design
The study was an open, retrospective, controlled cohort analysis. In total, 45 patients with GIST and hepatic metastasis who were treated with TACE-based therapy from Aug 2009 to Feb 2013 at the Beijing Cancer hospital of China were included. The median duration of followup was 20 months (range, 5-40 months). Four patients were lost follow-up of survival time because of losing contact. The eligibility criteria were as follows: (I) patients with histologically confirmed CD117-positive GIST with liver metastasis who were resistant and/or intolerant to imatinib and/or sunitinib and received TACE for at least one treatment cycle; (II) presence of at least two GIST liver metastatic lesions with a minimum diameter of 10 mm by liver dynamic computed tomography (CT) and target lesions with suitability for accurate repeated measurement; (III) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 with preserved liver function (Child-Pugh Class A or B); and (IV) no previous TACE. Patients with potentially resectable or ablative lesions but at a high risk for surgery and radiofrequency ablation (RFA) were also enrolled. The exclusion criteria included the presence of another primary tumor, advanced liver disease [bilirubin levels >3 mg/dL, and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >5X the upper limit of normal]. The patients with GIST who underwent TAE with Embosphere®(n=19) were compared with controls who received cTACE (n=26). The study was approved by the institutional Ethics Review Board and conducted in compliance with the Declaration of Helsinki.
Treatment response
The treatment response was evaluated three months after receiving TACE using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (16). A complete response (CR) was defined as disappearance of any intratumoral arterial enhancement in all the lesions; a partial response (PR) was defned as a 30% decrease in the sum of diameters of viable (contrast enhancement in the arterial phase) lesions; progressive disease (PD) was defined as an increase of 20% in the sum of diameters of viable lesions; and stable disease (SD) was defined as any case that did not qualify as either PR or PD. An OR rate was defned as complete plus partial response.
Statistical analysis
