超高压处理对牛半腱肌肌束膜和肌内膜胶原蛋白热力学特性影响
常海军,牛晓影,唐 翠,王 强
(1.重庆工商大学环境与生物工程学院,重庆高校催化理论与应用技术市级重点实验室,重庆 400067;2.重庆第二师范学院食品安全与营养研究所,生物与化学工程系,重庆 400067)
Meat quality characteristics and textural properties both have a crucial influence on the market success of meats and meat products. The quality of meats is determined by their sensory attributes, chemical composition, physical properties and so on[1-3]. The texture of meat is one of the most important quality properties, which has been studied for many years in different aspects. High pressure processing (HPP) is one of the emerging food processing high technology, which is nonthermal, and consists of submitting the foods to pressures above 100 MPa[4]. High pressure can modify the structure and function of meat proteins and those changes affect the textural and physiochemical properties of the muscle[5].
Changes of meat tenderness and texture during pressure processing are partly resulted from the changes of collagen characteristics, including collagen contents, solubility and thermal stability (thermal characteristics)[6]. Meat collagen characteristics have been analyzed to obtain information on meat tenderness, especially for collagen contents and solubility. In addition to, the thermal stability of connective tissue (perimysium and endomysium collagen) has been analyzed by measuring the onset (To) and peak (Tp) temperatures and enthalpy (ΔH) of thermal shrinkage when the role of intramuscular connective tissue (IMCT) in meat tenderness has been studied[7].
Thus, the objective of this study was to investigate the effects of high pressure processing (with different pressures and maintain time) on thermal characteristics of perimysium and endomysium collagen from beef semitendinosus muscle.
1 Materials and Methods
1.1 Materials
Beef semitendinosus samples were collected from 4 Chinese yellow bull (Nanyang × Simmental crossbreed) (live weight: (500 ± 30) kg; age: 24-30 months) carcasses slaughtered humanely in a commercial meat processing company (Lüqi Meat Co. Ltd., Henan, China) by the Halal method within 48 h postmortem, during which the carcasses were hung by Achilles tendon in a 4 ℃ chiller (The cooling process for meat ageing after slaughtering is a necessary process). The visible subcutaneous fat and epimysial connective tissue were trimmed off and sliced into 2.54 cm thick cubes, perpendicular to the direction of the fiber. The samples were prepared in triplicate.
1.2 Apparatus
HPP equipment (UHPF-750MPa, Kefa, Baotou, China); MC-DSC (Multi cell differential scanning calometer, TA instrument, USA); High-speed freezing centrifuge (Beckman Allegra 64R, Beckman-Coulter Company, USA); Freezedrying system (Heto Power Dry LL3000, Thermo Scientific, USA); Waring-basic blender (Ultra-Turrax T25, IKAWERKE, Germany); Drying oven (GZX-9076 MBE, Boxun, Shanghai, China); Water bath (HH-42, Guohua, Changzhou, China); Digital needle-tipped thermometer (HI145, Hanna Instruments, Italy); pH meter (Thermo scientific, England); Electronic Balance (AUY120, Shimadzu, Japan).
1.3 High pressure processing (HPP)
Beef semitendinosus muscle steaks (2.5 cm × 5.0 cm × 5.0 cm; weight: (100 ± 5) kg) were vacuum packed with polyethylene membrane layer (Beijing Huadun Xuehua Plastic Group Co. Ltd., China) to prevent contamination from the high-pressure transmission fluid (water). Raw meat (untreated) kept at about 20 ℃ was used as the control samples. Beef steaks were subjected to high pressure in a 2 L vessel from 200 to 600 MPa for 10 min and 20 min respectively at room temperature (20 ℃). The pressure level and time of pressurization were controlled by a computer program (BTNMC for HPP Control 1.0). Pressure holding time reported in present study does not include pressure come-up or release times. The pressure come-up rate was 350 MPa/min and pressures were released instantaneously. Six muscle steaks for each pressure processing. After processing, the surface water of muscle steaks were drained by the absorbent paper, the steaks were stored under 4 ℃ for index analysis after secondary vacuum packaging.
