Synthesis, Structure and Properties of a New Lead(II) Complex of 1,3-Benzenedicarboxylic Acid, 1,10-Phenanthroline and Its Thermal Decomposition to PbO Micro-crystal Particles①
LIU Cheng LIU Qi-Hui GE Shu-Bao WU Gang
(College of Material and Chemical Engineering, Chuzhou University, Anhui 239012, China)
1 INTRODUCTION
Due to diversity of organic ligand molecules and coordination models of metal ions, coordination polymers with versatile structures and properties were constructed, giving rise to extendedly potential applications, such as adsorptive, molecular separation/exchange, catalytic, electronic, magnetic, and optical properties, which have attracted wide attention from chemists and scientists from other fields,for example, material scientists[1-4]. A careful selection of metal, bridging ligand and the control of reaction conditions may lead to complexes with novel structures and properties[5-6]. Lead is localized in IVA group and sixth period, and is extendedly used, such as fuel additives, solder, roofing and batteries[7-8]. However, lead is a heavy toxic metal,and often presents as a pollutant in the environment.Therefore, researching Pb coordination properties is very important for understanding the Pb(II) physical and chemical properties. In addition, lead coordination polymers have aroused much attention because of their interesting structural features, resulting from the consequence of large radius, adoption of different coordination numbers, and occurrence of the stereo-chemically active lone pairs of electron[9].
On the other hand, PbO nano- or micro-sized particles have many applications. The nanopowders and micro-crystal powders of lead oxide can be widely used in the battery manufacturing industry,such as lead-acid batteries[10]. In some chemical reactions, it can be used as active catalyst[11]. Nanoand micro-crystalline PbO have been synthesized via different methods with controlled size and shape[12].It can be also used in dye and glass industry (lead glasses) and piezoelectric ceramics[13]. Therefore, we focus on the simple synthetic preparation of a new one-dimensional Pb(II) coordination polymer 1 of 1,10-phenanthroline and 1,3-benzenedicarboxylic acid, and it can convert into micro-crystal structured lead oxide by direct calcination at moderately elevated temperature.
2 EXPERIMENTAL
2. 1 General procedures
All commercially available chemicals were of reagent grade and used as received without further purification. Elemental analyses for C, H and N were carried out with a Perkin-Elmer 240C Elemental Analyzer. Infrared spectra were obtained with a Nicolet 6700 FT-IR Spectrophotometer by using KBr pellets in the range of 4000~400 cm-1.The fluorescent spectrum was recorded on a Perkin Elmer LS 55 spectrofluorimeter at room temperature with a xenon arc lamp as the light source in the solid state. In the measurements of the emission and excitation spectra, the passage width is 5.0 nm.Thermogravimetric analyses (TGA) were carried out with a SDT Q600 instrument under 100.0 mL/min flowing nitrogen, increasing the temperature at a rate of 20.00 ℃/min from 26 to 800 ℃. Powder X-ray diffraction (PXRD) measurements were performed on a Bruker D8 ADVANCE X-ray diffractometer with Cu-Kα monochromatized radiation at 40 kV and 40 mA. The sample shape was observed with a scanning electron microscope (SEM) (JEOL JSM 5600LV) with gold coating.
2. 2 Synthesis of complex [Pb(phen)(bdc)] (1)
A mixture of 1,3-benzenedicarboxylic acid (0.1 mmol, 16.6 mg), Pb(NO3)2(0.1 mmol, 33.1 mg) and 1,10-phenanthroline (0.3 mmol, 59.5 mg) in 6 mL N,N-dimethylacetamide (DMA) was stirred for 10 minutes at room temperature. Then this clear solution was sealed in a 25 mL Teflon-lined bomb at 85℃ for 72 h, and then cooled to room tem- perature.Yellow rod crystals of 1 were obtained (yield: 13.1 mg). Anal. Calcd. (%) for 1 (C24H16N6O6Pb(691.62)): C, 41.68; H, 2.33; N, 12.15. Found (%): C,41.63; H, 2.37; N, 12.18. IR bands (cm-1) for 1:3060(w), 1619(m), 1526(s), 1386(s), 1314(s),1264(s), 1208(m), 1095(m), 846(s), 717(s).
To prepare micro-structured powder sample, 1,3-benzenedicarboxylic acid 33.2 mg (0.2 mmol),Pb(NO3)2(0.2 mmol, 66.2 mg) and 1,10-phenanthroline (0.6 mmol, 119.0 mg) in 6 mL N,N-dimethylacetamide (DMA) was stirred for 10 minutes at room temperature. Then this mixture was sealed in a 25 mL Teflon-lined bomb at 85 ℃ for 72 h, and cooled to room temperature. Yellow powder was obtained. IR bands (cm-1) for powder sample:3048(w), 1621(m), 1523(s), 1385(s), 1311(s),1266(s), 1210(m), 1098(m), 841(s), 715(s).
Lead(II) oxide micro-crystal particles were synthesized from powder sample through calcination.Amount of powder sample (about 25 mg) was heated to 506 ℃ for 1 h in air. After cooling, black powder was obtained, and directly used to study the morphology, size and shape by means of PXRD and SEM.