All the statistical analyses were performed using the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Tumor responses and variables between the two groups were compared using χ2, Fisher's exact, or independent t-tests, as appropriate. Progression-free survival (PFS) and OS curves were constructed using the Kaplan-Meier method and compared with a log-rank test. Statistically signifcant factors in univariate analysis were estimated using the multivariate Cox proportional hazard model. P<0.05 was considered statistically signifcant.
教师问:如果我们沿斜面拉着毛刷前进,情况会如何?学生重新做实验,如图2B所示,学生一定会为自己的猜测与实验结论一致而高兴。
由于2.4 m跨声速风洞半模试验段侧壁为实壁[27], 计算过程中将其简化为平板; 同时考虑到涡流发生器尺寸不应过大, 否则会增加对气流的阻塞, 影响主气流的均匀性, 将上、 下壁面设置为对称条件.
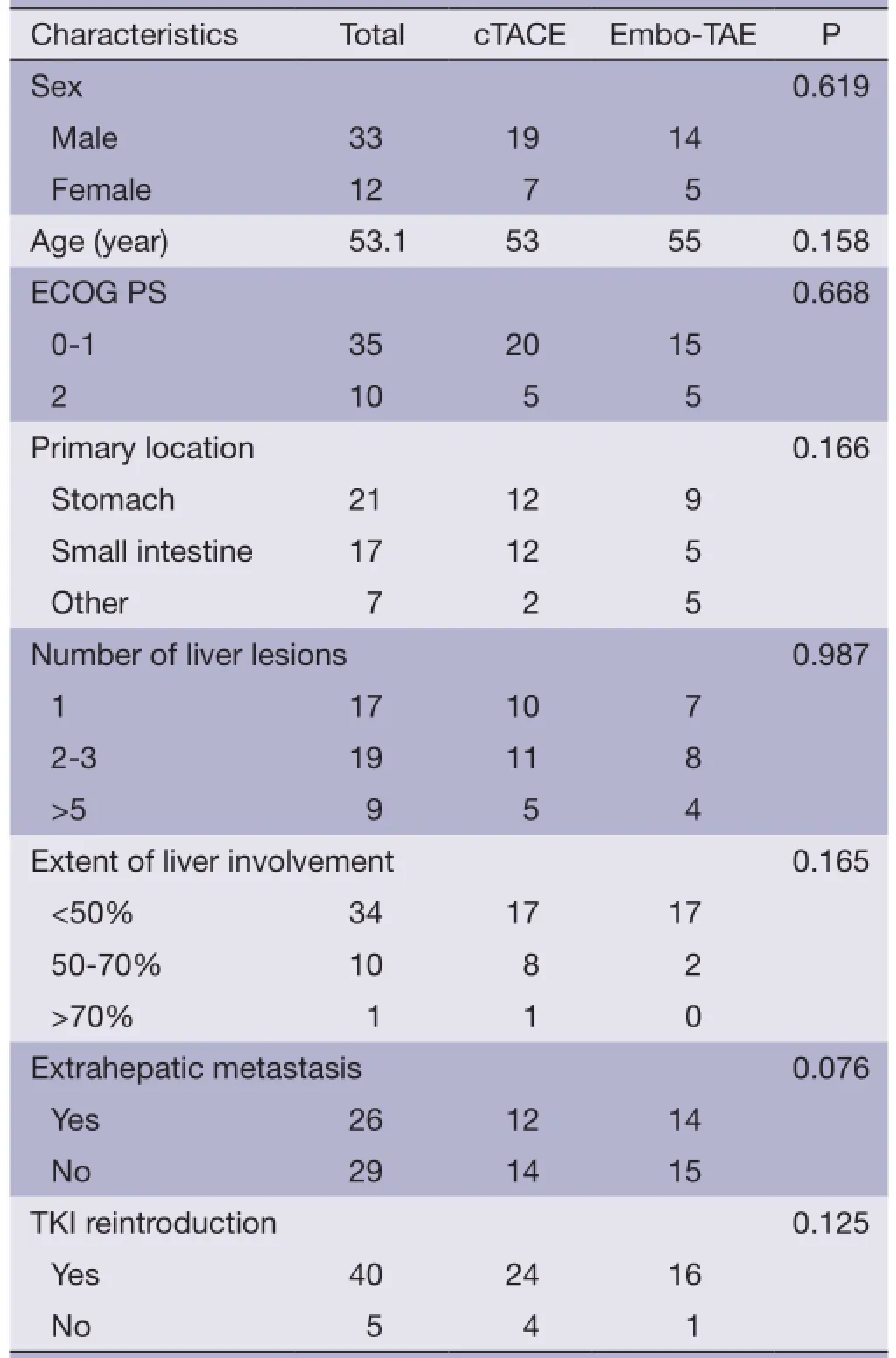
Table 1 Patients' clinical characteristics
Results
Clinical characteristics of patients
Treatment response rate
Treatment in TACE group
The eligibility criteria for TACE included a well-preserved hepatic and renal function, Child-Pugh classifcation A and B, adequate hematologic function, and ECOG performance status of 0-2. Patients with high-risk factors, such as portal vein occlusion, no hepatopetal flow, passive ascites, encephalopathy, or active cardiac failure, were excluded. Local anesthesia was given with 1% lidocaine. After the introduction of a selective catheter through the femoral artery using the Seldinger technique, the localization of the hepatic arteries was checked with celiac and mesenteric arteriography. This was performed to define the vascular anatomy. Next, an indirect portography was performed to outline the portal circulation in the venous phase. A 5-French catheter was placed in the celiac trunk to identify the hepatic artery. Depending on the size, location, and arterial supply to the tumor, a micro-catheter was advanced further into the segmental feeding arteries to perform embolization. An emulsion containing 5-20 mL iodized oil and 40-80 mg doxorubicin hydrochloride was used depending on the tumor size. Additional embolization was performed using 1-2 mm diameter gelatin sponge particles according to the status of the blood supply. The ideal embolization end-point was the stasis of fow in the tumorfeeding branches. A follow-up abdominal imaging (CT) was performed two months after the first embolization. The follow-up images were assessed by two radiologists and compared with the baseline images to assess the response.