1.4 Perimysial and endomysial collagen preparation
The perimysial and endomysial portions of raw and high pressure processed meat samples were prepared and extracted according to the procedures of Light and Champion[8]modified by Li Chunbao et al.[9]. Briefly, each meat sample (30 g wet weight) was cut into 1 cm cubes and homogenized in ice-cold 50 mmol/L CaCl2for 30 s at full speed (about 5000 r/min) in a Waring Blendor. The homogenate was filtered through a layer of nylon net (1 mm2perforations) and the retentate on the filter was rehomogenized in 50 mmol/L CaCl2and refiltered. The process was repeated twice and then the filtrates, containing endomysium, was collected. The retentate on the filter was mainly the perimysium. The perimysium was washed three times in 1 g/100 mL sodium dodecyl sulphate (SDS) for 30 min at room temperature and SDS was removed by dialysis at 4 ℃ against distilled water (24 h), 40% methanol (24 h) and then distilled water (24 h).
1.5 DSC analysis
DSC analysis of perimysium and endomysium were conducted as described by Chang Haijun et al.[10]with slight modifications. The purified perimysial and endomysial portions were dried by freeze-drying, and then the thermal shrinkage temperatures of perimysial and endomysial collagen were measured using DSC (Multi cell differential scanning calometer). The samples (10 mg) were accurately weighed in aluminum pans and hermetically sealed. The samples were heated from 20 to 100 ℃ at heating rate of 2 ℃/min. An empty sample pan was used as the reference. Thermal shrinkage temperatures (To: onset temperature; Tp: peak temperature; Te: end temperature) of perimysium and endomysium collagen were estimated from the thermogram using the software of Universal Analysis 2000 (TA instrument).
1.6 Statistical analysis
All measurements in the study were done in triplicate; the results reported here were the means of the three replicates (expressed as the mean ± standard deviation). Statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) 16.0 (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) and Duncan’s multiple-range test were carried out to determine significant differences in perimysial and endomysial portion contents, and thermal shrinkage temperatures (To, Tpand Te) of perimysial and endomysial collagen between pressure processed meat samples for 10 min and 20 min, and the effects were considered significant at P<0.05 (*) and P<0.01 (**).
2 Results and Analysis
2.1 Perimysial and endomysial portion contents
Perimysial and endomysial portion contents for beef semitendinosus muscle during high pressure processing are shown in Fig.1 A and B respectively. Compared with the control group, the perimysial portion content for pressure treated meat (pressure maintain time were 10 min and 20 min) were decreased. There was a gradual decrease in perimysial portion content with increasing in pressure when the pressures maintain time was 10 min. The endomysial portion content was showed the trend of increased firstly and then decreased. The contents of perimysial and endomysial portion from meat steak after pressure processed were exhibited the significant differences (P<0.05 or P<0.01) between pressure processed for 10 min and 20 min during some treatments.
Perimysial and endomysial were the main components of IMCT. Light et al.[11]have inferred that in muscle toughness, the amount of perimysial collagen is much more important than the amount of endomysial collagen. They also showed that the variations of the amount of IMCT among muscles are mainly because of the amount of perimysium, whereas, the amount of endomysium remains almost constant. Therefore, it seems essential to elucidate the mechanical properties of the perimysium network in order to understand the role of connective tissue in meat toughness.