2. 3 Single-crystal X-ray data collection and structure determination
Diffraction data for 1 were measured and collected on a Bruker Smart Apex II CCD diffractometer equipped with graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å) at 296(2) K. The collected data were reduced with the program SAINT[14]and the empirical absorption correction was done with SADABS program[15]. All structures were solved by direct methods and refined by full-matrix leastsquares method on F2with anisotropic thermal parameters for all non-hydrogen atoms[16]. Hydrogen atoms were added geometrically and refined using the riding model. Selected bond lengths and bong angles for complex 1 are listed in Table 1. Crystal data for 1: triclinic, space group P, a = 7.5595(6), b= 9.8199(8), c = 13.1663(11) Å, α = 69.7590(10), β= 80.6000(10), γ = 71.3180(10)o, V = 867.19(12) Å3,Z = 2, Mr= 551.51, Dc= 2.112 g/cm3, F(000) = 520,μ = 9.757 mm-1, the final R = 0.0304 and wR =0.0763 (w = 1/[σ2(Fo2) + (0.0547P)2+ 0.0000P],where P = (Fo2+ 2Fc2)/3) for 3427 observed reflections with I > 2σ(I) and R = 0.0365 and wR =0.0894 for all data. (Δ/σ)max= 0.000, (Δρ)max= 1.240 and (Δρ)min= –0.884 e/Å3. The goodness-of-fit indicator (S) is 1.022.
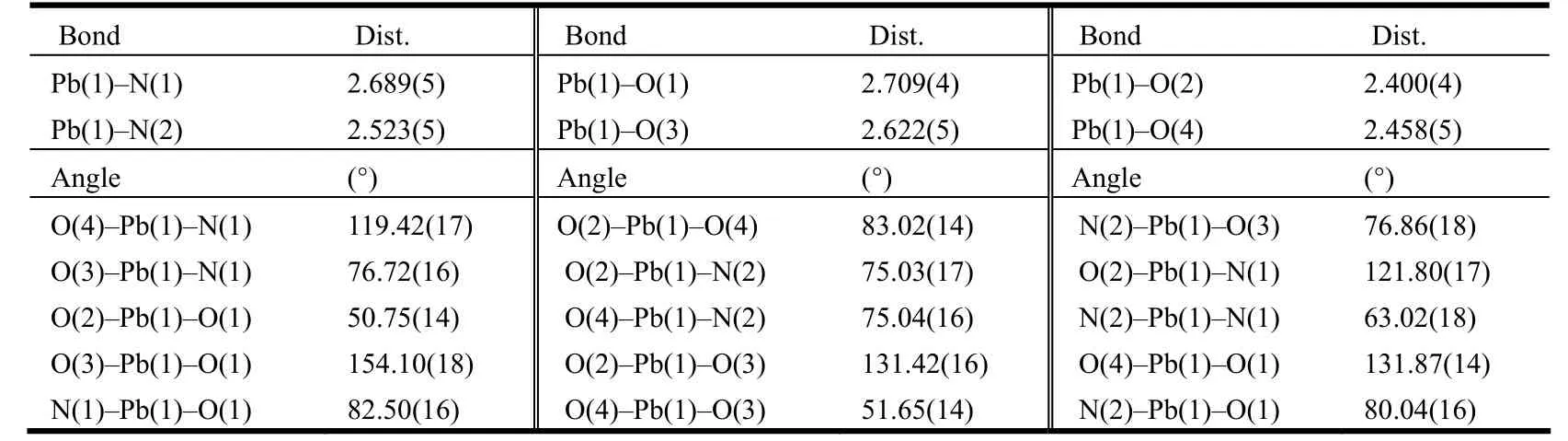
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
3. 1 IR spectrum
The infrared absorption bands are useful for the determination of the coordination mode of the ligand.The absence of absorbed peak around 1710 cm-1indicates that both two carboxyl groups of H2bdc are completely deprotonated, in good agreement with the crystal structure. The positions of the stretching COO vibrations, νas(COO) and νs(COO), are found at 1526 and 1386 cm-1, respectively. The corresponding Δv value of 140 cm-1suggests the presence of chelating COO groups[17-18].
3. 2 Crystal structure of 1
The title compound crystallizes in space group P.The crystallographic analysis revealed that the structure of 1 consists of one phen moiety, one deprotonated bdc2-anion, and one Pb2+cation in an asymmetric unit. The central Pb(II) atom is coordinated by two N(N(1), N(2)) atoms from one phen molecule, which acts a bidentate ligand chelating with a Pb(II) atom and four O (O(1),O(2), O(3),O(4)) atoms from two ligand bdc2-anions (Figs. 1 and 2). The lead atom is almost coplanar with the phen plane with deviation of 0.0188 Å. The bond lengths of two Pb–N are 2.689(5) and 2.523(5) Å,respectively. The bond lengths of Pb–O vary from 2.400(4) to 2.709(4) Å. The bond angles of O–Pb–O and N–Pb–O fall in the 50.75(14)~154.10(18)°range. The bond angle of N(1)–Pb–N(2) is 63.02(18)°. All these bond lengths are typical and comparable with those of other Pb(II) complexes in the literature (Table 1)[19-20].