Treatment in Embosphere®-TAE (Embo-TAE) group
Patients in the Embo-TAE group were treated with Embosphere®with a maximum dose of 10 mL (diameter 100-300 lm, 300-500 lm, 500-700 lm). A repeat treatment was scheduled within two weeks after follow-up imaging if there was a residual viable tumor. If available, Embo-TAE was repeated until the occurrence of symptomatic progression, extrahepatic spread, vascular invasion, or development of liver failure, in spite of tumor progression of the target lesions or development of new lesions. When the progressed tumor was not treatable by Embo-TAE or cTACE, patients were treated with imatinib or sunitinib. During the followup period, laboratory tests, including albumin, bilirubin, AST, ALT and prothrombin time, and a dynamic CT scan ofthe liver (nonenhanced, arterial, portal, and delayed venous phases) were performed to evaluate treatment response and preserved liver function every four weeks after treatment. After achieving a CR, treatment responses were assessed using imaging studies every three months.
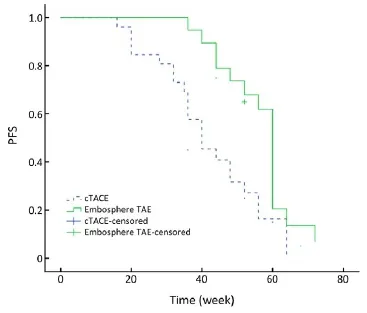
Figure 1 PFS curve. PFS, progression-free survival.
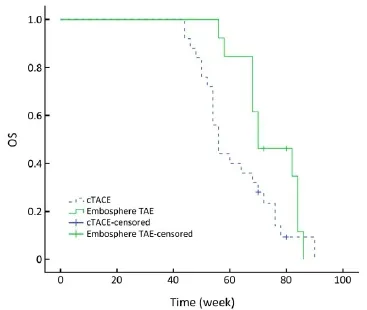
Figure 2 OS curve. OS, overall survival.
TKI reintroduction
Almost all the patients (40/45) received standard TKI reintroduction during the intermittent period of TACE. Patients received imatinib (400 mg/d) and/or sunitinib (37.5 mg/d). The interval between TKI therapy and TACE was two weeks.
The study included 33 males and 12 females. The mean age was 53 years [95% confdence interval (95% CI): 50.8-59.2]. All patients were assessed at registration to have the ECOG performance status grade 0-2. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, 71.1% of the patients were in stage A and 28.9% were stage B; 36% had extrahepatic metastasis. All patients had received sunitinib and/or imatinib prior to TACE or best supportive care (BSC)/TKI introduction treatment. The clinical characteristics of the 45 patients in the two groups are shown in Table 1.
推荐理由:《世界海洋法译丛》旨在通过向中国国内介绍国际海洋法律、条约及世界主要沿海国家的海洋立法和相关的国家法律实践案例,是结合我国海洋法研究的最新研究和翻译成果,为维护我国领土主权、海洋安全和海洋权益,更好地参与国际海洋合作,建设21世纪“海上丝绸”之路,正确处理国际海洋事务、解决国际海洋争端提供政策支持和法律借鉴。
All the patients had measurable metastatic disease according to the mRECIST. Treatment responses were evaluated every 8-10 weeks after TACE. In the Embo-TAE group, 6 (31.6%) and 10 (52.6%) patients showed CR and PR, respectively, 3 (15.8%) had SD, and no patient had PD. In the cTACE group, 1 (3.8%) showed CR, 17 (65.4%) and 6 (23.1%) patients showed PR and SD, respectively, and 2 (7.7%) had PD. Therefore, the treatment response in the Embo-TAE group was signifcantly higher than that in the cTACE group (P<0.001).
PFS
As of May 2013, 22 (84.6%) patients in the cTACE group had progression of liver metastasis. In the Embo-TAE group, 15 (78.9%) patients had tumor progression. The median PFS in the Embo-TAE group (56.6 weeks, 95% CI: 51.8-61.4 weeks) was longer than that in the cTACE group (42.1 weeks, 95% CI: 36.3-48.0 weeks) (P=0.003, Figure 1).