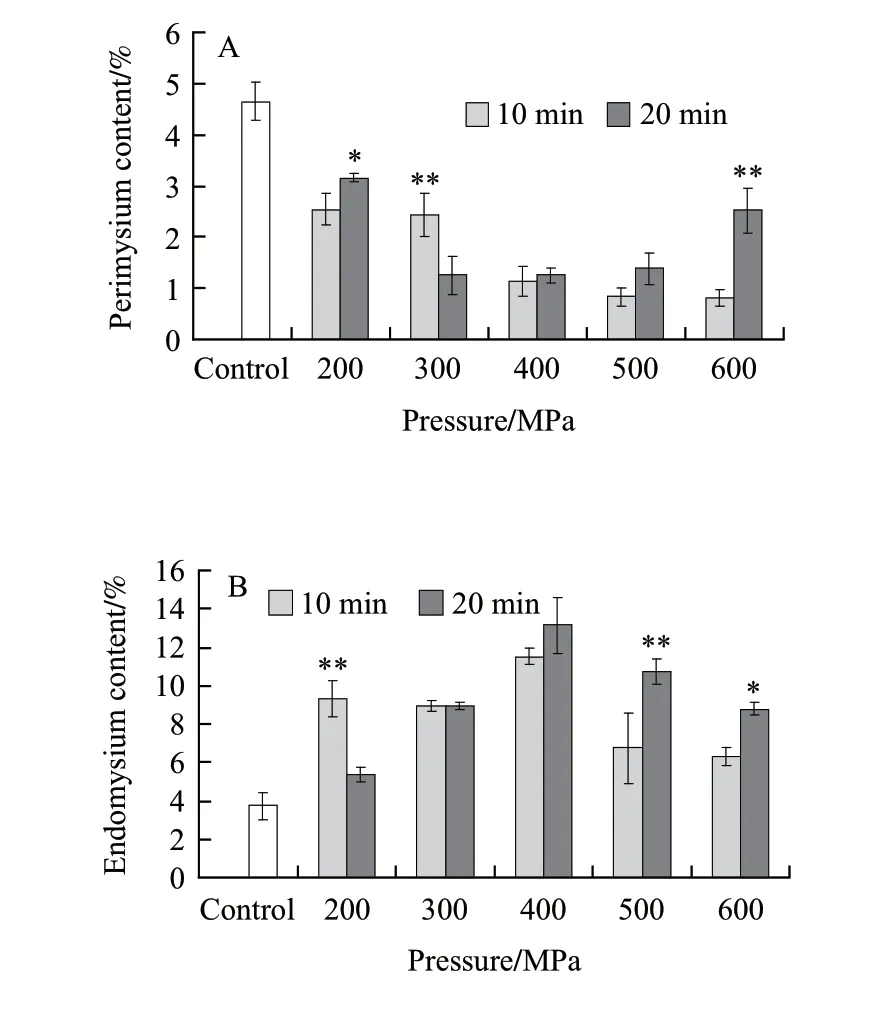
Fig.1 Changes in perimysial (A) and endomysial (B) portion content of intramuscular connective tissue from beef semitendinosus muscle following HPP treatment
2.2 Thermal characteristics of perimysium collagen
Thermal shrinkage temperatures, including onset (To), peak (Tp) and end (Te) temperature of perimysium collagen were shown in Fig.2 A, B and C respectively. As can be seen from the figures, Toand Tpof perimysium collagen were manifested the same variation trends during the high pressure processing. And the values of Toand Tpfor pressure processed meat steaks for 20 min were higher than treated for 10 min excepted at the 600 MPa. There were fewer changes in the Teof perimysium collagen for pressure processed beef meat samples.

Fig.2 Changes in thermal shrinkage temperatures of perimysium collagen of beef semitendinosus muscle following HPP treatment
2.3 Thermal characteristics of endomysium collagen
The thermal characteristics of endomysium collagen analyzed by DSC during high pressure processing are depicted in Fig.3.
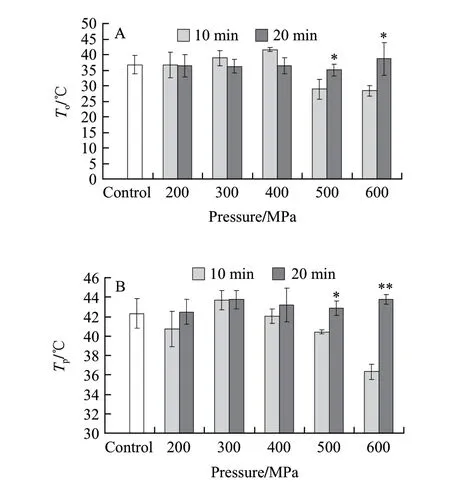
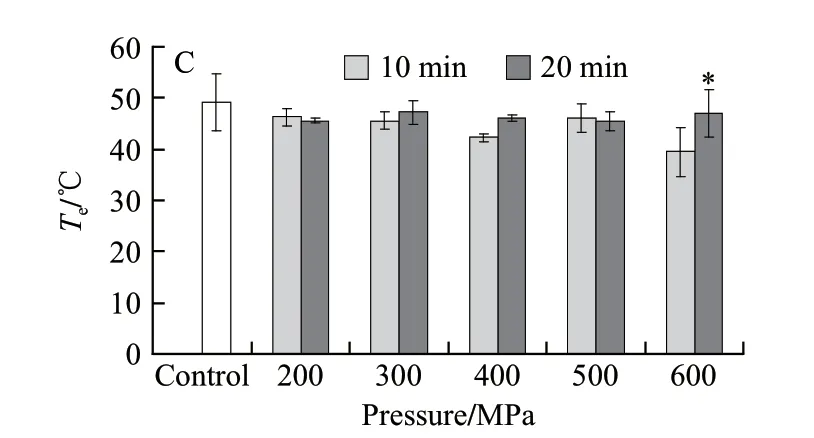
Fig.3 Changes in thermal shrinkage temperatures of endomysium collagen of beef semitendinosus muscle following HPP treatment
The effects of high pressure on thermal shrinkage temperatures of endomysium collagen were more significant than on perimysium collagen, especially for higher pressure (≥500 MPa) and longer maintain time (20 min) processing. When the pressure more than 500 MPa, the longer pressure processed time (maintain time) can lead to the higher shrinkage temperatures of endomysium collagen (mainly presented in Tp) (P<0.01), the peak temperature of endomysium collagen from meat steak after pressure processed for 20 min were higher than processed for 10 min when the pressure up to 500 MPa (P<0.05) and 600 MPa (P<0.01) respectively. This was probably resulted from the weakening of the average stability of collagen because of the pressure induced gelation and denaturation of endomysium portion collagen.
