超声波辅助提取田基黄多酚类和黄酮类化合物及其抗氧化活性研究
汤须崇,蔡婀娜
华侨大学化工学院,厦门 361021
Introduction
Hypericum japonicum,locally called‘Tian-ji-huang’,is prepared from the entire herb of H.japonicum Thunb.ex Murray (Hypericaceae).It is one of traditional Chinese medicines (TCM)widely distributed in the south of the Yangtze River,China[1].H.japonicum has been used for the treatment of bacterial diseases,infectious hepatitis,gastrointestinal disorder,internal hemorrhage and tumors[2-6].As reported previously,H.japonicum mainly contains xanthones[6,7],chromenes[8],flavonoids[9,10],dipeptide derivatives[11],polyphenols and phloroglucinol derivatives[12].Some of these constituents are known to exhibit pharmacological and biological activities[13].
It has long been recognized that polyphenol and flavonoid are an important class of antioxidants[14].Antioxidants play an indispensable role as health benefactors in human life and are also added to food to prevent or delay its oxidation[15].Synthetic antioxidants are widely used since they are more effective and cheaper than natural ones.However,the safety and toxicity of synthetic antioxidants have brought great concerns.Thus,it is essential to develop and utilize effective and natural antioxidant to protect the body[16].In the present study,the total phenol content (TPC)and total flavonoid content (TFC)of H.japonicum were chosen to determine their antioxidant activities.
Recently,ultrasonic extraction method has been widely employed to extract bioactive components from plant material due to its high extraction efficiency[17].Response surface methodology (RSM)is a relatively new method for optimizing experimental conditions.In the present study,the ultrasonic technique was employed to extract bioactive components from H.japonicum.Response surface methodology and Box-Behnken design were used to evaluate the effects of ultrasonic time,temperature and ethanol concentration,which were chosen according to the single factor study,on the extraction of bioactive components and free radical scavenging activity (determined by DPPH and ABTS+methods)of H.japonicum.
Materials and Instruments
Materials and chemicals
Hypericum japonicum was purchased from a drug store in Fujian Province of China and stored at 4 ℃till tested.Folin-ciocalteu reagent,1,1-Diphenyl-2-picrylhydrazyl (DPPH),2,2’-azinobis-3-ethylbenzothiazoline -6-sulfonic acid (ABTS),butylated hydroxytoluene(BHT),6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox)were purchased from Sigma company.Deionized water was used throughout the experiment.All other chemicals used were of analytical grade.
Instruments
UV-Visible spectra were measured using Spectra(Pharmaspec UV-2550,Shimadzu,Kyoto,Japan)spectrophotometer.The ultrasonic assisted extraction was carried out in a KQ-600E ultrasonic device (Changzhou Nuoji Instrument Company,China)with an ultrasonic power of 600 W,heating power of 800 W and frequency of 40 kHz,equipped with a digital time and a temperature controller.
Methods
Sample preparation
H.japonicum was dried at 60 ℃till constant weight.The dried material was pulverized to 100 meshes.The powdered H.japonicum was accurately weighted and then ultrasonically extracted once with ethanol (40 mL)for special time.The supernatant solution was combined,filtrated and then cooled down to room temperature.The solution was transferred to a 100 mL volumetric flask and topped up to the volume with ethanol.
Single factor experiments for TFC extraction
The effects of five experimental variables on extraction yield of TFC were investigated by single factor tests.Five experimental variables were liquid-to-solid ratio(10,15,20,25,30),ultrasonic time (20,30,40,50,60 min),power (50%,60%,70%,80%,90%,100%),temperature (30,40,50,60,70 ℃)and ethanol concentration (50%,60%,70%,80%,90%).
Determination of TFC content
The TFC was determined by the modified aluminum chloride colorimetric method[18]with rutin as standard.Each of the plant extracts (2 mL,1.6 mg/mL)or rutin (0.222 mg/mL)was added to 30% ethanol concentration (4 mL)and 5% NaNO2solution (1 mL).After 6 min,10% Al(NO3)3solution (1 mL)was added.After another 6 min,4% NaOH solution (10 mL)was added,and the volume was made up with 30%ethanol.The mixture was shaken thoroughly and measured at 510 nm.The results were expressed as milligram Rutin equivalents/g of dry plant material.