The arrangement of these ligands suggests a gap or hole in coordination geometry around the metal Pb(II) ions (the presence of gap is clear (angles >180o)), occupied possibly by a stereo-active lone pair of electrons on lead(II)[21](Fig. 1), which suggests that the coordination environment of lead(II)atom is holodirected. Therefore, the 6s orbital of the Pb(II) centre may be hybridized with the 6p orbitals to give a ‘‘stereochemically active’’ 6s electron pair(or stereochemically active lone electron pair,SALEP), occupying one position in the coordination sphere of the metal[22].
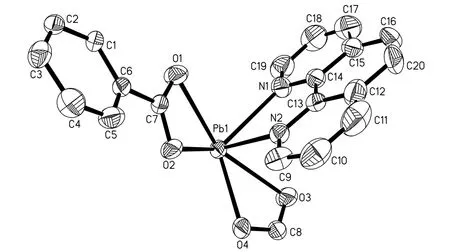
Fig. 1. ORTEP view of coordination environment of Pb(II) atom in 1 with 50%probability displacement. The hydrogen atoms are omitted for clarity

Fig. 2. Infinite 1D chain structure in complex 1
In complex 1, ligand bdc2-only takes one kind of coordination mode. Two carboxylate groups of bdc2-adopt the same μ1-η1:η1chelating coordination mode with Pb(II). Therefore, the whole ligand bdc2-acts as a μ2-bridge (Fig. 2) connecting two different Pb(II)atoms, resulting in a one-dimensional chain structure(Fig. 2).