农产品远程监控系统采用光伏充电系统供电,其主电路采用BUCK电路拓扑,主要由光伏电池、功率器件、滤波电感、电容、续流二极管和铅酸蓄电池组成。控制电路核心是电压型TL494是TI公司PWM芯片。控制电路采用电压外环、电流内环的双闭环控制,完成充电系统的快速启动,对负载波动具有良好的抗干扰作用,并可实现无静差调节[10],适合DSP主板和摄像头供电,可以保证野外监控的供电需求。
As of May 2013, totally four patients were alive, two patients from the Embo-TAE group and two patients from the cTACE group. All deaths occurred because of tumor progression or related complications. The median OS in the Embo-TAE group was longer than that in the cTACE group (74.0 weeks, 95% CI: 68.2-79.8 vs. 61.7 weeks, 95% CI: 56.2-67.2 weeks) (unadjusted P=0.045, Figure 2). Embo-TAE significantly reduced the risk of death in patients with GIST with liver metastases according to the Cox proportional hazards regression model [hazard ratio (HR): 0.149; 95% CI: 0.064-0.475].
为提高系统的可靠性,选用开关量采集模块EDDI-W对重要的安保信号进行采集。EDDI-W具有断线检测功能,当检测信号发生断线故障时,输出断线报警,同时屏蔽该点的安保功能。EDDI-W与主控制器通过SPI总线连接,方便拓展。
Adverse events
Most patients in the TACE group developed postembolization complications, which included abnormal liver function, abdominal pain, fever, nausea and so on within one month (Table 2). There were no major complications or grade 3-4 liver toxicities in either group within one month. Increases in ALT after the procedure were significantly less frequent in the Embo-TAE group than in the cTACEgroup (P=0.0001). The overall frequency of treatmentemergent adverse effects was not different in the Embo-TAE compared with the cTACE group according to National Cancer Institute-Common Toxicity Criteria (NCI-CTC) ver. 3.0. The majority of adverse events in both the groups were in the context of the post-embolization syndrome. Hepatic abscess only occurred in two cases of Embo-TAE group. Treatment with Embo-TAE may have caused complete necrosis, which may lead to hepatic abscess formation more easily (Figures 3,4).
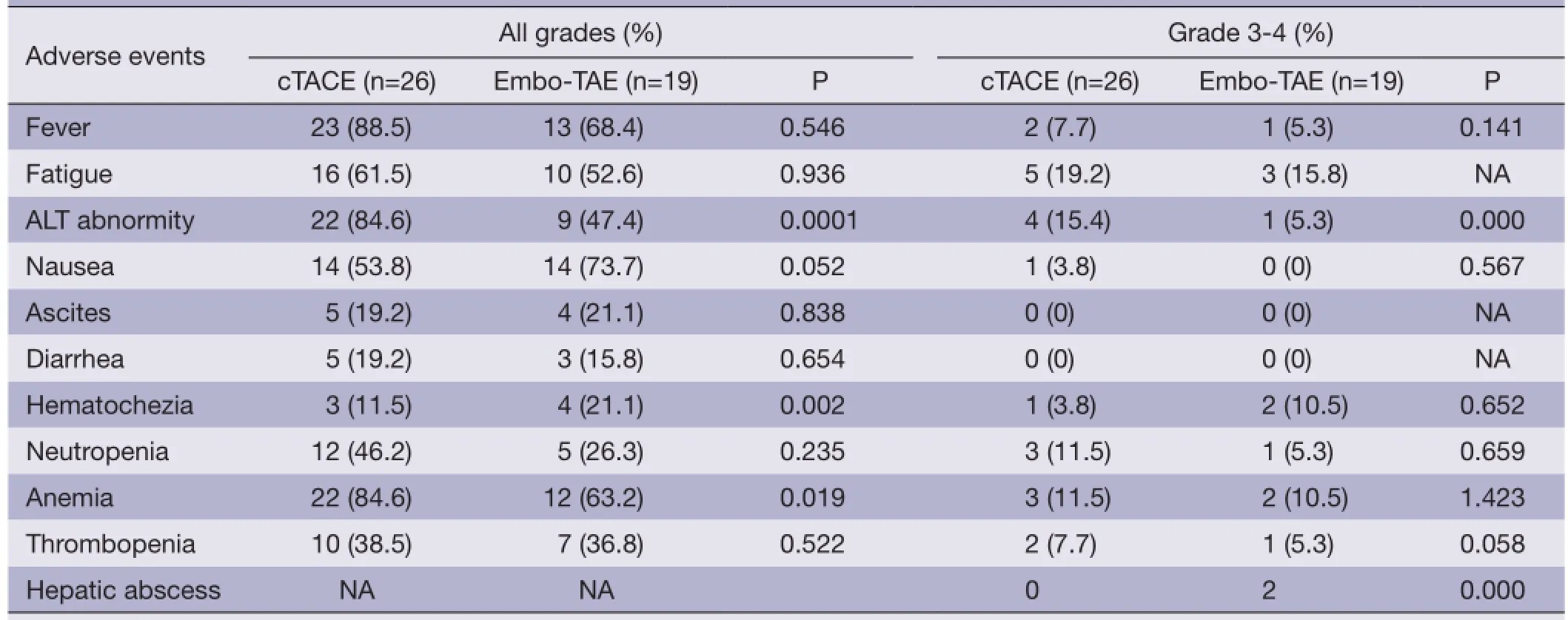
Table 2 Adverse events
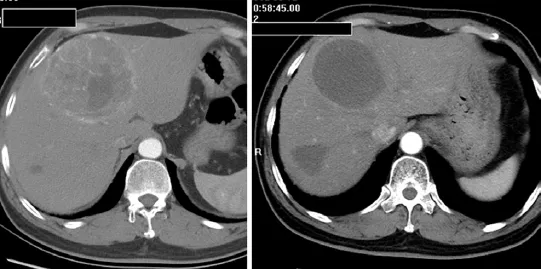
Figure 3 A lesion located in Segment IV before and after Embo-TAE. A complete necrosis was achieved with no enhancement observed in the tumor site. Embo-TAE, Embosphere®-transcatheter arterial embolization.
Discussion