The calibration curve of rutin was y=12.671x-0.0125,R2=0.9998 (0.00888-0.05328 mg/mL).Here,y=absorbance and x=concentration.All experiments were done in triplicate.
Determination of TPC content
The TPC was determined by the modified Folin-Ciocalteu method[19]with gallic acid as standard.Each of the plant extracts (1 mL,1.6 mg/mL)or gallic acid (150 μg/mL)was added to deionized water (10 mL)and Folin-Ciocalteu reagent (1.5 mL).After 30s,10%Na2CO3(6 mL)was added to the mixture.The mixture was shaken thoroughly and allowed to stand at 30 ℃for 2 h in the dark.The absorbance was measured at 765 nm.The results were expressed in mg Gallic acid equivalent/g of dry plant material.
The calibration curve of gallic acid was y=129.34x-0.0125,R2=0.9993 (0.000616-0.00616 mg/mL).Here,y=absorbance and x=concentration.All experiments were done in triplicate.
DPPH and ABTS+ free radical scavenging activity assay
DPPH assay
The DPPH free radical scavenging activity of the extracts was determined using the reported method[20].Equal volumes (800 μL)of different concentrations of the extracts and ethanol (1200 μL)were added with 0.1mM DPPH (2000 μL).The mixture was measured at 517 nm after 30 min of incubation at 37 ℃in the dark.The %DPPHsc was determined using the following formula:%DPPHsc=[1-(As-A0)/Ac]×100
As:sample (800 μL)+ethanol (1200 μL)+0.1mM DPPH (2000 μL)
A0:sample (800 μL)+ethanol (3200 μL)
Ac:ethanol (2000 μL)+0.1 mM DPPH (2000 μL)The IC50was calculated from the graph of scavenging effect percentage against extract concentration.Synthetic antioxidants (Trolox and BHT)were used as control,and all tests were performed in triplicate.
ABTS+· assay
ABTS+free radical was produced by reacting 2,2’-azinobis [3-ethylbenzothiazoline-6-sulphonic acid](ABTS)with potassium persulfate (K2S2O8).ABTSassay was carried out,according to the reported method[21]with slight modification.ABTSsolution (2000 μL)was added to each of the samples (800 μL),and mixed vigorously.The reaction mixture was kept at room temperature for 7 min before the absorbance was measured at 734 nm.The %ABTSsc was determined using the following formula:%ABTSsc=[1-(As-A0)/Ac]×100
As:sample (800 μL)+ethanol (1200 μL)+ABTS(2000 μL)
A0:sample (800 μL)+ethanol (3200 μL)
Ac:ethanol (2000 μL)+ABTS(2000 μL)
The IC50was calculated from the graph of scavenging effect percentage against extract concentration.Synthetic antioxidants (Trolox and BHT)were used as control,and all tests were performed in triplicate.
Experimental design
The extraction parameters were optimized using response surface methodology (RSM).A Box-Behnken design (BBD)was employed for experimental design,data analysis and model building.Three variables used in the study were ultrasonic time (X1),ethanol concentration (X2)and temperature (X3).The symbols and levels presenting in Table 1 were based on single factor pre-test.TFC,TPC,%DPPHsc,%ABTSsc were selected as the responses for the combination of the independent variables given in Table 2.Three triplicate experiments were carried out at each experimental design point and the mean values were stated as observed responses.Experimental runs were randomized,to minimize the effects of unexpected variability in the observed responses.The variables were coded according to the following equation:X=(Xi-Xo)/△X
Where X is the coded value,Xi is the corresponding actual value,Xo is the actual value in the center of the domain,and △X is the increment of Xi corresponding to a variation of 1unit of X.The mathematical model corresponding to the Box-Behnken design is:Y=β0+β1X1+β2X2+β3X3+β11X12+β22X22+β33X32+β12X1X2+β13X1X3+β23X2X3+ε
Where Y is the dependent variable (TFC,TPC,%DPPHsc,%ABTSsc),β is the model constant,βi,βii and βij are the model coefficients,and ε is the error.They represent the linear,quadratic and interaction effects of the variables.Analysis of the experimental design data and calculation of predicted responses were carried out using Design Expert software (version7.0,stat-Ease,Inc.,Minneapolis,MN).Additional confirmation experiments were subsequently conducted to verify the validity of the statistical experimental design.