It is noticeable that, in ligand bdc2-, the two carboxylate groups and the central benzene ring are not in the same plane. The dihedral angle between the carboxylate group (O(1), C(7), O(2)) and the benzene ring (C(1), C(2), C(3), C(4), C(5), C(6)) is 8.78°, and that between another carboxylate group(O(3), C(8), O(4)) and the same benzene ring is 7.59°.
The individual one-dimensional chains in 1 are almost parallel and further linked by Obdc2-··H–Cphen(O(1)··H(10)A–C(10)) weak hydrogen bonding interactions with a bond distance of 3.331(11) Å and an angle of 157o(Table 2) to generate a two-dimensional layer structure (Fig. 3). Further, the adjacent two-dimensional layers are linked together by C(17)–H(17A)··O(3) hydrogen bond to generate a three-dimensional structure (Table 2, Fig. 4).
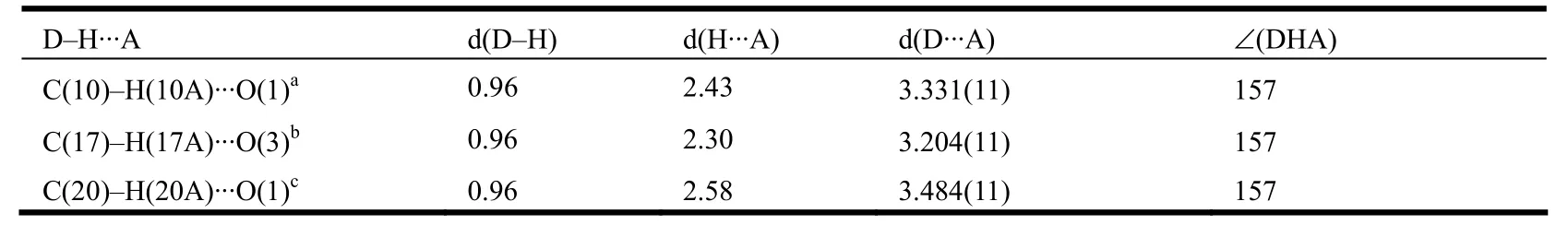
Table 2. Hydrogen Bond Distances (Å) and Angles (°) for Complex 1
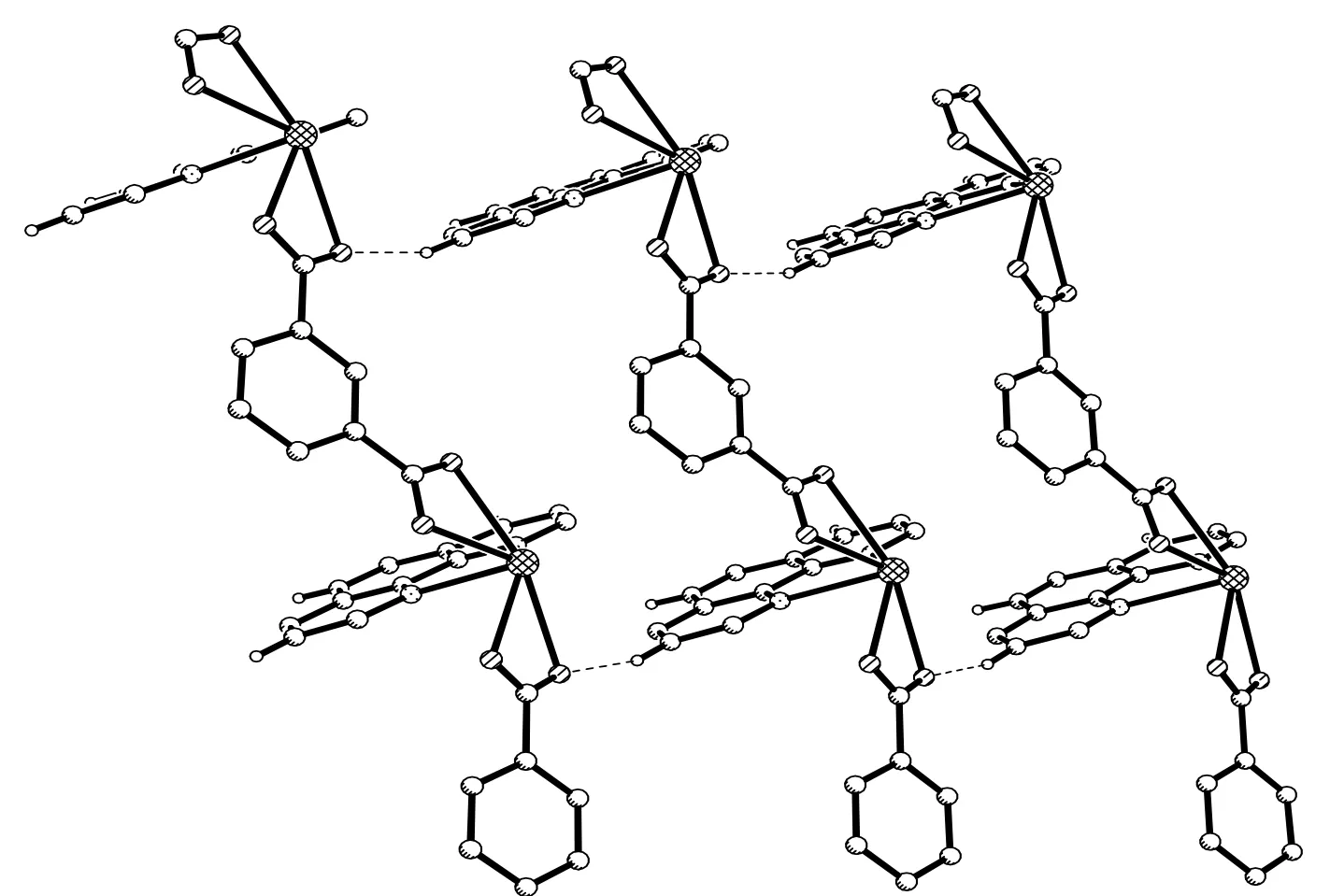
Fig. 3. 2D layer structure of 1 formed by C–H··O hydrogen bonds
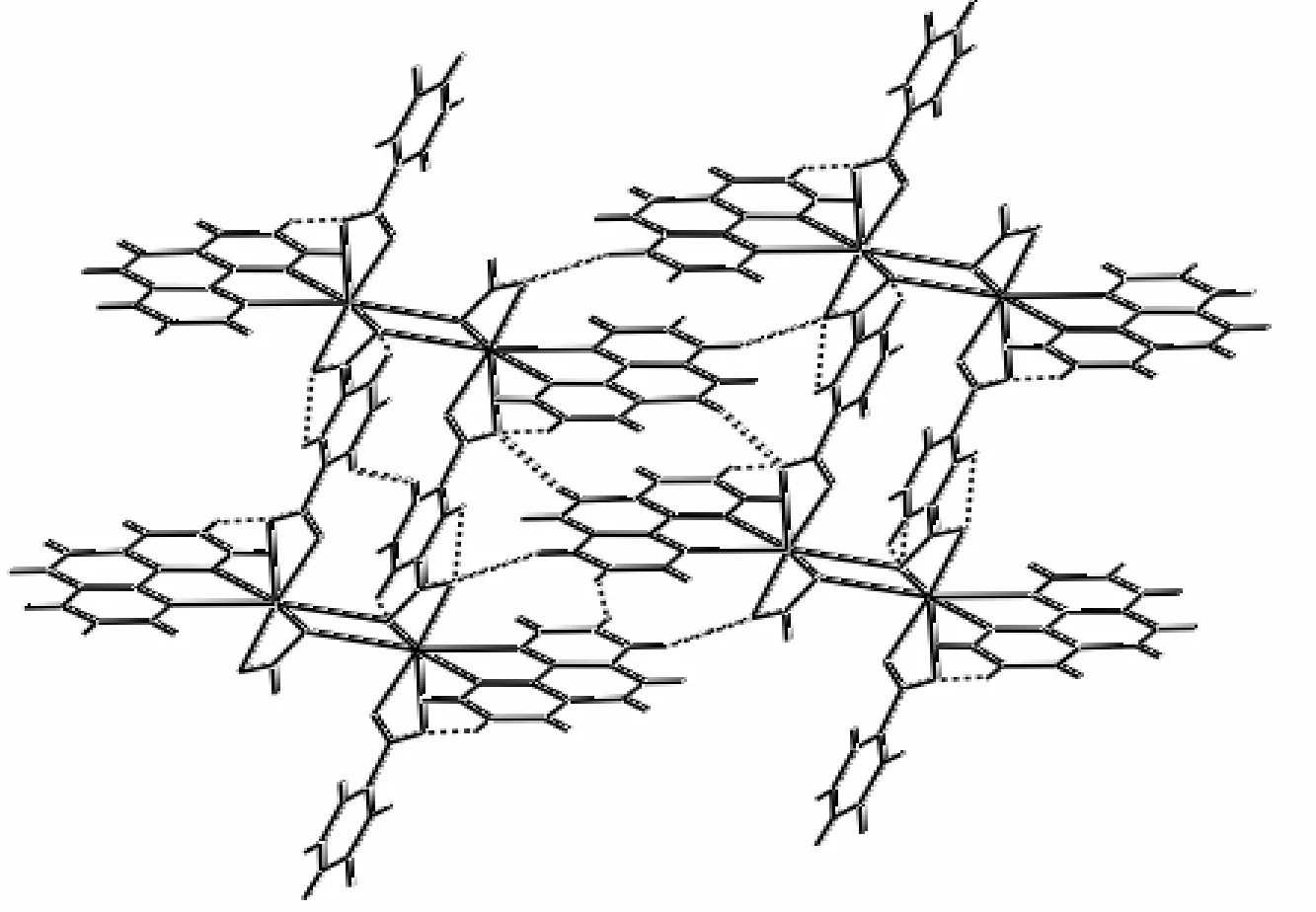
Fig. 4. Crystal packing diagram of 1
3. 3 Fluorescent property of 1
The solid-state photoluminescent spectrum of complex 1 was recorded at room temperature under 314 nm excitation. Complex 1 showed one maximum emission spectrum centered at 410 nm (Fig. 5).To ascertain the adscription of emission spectra, the photoluminescence of pure H2bdc and phen was measured under the same conditions. The emission band of compound 1 may be assigned to π-π*intraligand fluorescence.