According to Bailey et al.[12]reports, Tpof collagen from mammals is around 65 ℃ but it is different for different muscles and animal species. They reported that Tois considered to describe the least stable collagen and the Tpis a measure of the average stability of collagen. In this study, perimysial and endomysial portions were not purely the connective tissue collagen portions, this was because that the mixed myofibrillar and connective tissue proteins make it more difficult to homogenate and separate perimysial and endomysial protein from the mixed components perfectly. Although the DSC samples were not pure collagen in this study, the thermal shrinkage temperatures of collagen were similar to that reported on whole meat. Nevertheless, minor difference also existed because of the different muscles, animal species and collagen kinds.
High hydrostatic pressurization is one of the new techniques for tenderizing meat. The changes in the ultrastructure of the pressurized muscle have been reported by many workers[13-15]. The effects of high pressure processing on meat tenderness (or toughness) resulted form the changes of both “actomyosin toughness” and “background toughness”. Actomyosin toughness is the toughness attributed to the myofibrillar protein, whilst the background toughness is the toughness due to the presence of connective tissue collagen and other stromal protein[16].
High hydrostatic had a significant effect on meat histological structure and the texture of treated meat relies on the gel network formed with the melted collagen, denatured and aggregated myofibrillar proteins and sarcoplasmic proteins. Pressure processing can lead to the loss of the structural continuity of muscle due to the rupture of I-filaments, loss of M-line protein and cleavage of A-filaments[16], as well as the solubilization and gelation of IMCT collagen (mainly comprising perimysial and endomysial collagens). Based on those viewpoints, the authors concluded that the changes in thermal shrinkage temperatures of perimysial and endomysial collagen for pressure processed beef muscle were attributed to the pressure-induced changes in thermal characteristics (thermal stability) of connective collagen.
2.4 Correlation analysis
Correlation coefficients among the traits of perimysium and endomysium portion contents and thermal characteristics (Tp) were listed in Table 1. The results showed that there were no significant correlation between contents and thermal shrinkage temperature, however, perimysium content were correlated negatively (P<0.01) with endomysium portion content for pressure processed beef muscle (r =-0.526).
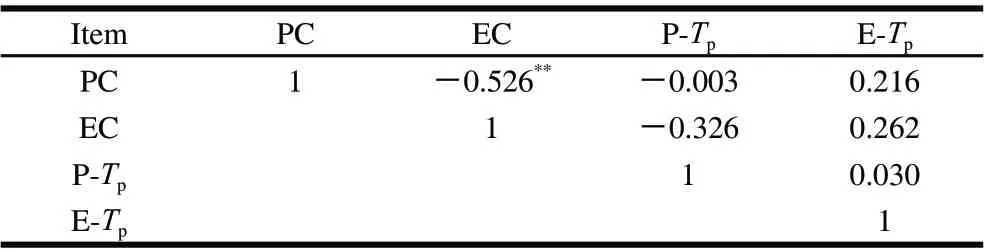
Table 1 Correlation between thermal characteristic changes of perimysium and endomysium portion collagen following HPP treatment (n=33)
3 Conclusion
According to these results, the thermal stabilities (expressed as thermal shrinkage temperatures) of perimysium and endomysium collagen in beef semitendinosus muscle changed during high pressure processing. Due to the pressure exposure, perimysium and endomysium collageneous tissues denatured and melted when the pressure up to 500 MPa, and 500—600 MPa was critical treating pressure which affects thermal shrinkage temperatures of perimysium and endomysium collagen during processed for both 10 min and 20 min. Based on those viewpoints, it is concluded that the changes in thermal shrinkage temperatures of perimysial and endomysial collagen for pressure processed beef muscle were attributed to the pressure-induced changes in thermal characteristics (thermal stability) of connective tissue collagen.