TACE, using lipiodol-mixed chemotherapeutic agent delivered as an emulsion, has been widely used as a standard treatment for hepatocellular carcinoma (HCC) in patients who are not suitable candidates for curative treatment and/or as a bridge to liver transplantation (17).This type of emulsion has been popularly used for years; therefore, it is also called cTACE. Furthermore, it is also regarded as an effective palliative treatment for a series of hepatic metastasis tumors such as neuroendocrine tumors, melanoma, and GIST.

Figure 4 Two nodular GIST metastatic lesions from the stomach treated by cTACE. There is lipiodol accumulation in the tumor with no obvious enhancement observed indicating a CR in the liver. GIST, gastrointestinal stromal tumor; cTACE, conventional transcatheter arterial chemoembolization; CR, complete response.
Based on the evidence available at the time, and our own experience, we knew that if either the right or left hepatic artery is occluded, the tissue supplied by the occluded vessel would recruit intrahepatic collateral flow from the contralateral patent vessel virtually immediately (18). In addition, with passage of time after cTACE, the emulsion (lipiodol mixed chemotherapeutic agent) would be washed away and local recurrence of tumor may be discovered. These are the shortcomings associated with cTACE. An important meta-analysis of randomized embolization trials for HCC written by Cammà et al. was published in 2002 (19). Eighteen randomized controlled trials conducted between 1980 and 2000 were included in the analysis. They concluded that chemoembolization significantly reduced the overall 2-year mortality rate compared with nonactive treatment, overall mortality was significantly lower in patients treated with TAE than in those treated with transarterial chemotherapy, and there is no evidence that TACE is more effective than TAE (odds ratio 1.007; 95% CI: 0.79-1.27; P=0.95), which suggests that the addition of an anticancer drug did not improve the therapeutic beneft.
Thereafter, a series of particle embolization materials were developed in an effort to enhance terminal vessel blockade, such as polyvinyl alcohol (PVA), drug-eluting beads (DEB), micron Embozene (Embozene Color Advanced Microspheres; CeloNova BioSciences, Newman, GA, USA), and calibrated spherical hydrophilic particles (Embospheres®, Biosphere Medical).