Table 1 Three factors and three levels design of RSM experiment
Results and Discussion
Single factor experiments for TFC extraction
Effect of liquid-to-solid ratio on the extraction yield of TFC
In order to evaluate the effect of liquid-to-solid ratio on the extraction yield of TFC,different liquid-to-solid ratios (10,15,20,25 and 30)were tested.Other experimental parameters were set as follows:70% ethanol concentration;60 ℃ultrasonic temperature;100% ultrasonic power;30 min ultrasonic time.The results were shown in Fig.1.

Fig.1 Effect of liquid-to-solid ratio on the extraction yield of TFC
The extraction yield of TFC increased from 35.14 mg RE/g DW to 110.06 mg RE/g DW as the liquid-tosolid ratio increased within the range of 10~25 (V/W).When the liquid-to-solid ratio increased to 30 (V/W),the yield of TFC increased to 88.64 mg RE/g DW.The results indicated that 1∶25 was more suitable for the extraction of TFC.
Effect of ethanol concentration on the extraction yield of TFC
Ethanol concentration was the most important step towards parameter optimization,which had a strong impact on extraction yield of TFC.Different ethanol concentrations (50%,60%,70%,80% and 90%)were tested in the experiment.Other experimental parameters were set as follows∶1∶20 liquid-to-solid ratio;60 ℃ultrasonic temperature;100% ultrasonic power;30min ultrasonic time.The results were shown in Fig.2.

Fig.2 Effect of ethanol concentration on the extraction yield of TFC
The extraction yield of TFC increased from 85.09 mg RE/g DW to 87.55 mg RE/g DW as the ethanol concentration increased from 50% to 60%.When the ethanol concentration continued to increase up to 90%,the yield of TFC decreased to 61.90 mg RE/g DW.The results indicated that 60% ethanol concentration was suitable for the extraction of TFC.
Effect of ultrasonic power on the extraction yield of TFC
The yield from the TFC extraction could be influenced by the ultrasonic power.Different ultrasonic powers(100%,90%,80%,70%,60% and 50%)were tested in the experiment.Other experimental parameters were set as follows∶1∶25 liquid-to-solid ratio;70% ethanol concentration;60 ℃ ultrasonic temperature;30 min ultrasonic time.The results were shown in Fig.3.

Fig.3 Effect of ultrasonic power on the extraction yield of TFC
The extraction yield of TFC changed within the range of 85.87 mg RE/g DW to 91.22 mg RE/g DW as the ultrasonic power increased from 50% to 100%.The ultrasonic power had little impact on the extraction yield of TFC.
Effect of ultrasonic temperature on the extraction yield of TFC
Ultrasonic temperature was a factor that would significantly influence the extraction efficiency of TFC.Different ultrasonic temperatures (30,40,50,60 and 70 ℃)were tested in the experiment.Other experimental parameters were set as follows∶1∶25 liquid-to-solid ratio;60%ethanol concentration;100%ultrasonic power;30min ultrasonic time.The results were shown in Fig.4.

Fig.4 Effect of ultrasonic temperature on the extraction yield of TFC
The extraction yield of TFC increased from 86.63 mg RE/g DW to 97.11mg RE/g DW as ultrasonic temperature increased from 30 ℃to 70 ℃.It indicated ultrasonic temperature had a significant impact on the extraction yield of TFC.The results showed that 70 ℃was suitable for the extraction of TFC.
Effect of ultrasonic time on the extraction yield of TFC
Different ultrasonic time (20,30,40,50,60 min)was tested in the experiment.Other experimental parameters were set as follows∶1∶25 liquid-to-solid ratio;60% ethanol concentration;100%ultrasonic power;60 ℃ultrasonic temperature.The results were shown in Fig.5.

Fig.5 Effect of ultrasonic time on the extraction yield of TFC
The extraction yield of TFC significantly increased from 97.73 mg RE/g DW to 102.66 mg RE/g DW as ultrasonic time increased from 20 min to 50 min.However,as ultrasonic time increased up to 60 min,the yield of TFC decreased to 94.64 mg RE/g DW.The result showed that 50 min was suitable for the extraction of TFC.
Optimization of bioactive components and antioxidant activities by Response Surface Methodology(RSM)
Three variables[ultrasonic time (X1),ethanol concentration (X2)and temperature(X3)],which had higher impact on the extraction yield of TFC,were selected according to single factor tests.The four responses variables were TFC,TPC,% DPPHsc,% ABTSsc.The results of 15 runs using BBD design were shown in Table 2.