3. 4 Thermogravimetric analyses
The thermal stability of complex 1 (Fig. 6) has been determined from room temperature to 800 ℃in a N2atmosphere by thermogravimetry (TG).Complex 1 is stable up to 311 ℃, at which the phen molecule begins to remove. The experimental mass loss of 31.74% is consistent with the calculated value of 32.68% for the elimination of one phen molecule. The solid residue formed between 311 and around 360 ℃ is suggested to be Pb(bdc). At higher temperature, the decomposition of the residue occurs with an exothermic effect at 346 ℃. Mass loss calculations of the end residue show that the final decomposition product is PbO.
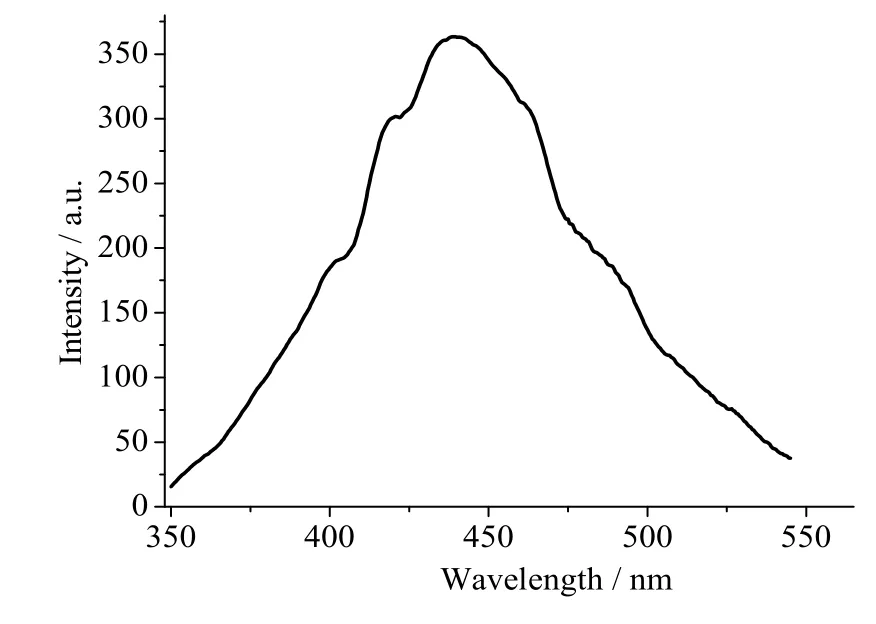
Fig. 5. Emission spectrum of complex 1 in the solid state at room temperature
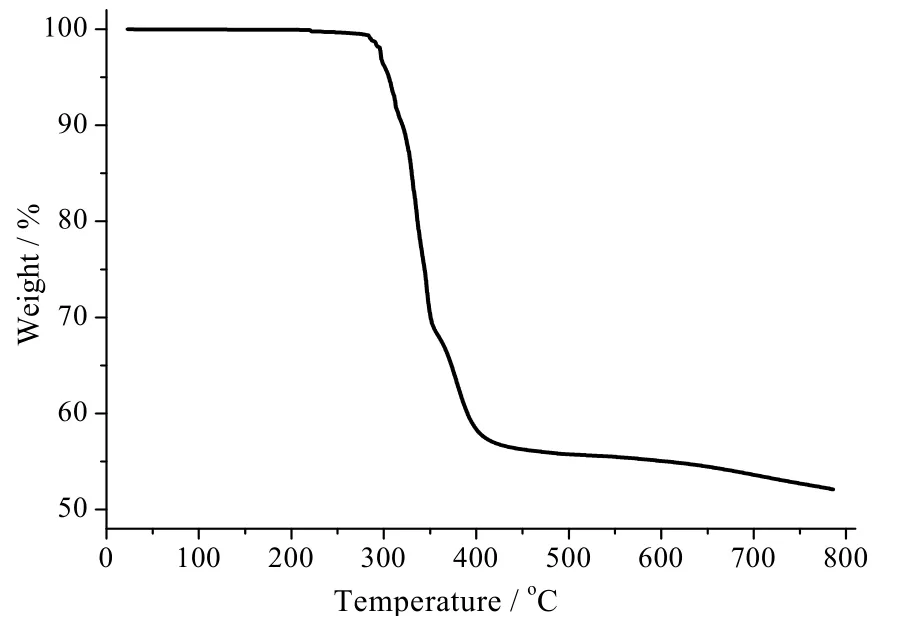
Fig. 6. TG curve of 1
3. 5 Micro-crystal structure of PbO
Fig. 7 shows the stimulation XRD patterns from the single crystal data of 1 and powder sample. A complete match is observed, suggesting that the powder sample obtained has a pure single phase with the same structure of 1. The shape and size of the powder sample particles were further investigated with SEM (Fig. 8). They are irregular sheets in ~0.39 μm thickness.