[1] LAWRIE R A. The eating quality of meat[M]//Lawrie’s meat science. Press: Woodhead Publishing Limited and CRC Press LLC, 2006: 279-341.
[2] PROBOLA G, ZANDER L. Application of PCA method for characterisation of textural properties of selected ready-to-eat meat products[J]. Journal of Food Engineering, 2007, 83(1): 93-98.
[3] CHANG Haijun, CAO Yingying, WANG Qiang, et al. Effects of thermal treatment mode and temperature on meat quality of beef semitendinosus muscle[J]. Food Science, 2010, 31(11): 42-46.
[4] SIKES A, TORNBERG E, TUME R. A proposed mechanism of tenderizing post-rigor beef using high pressure-heat treatment[J]. Meat Science, 2010, 84(3): 390-399.
[5] RUTH A M, BEGONYA M, JOSEPH P K, et al. Influence of HPP conditions on selected beef quality attributes and their stability during chilled storage[J]. Meat Science, 2011, 87(3): 274-281.
[6] CHANG Haijun, WANG Qiang, XU Xinglian, et al. Analysis of relationship between collagen variation and meat tenderness postmortem[J]. Science and Technology of Food Industry, 2010, 31(8): 404-408.
[7] CHANG Haijun, WANG Qiang, XU Xinglian, et al. DSC analysis of heat-induced changes of thermal characteristics for connective tissue collagen from beef semitendinosus muscle[J]. Food Science, 2011, 32(13): 49-53.
[8] LIGHT N, CHAMPION A E. Characterization of muscle epimysium perimysium and endomysium collagens[J]. Biochemistry Journal, 1984, 219(6): 1017-1026.
[9] LI Chunbao, ZHOU Guanghong, XU Xinglian. Changes of meat quality characteristics and intramuscular connective tissue of beef semitendinosus muscle during postmortem aging for Chinese Yellow bulls[J]. International Journal of Food Science and Technology, 2008, 43(5): 838-845.
[10] CHANG Haijun, WANG Qiang, ZHOU Guanghong, et al. Influence of weak organic acids and sodium chloride marination on characteristics of connective tissue collagen and textural properties of beef semitendinosus muscle[J]. Journal of Texture Studies, 2010, 41(3): 279-301.
[11] LIGHT N, CHAMPION A E, VOYLE C, et al. The role of epimysial, perimysial and endomysial collagen in determining texture in six bovine muscles[J]. Meat Science, 1985, 13(3): 137-149.
[12] BAILEY A J, LIGHT N D. Connective tissue in meat and meat products[M]. London: Elsevier Applied Science, 1989: 114.
[13] MA Hanjun, LEDWARD D A. High pressure/thermal treatment effects on the texture of beef muscle[J]. Meat Science, 2004, 68(3): 347-355.
[14] BEILKEN S L, MACFARLANE J J, JONES P N. Effect of high pressure during heat treatment on the Warner-Blatzler shear force values of selected beef muscles[J]. Journal of Food Science, 1990, 55(1): 15-18; 42.
[15] GUDBJORNSDOTTIR B, JONSSON A, HAFSTEINSSON H, et al. Effect of high-pressure processing on Listeria spp. and on the textural and microstructural properties of cold smoked salmon[J]. LWT-Food Science and Technology, 2010, 43(2): 366-374.
[16] SUZUKI A, WATANABE M, IKEUCHI Y, et al. Effects of high pressure treatment on the ultrastructure and thermal behaviour of beef intramuscular collagen[J]. Meat Science, 1993, 35(1): 17-25.