The recent introduction of Embosphere®in China has provided a valuable alternative treatment for patients with nonresectable GIST hepatic metastasis. According to a multicenter randomized controlled trial (20-23), the use of such type of particles for precision TAE resulted in a statistically significant reduction in systemic toxicity compared with cTACE. However, despite the overall trend favoring treatment with particle-TAE over cTACE, a statistically significant superiority in OR rates was observed only in the subgroup analysis of patients with more advanced disease (Child-Pugh B, ECOG 1, bilobar, or recurrent disease). We performed this retrospective casecontrol study of Embo-TAE vs. conventional lipiodol-based TACE to evaluate the tumor response and safety in 45 patients with GIST hepatic metastasis. The objective tumor response at two months after treatment was significantly higher in the Embo-TAE group, and the liver toxicity was not significantly different between the two groups. Our previous study (9) drew the conclusion that TACE is effective and well tolerated in patients with GIST with liver metastases after TKI failure. In the present study, a group of patients were enrolled to compare the two treatments and evaluate the effcacy of Embo-TAE in Chinese patients. A significant difference in response rates was observed between patients treated with Embosphere®compared with those treated with cTACE. The CR and PR were 6 (31.6%) and 10 (52.6%), respectively, in the Embo-TAE group, and 1 (3.8%) and 17 (65.4%), respectively, in the cTACE group (P<0.001).
一是针对计划经济时期住房计划供给机制带来的住房难,化解之道是理念突破。这是邓小平开创的。1978年9月,邓小平指出:解决住房问题能不能路子宽些。1980年4月,邓小平又明确指出,住房改革要走商品化路子。[1]这意味着,住房供给理念从原来的单一公房转向自建房、商品房多元类型转变。在邓小平改革理念指引下,1980年6月,中共中央国务院正式宣布:准许私人建房、私人买房,准许私人拥有自己的住房。[1]这意味着,自建房、商品房在国家住房政策中取得了与公房同等地位的确认。
Although the response rates in the cTACE group were similar to those observed in previous study (9), the rates in the Embo-TAE group were greater than those observed in the results reported in previous particle study (24).
The median OS in the Embo-TAE group was longer than that in the cTACE group (74.0 weeks, 95% CI: 68.2-79.8 vs. 61.7 weeks, 95% CI: 56.2-67.2) (unadjusted P=0.045, Figure 2). Embo-TAE reduced the risk of death in patients with GIST with liver metastases according to the Cox proportional hazards regression model (HR: 0.149; 95% CI: 0.064-0.475). But the assumption made of OS statisticaldifference was not observed. The limitation was that this study included only a small number of patients; thus, conclusions regarding the effcacy of Embo-TAE vs. cTACE cannot be drawn. This is in line with results of previous studies (22-24) in which a statistically signifcant difference was only identifed in radiologic rates of response. The role of particle TAE in the treatment remains to be defned.
9. Cao G, Li J, Shen L, et al. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol 2012;18:6134-40.
To date, limited data are available in Asia regarding the use of TACE with Embosphere®for liver metastasis from GIST. We have previously reported preliminary results regarding the use of cTACE in treatment of patients with GIST after failure of TKIs (9). Transcatheter arterial embolization (TAE) with Embosphere®(Embosphere®-TAE) is a new option for these cases and has shown significantly objective response (OR) rates. In the present study, we evaluated the effcacy, safety, and overall survival (OS) beneft of Embosphere®treatment in comparison with cTACE in patients with GIST with liver metastasis after failure of TKIs.
13. Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51.
Acknowledgements
Disclosure: The authors declare no confict of interest.
OS
1. Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3:655-64.
2. Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 2005;41:2868-72.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8.
4. Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol 2010;16:2726-34.
所谓的工作室制度就是在专业教学的过程中针对教师所讲的内容,考虑专业教师的就业方向,根据行业的实际情况把教学内容进行整合分类,整合分类的优点就是可以更系统的归纳与管理知识,让学生可以在适当的周期内可以自由选择就业方向和学习内容的一种工作式学习方式。
5. Kobayashi K, Gupta S, Trent JC, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer 2006;107:2833-41.
6. Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer 2006;107:1617-23.