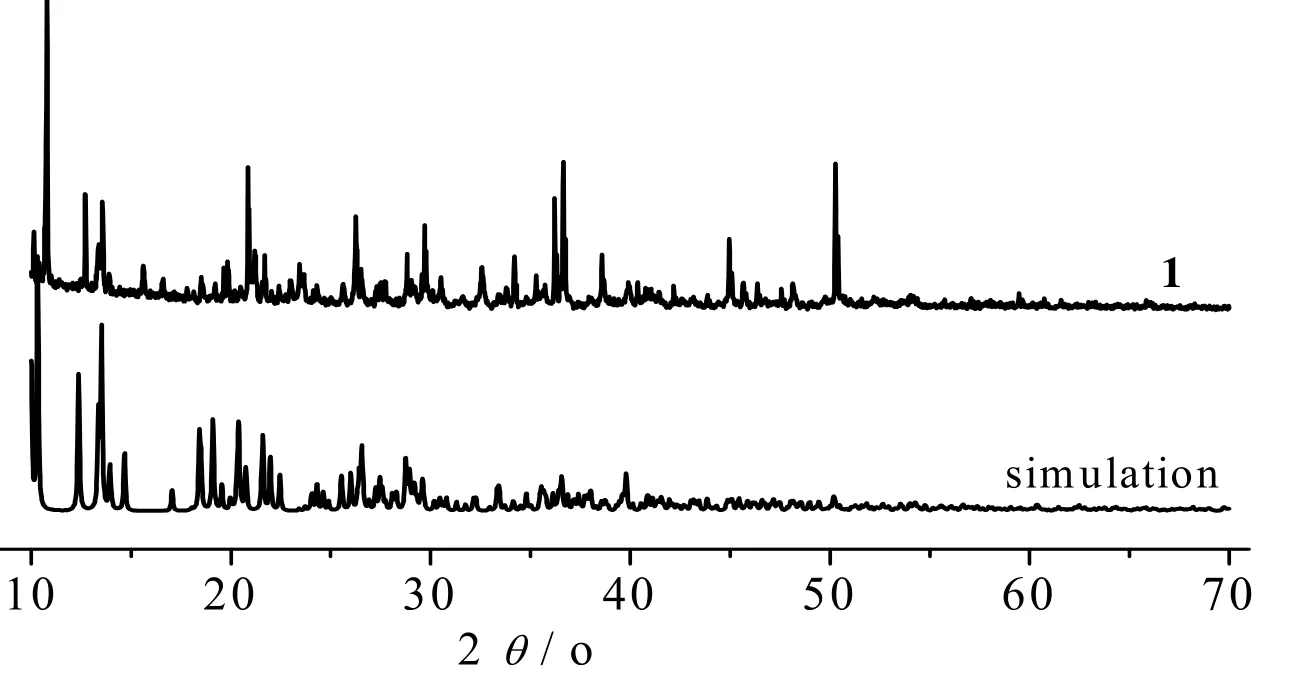
Fig. 7. XRD patterns of a simulation from single-crystal X-ray data of complex 1 and powder sample

Fig. 8. SEM photographs of the ground powder of sample 1
PbO micro-crystal particles were synthesized from the decomposition of the powder precursor 1 at 506 ℃ under air atmosphere. The phase purity of the as-prepared black orthorhombic PbO microparticles is completely obvious and all diffraction peaks are perfectly indexed to the orthorhombic PbO structure. G = P4/nmm, which are in JCPDS card file no. 05–0561. No characteristic peaks of impurity are detected in the XRD pattern (Fig. 9).
The shape and size of the as-prepared PbO samples were further investigated using SEM (Fig.10). Fig. 10 shows the SEM image of PbO microcrystal particles. Compared with Fig. 8, the shape of PbO is similar to that of its precursor. As it can be seen, there are nonuniform micrometer scale particles. The average thickness of PbO is about 0.4 μm.