Table 2 BBD with observed responses for TFC,TPC,%DPPHsc and %ABTSsc

aMean of triplicate determinations.

Table 3 ANOVA for response surface quadratic model:estimated regression model of relationship between response variables and independent variables (X1,X2and X3)
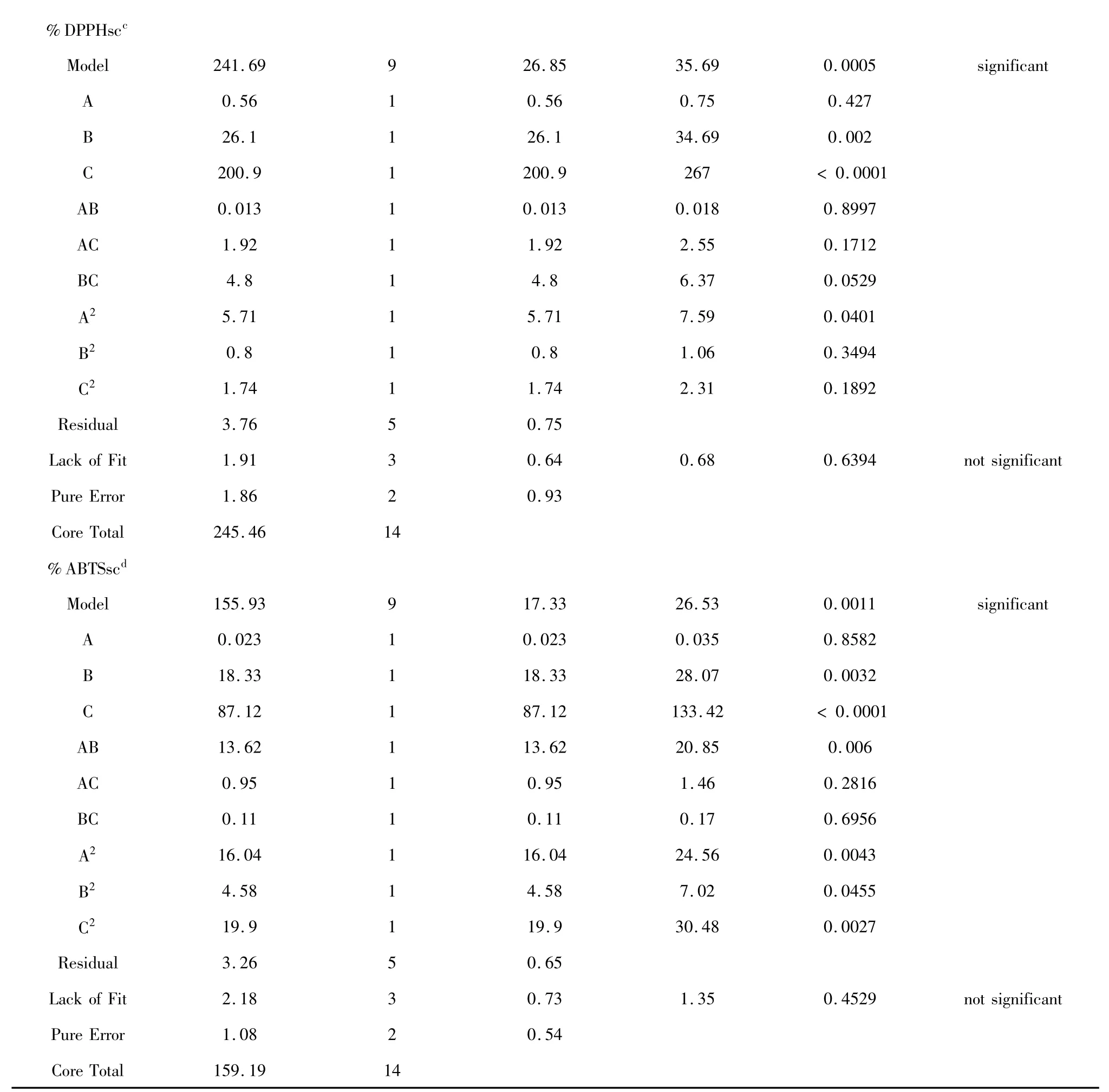
a.The coefficient of determination (R2)of the model was 0.9723.b.The coefficient of determination (R2)of the model was 0.9358.c.The coefficient of determination (R2)of the model was 0.9812.d.The coefficient of determination (R2)of the model was 0.9767.
Model fitting
Table 3 showed the results of fitting quadratic models to the data.The results of analysis of variance (ANOVA)indicated that the contribution of the quadratic model was significant.The significance of each coefficient was determined using the F-test and P-value in Table 3.The lack of fit was also used to verify the adequacy of the model.ANOVA for the lack of fit was not significant (P >0.05)for the model,indicating that the model can adequately fit the experiment data.Coefficient (R2)of determination was defined as the ratio of the explained variation to the total variation and was a measurement of the degree of fitness.The small value of R2indicated the poor relevance of the dependent variables in the model.The model can fit well with the actual data approaches unity.
The mathematical models representing TFC,TPC,%DPPHsc,%ABTSsc as a function of the response variables within the region under investigation were expressed by the following equation:

TFC
It can be seen that the variable with the largest effect on TFC extraction was the quadratic term of)followed by the linear terms of ultrasonic temperature(X3)and the quadratic terms of ultrasonic temperature)and ultrasonic time ()(P <0.01),the linear term of ethanol concentration (X2)and the quadratic term of)were not significant,indicating that the relationship between response variable (TFC)and the process variables was not simply a linear one.The Model F-value of 19.50 implied the model was significant.The "Lack of Fit F-value" of 0.64 implied that the Lack of Fit was not significantly relative to the pure error.Non-significant lack of fit was good.R2value of the model for TFC was determined to be 0.9723.These values gave a relative good fit to the mathematic model in Fig.6(A-C).
TPC
It can be seen the variable with the largest effect on TPC extraction was the linear terms of ultrasonic temperature(X3)(P <0.01)and ethanol concentration(X2)followed by the quadratic term of ultrasonic time(),the linear of (X1)(P <0.05),while the interaction terms were not significant (P >0.05),indicating that the change of X1,X2,X3,had significant effects on TPC extraction.The Model F-value of 8.09 implied the model was significant.The "Lack of Fit Fvalue" of 0.18 implied the Lack of Fit was not significant relative to the pure error. Non-significant lack of fit was good.R2value of the model for TFC was determined to be 0.9358.These values would give a relative good fit to the mathematic model in Fig.6(DF).
%DPPHsc
It can be seen the variable with the largest effect on DPPH scavenging activity was the linear terms of ultrasonic temperature (X3)and the quadratic term of ultrasonic time (),the interaction (X2X3)(P <0.01),and (X1X3),the linear term of (X1).It indicated that the relationship between response variable(%DPPHsc)and the process variables was not simply a linear one.The Model F-value of 29.01 implied the model was significant.The " Lack of Fit F-value" of 2.08 implied the Lack of Fit was not significant relative to the pure error.Non-significant lack of fit was good.R2value of the model for %DPPHsc was determined to be 0.9812.These values gave a relative good fit to the mathematic model in Fig.6(G-I).
%ABTSsc
It can be seen the variable with the largest effect on ABTS scavenging activity was the linear terms of (X3)and (X2)followed by the quadratic terms of ultrasonic temperature ()and ethanol concentration()(P<0.01),the interaction (X1,X2)and (X1,X3)(P<0.05),indicating that the relationship between response variable (%ABTSsc)and the process variables was not simply a linear one.The Model F-value of 23.29 implied the model was significant.The "Lack of Fit F-value" of 0.79 impliesd the Lack of Fit was not significant relative to the pure error.Non-significant lack of fit was good .R2value of the model for %ABTSsc was determined to be 0.9767.These values gave a relative good fit to the mathematic model in Fig.6(J-L).
Interpretation of response surface method
Graphs of RSM directly reflected the impact of factors on the response value,which the extraction yield was corresponding to the factor X1,X2,X3consisting of a Three-dimensional response surface plot and two-dimensional contour plot.Its interactions during the procedure can be found from the response surface plot.The contour plot and response surface graph of TFC,TPC,% DPPHsc,%ABTSsc were shown in Fig.6.