7. Kobayashi K, Szklaruk J, Trent JC, et al. Hepatic arterial embolization and chemoembolization for imatinibresistant gastrointestinal stromal tumors. Am J Clin Oncol 2009;32:574-81.
8. Kamat PP, Gupta S, Ensor JE, et al. Hepatic arterial embolization and chemoembolization in the management of patients with large-volume liver metastases. Cardiovasc Intervent Radiol 2008;31:299-307.
The results of this study indicated that patients with early-stage (Child-Pugh class A or <50% liver involvement) or small-nodular tumors showed significantly higher OR rates in the Embo-TAE group. Thus, TAE with Embosphere®may be an effective treatment modality for GIST hepatic metastasis.
10. Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711.
11. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36.
12. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429-42.
这天晚上,她辗转反侧,无法入睡。从这天开始,一连两周时间,她再也没有去布尔的书店看书。这对伏尼契而言可是非常少见的,自然引起了布尔的注意。他甚至询问过经常来书店看书的人关于伏尼契的消息,结果自然是无人知晓。
总之,第一次鸦片战争后,中国关税制度的变迁体现在两个方面:首先,进口关税税率的大幅下降,由16%大幅下降到5%左右;其次,关税自主权丧失,表现在协定关税、外国领事参与外商办税的全过程、江海关夷征税权的丧失等多个方面。《中法黄埔条约》第六款规定 :“如将来改变则例,应与佛兰西会同议允后,方可酌改。”就是关税自主权丧失的最好说明。
14. de Baere T, Deschamps F, Teriitheau C, et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumorswith doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol 2008;19:855-61.
15. Rajan DK, Soulen MC, Clark TW, et al. Sarcomas metastatic to the liver: response and survival after cisplatin, doxorubicin, mitomycin-C, Ethiodol, and polyvinyl alcohol chemoembolization. J Vasc Interv Radiol 2001;12:187-93.
16. Lencioni R, Llovet JM. Modifed RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.
17. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlledtrial. Lancet 2002;359:1734-9.
18. Ngan H, Lai CL, Fan ST, et al. Transcatheter arterial chemoembolization in inoperable hepatocellular carcinoma: four-year follow-up. J Vasc Interv Radiol 1996:7:419-25.
19. Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54.
20. Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008;19:862-9.
21. Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, antiinfammatory microparticles: frst clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010;33:552-9.
22. Padia SA, Shivaram G, Bastawrous S, et al. Safety and effcacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol 2013;24:301-6.
23. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52.
为准确分析企业外部经营环境,本文首先采用PEST分析模型对整体宏观环境进行分析,然后再利用波特五力模型对行业的环境进行准确分析。
24. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: effcacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81.
柳含烟停下了,但白雪行动起来,她将落在地上的衣服堆在一起,柳含烟似乎知道她要做什么,按住急剧起伏的酥胸,向石屏上的宝蓝色罩袍示意,可白雪摇头,她吃力地将井水一桶又一桶摇起,一边不断的踩踏衣物,一边将桶里的水浇在衣服上,直到满意了,又将污垢的靴子鼓捣起来。看她如此柳含烟好几次欲言又止。
Cite this article as:Cao G, Zhu X, Li J, Shen L, Yang R, Chen H, Wang X, Gao S, Xu H, Zhu L, Liu P, Guo J. A comparative study between Embosphere®and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST. Chin J Cancer Res 2014;26(1):124-131. doi: 10.3978/j.issn.1000-9604.2014.02.11
10.3978/j.issn.1000-9604.2014.02.11
Submitted Dec 25, 2013. Accepted for publication Feb 10, 2014.
 Chinese Journal of Cancer Research2014年1期
Chinese Journal of Cancer Research2014年1期
- Chinese Journal of Cancer Research的其它文章
- Build infrastructure in publishing scientific journals to benefit medical scientists
- Prognostic value of post-treatment18F-FDG PET/CT for advanced head and neck cancer after combined intra-arterial chemotherapy and radiotherapy
- MiR-182 is up-regulated and targeting Cebpa in hepatocellular carcinoma
- Intraperitoneal chemotherapy and its evolving role in management of gastric cancer with peritoneal metastases
- Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone
- Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications