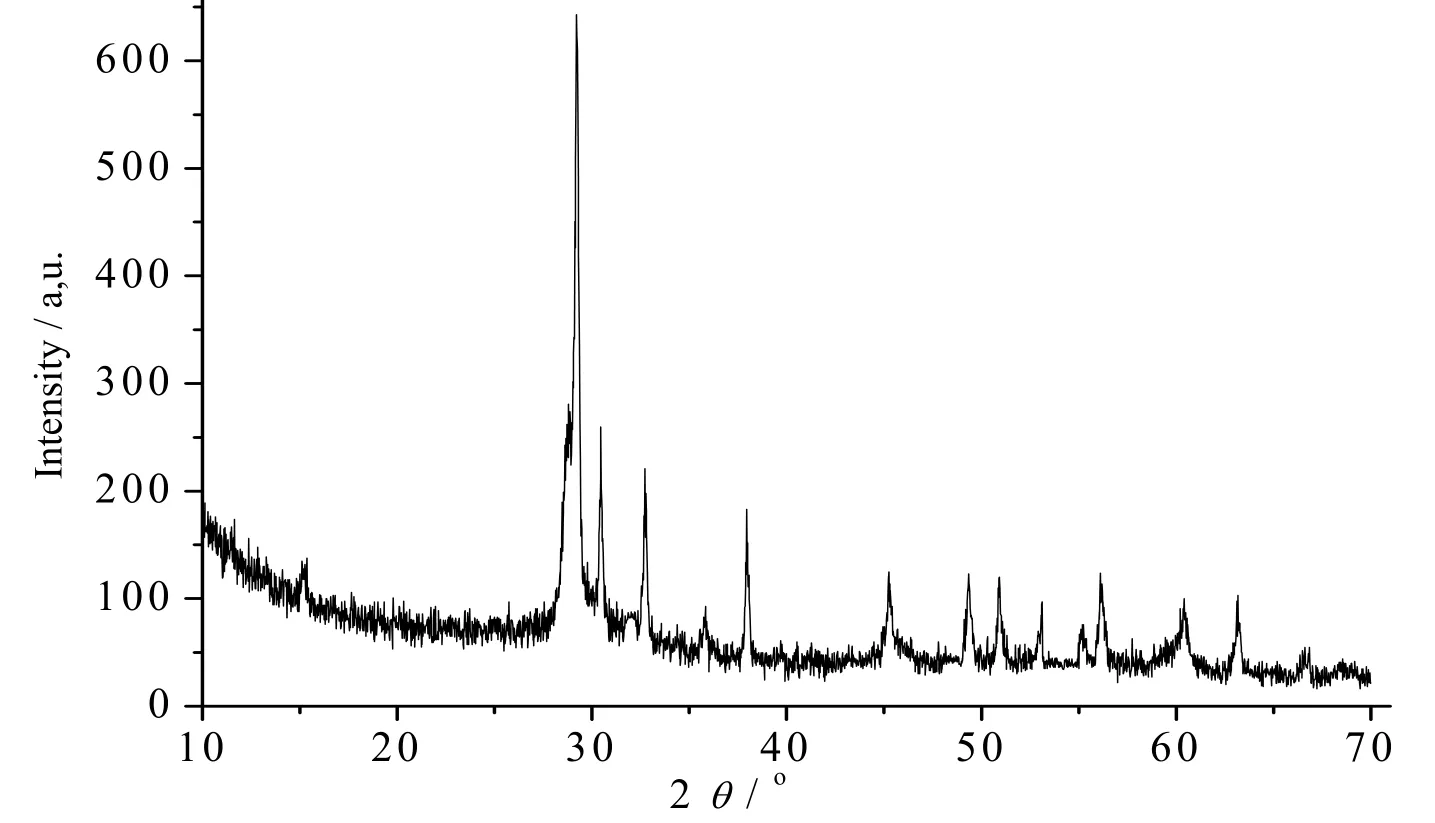
Fig. 9. XRD pattern of PbO

Fig. 10. SEM photograph of as-prepared PbO produced by calcinating precursor
4 CONCLUSION
In this work, a new 1D Pb(II) coordination polymer and PbO micro-particles were prepared and described. In complex 1, the coordination geometry of Pb(II) and the arrangement of the ligands suggested a gap in the coordination geometry around the metal ions, possibly occupied by a ‘‘stereoactive’’ lone pair of electrons of lead(II). Whole bdc2-acts as a μ2-bridge linking the Pb(II) center to generate a 1D chain structure. The two-dimensional layers and three-dimensional stacking structure are formed by C–H··O hydrogen bonding interaction.The thermal stable and fluorescent properties of 1 were investigated. PbO micro-crystal particles are then produced by calcination of powder of complex 1. The obtained PbO is characterized by XRD and SEM analyses.
(1) Zhang, J. P.; Zhang, Y. B.; Lin, J. B.; Chen, X. M. Supramolecular coordination assemblies constructed from multifunctional azole-containing carboxylic acids. Chem. Rev. 2012, 112, 1001-1033.