Fig.6 Response surface plots for extraction yield of TFC (A-C),extraction yield of TPC (D-F),%DPPH (G-I)and %ABTS (J-L)
Optimization ultrasonic conditon by RSM
Table 4 showed the optimal conditions for each individual response with the predicted and experimental values.Optimal conditions for TFC were:ultrasonic time of 50.58 min,ethanol concentration of 65.83% and ultrasonic temperature of 59.96 ℃.Optimal conditions for TPC were ultrasonic time of 49.59 min,ethanol concentration of 60.15% and ultrasonic temperature of 70.00 ℃.Optimal condition for %DPPHsc was ultrasonic time of 47.84 min,ethanol concentration of 80.00% and ultrasonic temperature of 70.00 ℃.Optimal condition for % ABTSsc was ultrasonic time of 41.78 min,ethanol concentration of 60.00% and ultrasonic temperature of 70.00 ℃.The conditions gave

Table 4 Predicted and experimental values under optimal conditions based on individual response (TFC,TPC,%DPPHsc,%ABTSsc)
TFC,TPC,%DPPHsc and %ABTSsc values of 107.42 mg RE/g DW,51.98 mg GAE/g DW,63.06% and 66.40%,respectively.
Table 5 showed that the three optimal conditions were based on combination of all responses.The optimal condition was ultrasonic time of 48.89 min,ethanol concentration of 63.72% and ultrasonic temperature of 66.92 ℃.The condition gave TFC,TPC,% DPPHsc and %ABTSsc values of 105.06 mg RE/g DW,51.75 mg GAE/g DW,58.81% and 64.99%.

Table 5 Predicted and experimental values under optimal conditions based on combination of responses (TFC,TPC,%DPPHsc and %ABTSsc)
Antioxidant activity
The extract of H.japonicum was chosen for the DPPH and ABTSscavenging assay,Trolox and BHT were used as positive control.The results were shown in Fig.7 and Fig.8.The IC50values of H.japonicum,BHT and Trolox in DPPH scavenging assays were 34.07 μg/mL,22.79 μg/mL and 2.78 μg/mL,respectively.The IC50values of H.japonicum,BHT and Trolox in ABTSscavenging assays were 25.48 μg/mL,6.59 μg/mL and 1.39 μg/mL.The results showed that the DPPH and ABTS free radical scavenging activities of H.japonicum were lower than the positive control(BHT and Trolox).

Fig.7 DPPH free radical scavenging activity of H.japonicum,BHT and Trolox
Conclusion
The response surface methodology and Box-Behnken design were applied to evaluate the effects of three independent variables (ultrasonic time,ethanol concentration,ultrasonic temperature)on the extraction of TFC,TPC and the scavenging activities to DPPH and ABTS+free radicals.The analysis of variance (ANOVA)indicated that the relationship between response variable (TFC,TPC,% DPPHsc,% ABTSsc)and the process variables was not simply linear one.

Fig.8 ABTS + free radical scavenging activity of H.japonicum,BHT and Trolox
From the data of the 3D response plots and model equations of TFC,TPC,%DPPHsc,%ABTSsc,the optimal conditions of each individual response and all responses were determined to be:
The optimal conditions of each individual response:Optimal conditions for TFC were:ultrasonic time of 50.58 min,ethanol concentration of 65.83% and ultrasonic temperature of 59.96 ℃.Optimal conditions for TPC were ultrasonic time of 49.59 min,ethanol concentration of 60.15% and ultrasonic temperature of 70.00℃.Optimal conditions for % DPPHsc were ultrasonic time of 47.84 min,ethanol concentration of 80.00%and ultrasonic temperature of 70.00 ℃.Optimal conditions for %ABTSsc were ultrasonic time of 41.78 min,ethanol concentration of 60.00% and ultrasonic temperature of 70.00 ℃.Under these optimized conditions,The yields of TFC and TPC were 107.42 mg RE/g DW and 51.98 mg GAE/g DW.The %DPPHsc and%ABTSsc values were 63.06% and 66.40%.
The optimal conditions of all responses:ultrasonic time of 48.89 min,ethanol concentration of 63.72% and ultrasonic temperature of 66.92 ℃.The optimal conditions gave TFC,TPC,%DPPHsc and %ABTSsc values of 105.06 mg RE/g DW,51.75 mg GAE/g DW,58.81% and 64.99%,respectively.
Under these optical conditions,the experimental values agreed with the predicted values.It indicated the high fitness of four models used and the success of response surface methodology for optimizing the extraction of TFC and TPC,for maximizing scavenging activities of H.japonicum on DPPH and ABTS+free radicals.
1 State Administration of Traditional Chinese Medicine "Chinese Material Medical" editorial board.Zhong Hua Ben Cao.Shanghai:Shanghai Science and Technology Press,1999.598-601.
2 Writing group of the compilation of Chinese herbal medicine.Compilation of Country wide Herbal Medicine of China.Beijing:People's Medical Publishing House,1996.4.
3 Gu GM,Feng SZ,Wang XY.The isolation and structure of Japonicine A,B,C,D.Acta Chim Sin,1988,3:246-251.
4 Ishiguro K,Yamaki M,Kashihara M,et al.Sarothralen A and B,new antibiotic compounds from Hypericum japonicum.Plant Med,1986,4:288-290.
5 Jiangsu New Medical College.Dictionary of Traditional Drugs.Shanghai:Shanghai Scientific and Technical Publishers,1977.84-85.
6 Wu QL,Wang SP,Du LJ,et al.Xanthones from Hypericum japonicum and H-Henryi.Phytochemistry,1998,49:1395-1402.
7 Ishiguro K,Nagata S,Oku H,et al.Bisxanthones from Hypericum japonicum:Inhibitors of PAF-induced hypotension.Planta Med,2002,68:258-261.
8 Ishiguro K,Yamaki M,Kashihara M,et al.An isopentenylated flavonol from Hypericum-Japonicum .8.phloroglucinol derivatives from Hypericum-Japonicum.Phytochemistry,1994,35:469-471.
9 Ishiguro K,Yamaki M,Kashihara M,et al.Phloroglucinol derivatives from Hypericum-Japonicum .9.A 2-pyrone derivative from Hypericum-Japonicum.Phytochemistry,1994,37:283-284.
10 Ishiguro K,Nagata S,Fukumota H.A flavanonol rhamnoside from Hypericum-Japonicum.7.an isopentenylated flavonol from Hypericum-Japonicum.Phytochemistry,1993,32:1583-1585.
11 Ishiguro K,Nagata S,Fukumota H.A phloroglucinol derivative from cell suspension cultures of Hypericum patulum.Phytochemistry,1998,47:1041-1043.
12 Hu LH,Khoo CW,Vittal JJ,et al.Phloroglucinol derivatives from Hypericum japonicum.Phytochemistry,2000,53:705-709.
13 Wu QL,Wang SP,Du LJ,et al.Chromone glycosides and flavonoids from Hypericum japonicum.Phytochemistry,1998,49:1417-1420.
14 Olfa B,Jihene C,Rym N,et al.Antimicrobial and antioxidant activities of methanol extracts of Evax pygmaea (Asteraceae)growing wild in Tunisia.World J Microbiol Biotechnol,2008,24:1289-1296.
15 Ghasemzadeh A,Jaafar HZE,Rahmat A.Antioxidant activities,total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe).Molecules,2010,15:4324-4333.
16 Lai FR,Wen QBA,Li L,et al.Antioxidant activities of watersoluble polysaccharide extracted from mung bean (Vigna adiata L.)hull with ultrasonic assisted treatment.Carbohydrate Polymers,2010,81:323-329.
17 Li YH,Jiang B,Zhang T,et al.Antioxidant and free radicalscavenging activities of chickpea protein hydrolysate(CPH).Food Chem,2008,106:444-450.
18 Kim DO,Jeong SW,Lee CY.Antioxidant capacity of phenolic phytochemicals from various cultivars of plums.Food Chem,2003,81:321-326.
19 Shui GH,Leong LP.Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals.Food Chem,2006,97:277-284.
20 Nsimba RY,West N,Boateng AA.Structure and radical scav-enging activity relationships of pyrolytic lignins.Agric Food Chem,2012,60:12525-12530.
21 Jorge AJ,Heliodoro de LG,Alejandro ZC,et al.The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica)skin in a reflux system using response surface methodology.Asian Pac J Trop Biomed,2013,3:436-442.