(2) Ni, J.; Wei, K. J.; Liu, Y.; Huang, X. C.; Li, D. Silver coordination polymers based on neutral trinitrile ligand: topology and the role of anion. Cryst.Growth Des. 2010, 10, 3964-3976.
(3) Fang, S. M.; Ma, S. T.; Guo, L. Q.; Zhang, Q.; Hu, M.; Zhou, L. M.; Gao, L. J.; Liu, C. S. A photoluminescent 3D silver(I) polymer with mixed 2-naphthol-5-carboxylate and hexamethylenetetramine ligands, showing an unusual (3,4)-connected (6,7,8)2(4,6,7)2(4,7,8)2(72,8)2(62,7,83)(62,72,8,10)topology. Inorg. Chem. Commun. 2010, 13, 139-144.
(4) Yuan, N.; Sheng, T. L.; Hu, S. M.; Fu, R. B.; Wu, X. T. Two new coordination polymers assembled with a functionalized ligand: syntheses and crystal structures. Chin. J. Struct. Chem. 2012, 115-121.
(5) Blake, A. J.; Champess, N. R.; Crew, M.; Hanton, L. R.; Parsons, S.; Schröder, M. A new CuI(SCN) structural motif: synthesis of an uncharged three-dimensional co-ordination network. J. Chem. Soc., Dalton Trans. 1998, 10, 1533-1534.
(6) Wang, J. Y.; Wang, W. Z.; Liu, X.; Liao, D. Z.; Jiang, Z. H.; Yan, S. P.; Wang, G. L. From dinuclear to mononuclear: Co(II) complexes with pyridine-2,6-dicarboxylic acid (H2PDA). Chin. J. Struct. Chem. 2012, 31, 67-72.
(7) Strasser, A.; Vogler, A. Optical properties of thallium(I), lead(II) and bismuth(III) hexafluoroacetylacetonates, intraligand phosphorescence under ambient conditions. Inorg. Chem. Commun. 2004, 7, 528-530.
(8) Hou, X. Y.; Wang, X.; Fu, F.; Wang, J. J. Syntheses, structures and characterization of two new PbIIpolymers built on a kinked flexible thiodiacetic acid ligand. Chin. J. Struct. Chem. 2013, 32, 1339-1347.
(9) Panjehpour, A.; Morsali, A. Sonochemical synthesis of a new lead(II) coordination polymer with 2-quinoline carboxylate ligand: a precursor for the preparation of nano-structured lead(II) oxide. J. Inorg. Organomet. Polym. 2012, 22, 938-945.
(10) Martos, M.; Morales, J.; Sanchez, L.; Ayouchi, R.; Leinen, D.; Martin, F.; Ramos, J. R. Electrochemical properties of lead oxide films obtained by spray pyrolysis as negative electrodes for lithium secondary batteries. Electrochim. Acta 2001, 46, 2939-2948.
(11) Cao, M.; Meng, Y.; Lu, Y. Synthesis of diphenyl carbonate from dimethyl carbonate and phenol using O2-promoted PbO/MgO catalysts. Catal.Commun. 2005, 6, 802-807.
(12) Yang, J.; Zeng, J. H.; Yu, S. H.; Yang, L.; Zhou, G. E.; Qian, Y. T. Formation process of CdS nanorods via solvothermal route. Chem. Mater. 2000,12, 3259-3263.
(13) Coondoo, I.; Panwar, N.; Kholkin, A. Lead-free piezoelectrics: current status and perspectives. J. Adv. Dielect. 2013, 3, 1330002(1-22).
(14) SAINT Software for SMART Detector, Bruker Axs Inc. Madison. Wisconsin, USA.
(15) Sheldrick, G. M. SADABS, Program for Empirical Absorption Correction of Area Detector Data. University of Göttingen, Germany 1996
(16) (a) Sheldrick, G. M. SHELXS-97, Program for the Solution of Crystal Structure. University of Göttingen 1997;(b) Sheldrick, G. M. SHELXL-97, Program for the Refinement of Crystal Structure, University of Göttingen 1997.
(17) Deacon, G. B.; Phillips, R. J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227-250.
(18) Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed., Wiley, New York 1997, pp. 59-62.
(19) Mahjoub, A. R.; Morsali, A. Direct synthesis of a dimeric mixed-anion lead(II) complex, crystal structure of [Pb(phen)(2OCCH3)(NCS)]2. Polyhedron 2002, 21, 1223-1227.
(20) Zhang, Q. Z.; Lu, C. Z. Synthesis and characterization of a lead(II) complex [Pb(phen)(H2O)(NO3)2] (phen = 1,10-phenanthroline). J. Chem.Crystallogr. 2005, 35, 795-798.
(21) Li, C.; Yarg, X.; Yarg, B.; Yan, Y.; Qian, Y. Growth of microtubular complexes as precursors to synthesize nanocrystalline ZnS and CdS, J. Cryst.Growth 2006, 291, 45-51.
(22) Shimoni-Livny, L.; Glusker, J. P.; Bock, C. W. Lone pair functionality in divalent lead compounds. Inorg. Chem. 1998, 37, 1853-1867.
- 结构化学的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid

