Polarity,Continuity,and Alignment in Plant Vascular Strands
Megan G.Sawchuk and Enrico Scarpella
Department of Biological Sciences,University of Alberta,Edmonton Alberta,Canada T6G 2E9
Introduction
In its most basic form,the body of a seed plant can be viewed as a shoot‐root axis that grows at both the shoot pole and the root pole;the shoot‐root axis is thus a bipolar structure(Groff and Kaplan 1988)(Figure 1).The shoot pole forms lateral organs,which arise from external layers of the shoot pole.The root pole forms no lateral organs;instead,lateral roots arise far from the root pole from internal layers of the root.Shoot organs are connected with roots by vascular strands(Figure 1):bundles of vascular cell files that mainly transport photosynthesis products from shoot organs to roots,and water and minerals from roots to shoot organs.
The specialized transport function of vascular strands is supported by their relation with the parts of the plant and by the relations between the parts of the strand(Figure 1).First,vascular strands primarily connect shoot organs with roots;vascular strands do connect shoot organs with one another and roots with one another,but they do so indirectly,by making contact with vascular strands that ultimately connect with roots or shoot organs(Dengler 2006).It follows that vascular strands are unequal at their ends—one end connects to shoot tissues,the other to root tissues—and are thus polar.Second,vascular strands are continuous.Third,within vascular strands cells are aligned with one another(Esau 1942);put differently,vascular cells have an axis that continues from one cell to another and coincides with the axis of the strand.
Emphasis here will be on the mechanisms that control the formation of polar,continuous,and aligned vascular strands;other aspects of vascular strand formation have been comprehensively reviewed elsewhere(e.g.,Sachs 1983,1984,1989;Nelson and Dengler 1997;Berleth 2000;Berleth and Mattsson 2000;Ullrich and Aloni 2000;Aloni 2001;Berleth and Sachs 2001;Dengler and Kang 2001;Ye 2002;Sachs 2003;Turner and Sieburth 2003;Fukuda 2004;Sachs 2005;Sieburth and Deyholos 2006;Berleth et al.2007;Rolland‐Lagan 2008;Dettmer et al.2009;Cano‐Delgado et al.2010;Ohashi‐Ito and Fukuda 2010;Aloni 2013;Lucas et al.2013;Sack and Scoffoni 2013).

Figure 1.Vascular strands:relations between their parts and to the parts of the plant.The plant body is a bipolar axis with a shoot pole(green)and a root pole(white).Shoot organs are connected with roots by vascular strands(blue lines):continuous files of vascular cells(blue fill)whose axis is aligned along the axis of the strand.Because one end of the strand contacts shoot tissue and the other end contacts root tissue,vascular strands are polar.
Induction of Vascular Strand Formation in Mature Tissue
Evidence of a mechanism that controls the formation of polar,continuous,and aligned vascular strands was first provided by experiments in which auxin had been locally applied to mature tissue(Kraus et al.1936;Jost 1942;Jacobs 1952;Sachs 1968a).Not only did the applied auxin promote the differentiation of vascular cells,but it aligned such differentiation along continuous lines to form vascular strands,a complex response with unique properties(Sachs 1981;Berleth et al.2000)(Figure 2A).First,the response is local,as it occurs at the site of auxin application.Second,the response is polar,as it is oriented toward the pre‐existing vasculature basal to the site of auxin application—in other words,toward the roots.Third,the response is continuous,as it generates uninterrupted vascular strands.Fourth,the response is spatially constrained,as vascular differentiation is restricted to strips of cells.The axis of these cells is not along the axis of the strand,as in normal development(Esau 1942)(Figure 1),but along the shoot‐root axis of the tissue(Jost 1942)(Figure 2A).And though divisions parallel to the axis of the developing strand are among the defining features of vascular cells formed in normal development(Esau 1942)(Figure 1),the auxin‐induced vascular differentiation does not require cell division(Roberts and Baba 1968).Auxin application can induce and orient vascular cell division but only in tissue that has retained the capability to divide(Kirschner et al.1971)(Figure 2B),suggesting that other factors in addition to auxin are required in normal vascular development.Fifth,the auxin‐induced vascular‐differentiation response depends on polar auxin transport,as it requires polarly transported auxins(Dalessandro and Roberts 1971)and is obstructed by inhibitors of polar auxin transport(Gersani 1987),suggesting that the underlying mechanism recruits the machinery that polarly transports auxin.
Auxin is produced in large amounts in immature shoot‐organs(Thimann and Skoog 1934;Avery 1935)and is transported to the roots through vascular strands(Went 1928;Wangermann 1974)(Figure 2C).Because immature shoot‐organs can replace auxin in inducing vascular strand formation(Simon 1930),auxin is at least one of the signals by which shoot organs control formation of the vascular strands that connect them with roots.Roots,on the other hand,orient formation of vascular strands toward themselves by acting as preferred sinks of the auxin that originates in the shoot(Sachs 1968b;Kerk et al.2000).The formation of polar vascular strands could thus be accounted for by the unequal action of shoot organs and roots on auxin production and consumption,and by the shoot‐to‐root,apical‐basal polarity of auxin transport.
The apical‐basal polarity of auxin transport is thought to derive from the localization of auxin efflux proteins at the basal end of auxin‐transporting cells(Rubery and Sheldrake 1974;Raven 1975)(Figure 2C).As a weak acid,in fact,auxin is negatively charged at the neutral intracellular pH and can only leave the cell through specialized efflux proteins(Figure 2C).This picture is certainly an oversimplification,but calculations based on known parameters suggest that it can account for the observed polar transport(Mitchison 1980a);can it also account for the unique properties of the auxin‐induced vascular‐differentiation response?The “auxin canalization hypothesis”proposes that it can,provided positive feedback exists between auxin movement through a cell and localization of auxin efflux proteins to the site where auxin leaves the cell(Sachs 1991a,2000)(Figure 2D).The applied auxin would initially move by diffusion with no preferred orientation,and auxin efflux proteins would be randomly distributed.By efficiently transporting auxin along the original,apical–basal auxin‐transport polarity of the tissue,the pre‐existing vasculature would act as an auxin sink and orient auxin movement in neighboring cells,polarizing the localization of auxin efflux proteins in these cells.The initiation of polar auxin transport in these cells would be gradually enhanced by positive feedback between auxin transport and efflux protein localization.By draining auxin in an increasingly more efficient and polar manner,these cells would in turn induce polar auxin transport and polarization of efflux protein localization in the cells above them,and inhibit the same processes in their lateral neighbors.Iteration of these events would result in preferential transport of auxin through limited cell files,which would eventually differentiate into vascular strands.During this process,chance localization of efflux proteins would be stabilized by positive feedback between auxin transport and efflux protein localization,resulting in random elements in the course of the selected cell files and deviations from the shortest routes for auxin transport.

Figure 2.Induction of vascular strand formation by auxin and polar auxin transport.(A)Lateral application of auxin(brown)to mature tissue induces differentiation of vascular cells in continuous lines to form vascular strands(blue lines)that connect the applied auxin to the pre‐existing vasculature basal to the application site.In the auxin‐induced vascular strands,cells are not aligned along the axis of the strand as in Figure 1,but along the shoot‐root axis of the tissue(green‐to‐white gradient).After Sachs(1968a,1991b).(B)Lateral application of auxin(brown circle)to vascular cells that have retained the capability to divide induces divisions perpendicular to the original axis of the tissue;daughter cells elongate by intrusion along the new axis.Arrows connect successive stages.After Neeff(1914).(C)Left:auxin(brown fill)is produced in large amounts in immature shoot‐organs and transported(brown arrows)to the roots by vascular strands.Top‐right:the shoot‐to‐root,apical‐basal polarity of auxin transport derives from the polar localization of efflux carriers of the PIN‐FORMED family(brown)at the basal plasma‐membrane of vascular cells.Bottom‐right:specialized efflux carriers are required for auxin to leave the cell(brown arrows)as auxin is negatively charged at intracellular pH;by contrast,auxin is electrically neutral at extracellular pH and can thus diffuse into the cell(gray arrows).(D)Successive stages(connected by gray arrows)of vascular strand formation in response to lateral application of auxin(brown circle)according to the “auxin canalization hypothesis.”Positive feedback between cellular auxin efflux(brown arrows)and localization of efflux carriers to the cellular site of auxin exit gradually polarize auxin transport(increasingly thicker brown‐arrows);this occurs first in cells in contact with the pre‐existing vasculature(gray fill),which transports auxin along the original,apical‐basal polarity of the tissue and thus orients auxin transport toward itself.Large polar‐auxin‐transport capacity in selected cells leads to vascular differentiation(blue fill)and drains auxin away from neighboring cells,thus inhibiting their differentiation.Iteration of the process forms a continuous vascular strand that connects the applied auxin to the pre‐existing vasculature basal to the site of auxin application.Figure inspired by Sachs(1991a).(E)Top:through the auxin transport polarity of the tissue(brown arrows),the polarity of vascular strands(blue lines)is normally aligned with the shoot‐to‐root polarity of the tissue(green‐to‐white gradient).Bottom:disruption of the existing auxin‐transport polarity allows induction of a new auxin‐transport polarity,which can be different from—even opposite to—the original shoot‐to‐root polarity of the tissue;it is along this new auxin‐transport polarity that new vascular strands will form.In these new vascular strands,cells will not be aligned along the axis of the strand as in Figure 1,but along the shoot‐root axis of the tissue as in(A).After Sachs(1981).
The positive feedback between auxin transport and efflux protein localization can thus account for the unique properties of the auxin‐induced vascular‐differentiation response.But it can also account for the seemingly conflicting coexistence of stability and flexibility in the alignment between vascular strand polarity and the shoot‐to‐root polarity of the tissue.Shoot‐to‐root polarity and auxin transport polarity are normally aligned with each other(Went 1928;Wangermann 1974)(Figure 2C).According to the auxin canalization hypothesis,vascular strands would normally form along the existing auxin‐transport polarity of the tissue—and thus along the shoot‐to‐root polarity of the tissue(Figure 2E).Induction of a new auxin‐transport polarity would require auxin diffusion,but auxin diffusion would be limited,or dominated,by the existing auxin‐transport polarity.If,however,the existing auxin‐transport polarity were disrupted—for example,by wounding—auxin diffusion would no longer be limited,and a new auxin‐transport polarity could be gradually induced.New vascular strands would form along the new auxin‐transport polarity,which may even be opposite to the original shoot‐to‐root polarity of the tissue(Figure 2E).Perturbations of the alignment between vascular strand polarity and the shoot‐to‐root polarity of the tissue are not limited to abnormal growth conditions(e.g.,Sachs 1981)but also occur in normal development(e.g.,Sachs 1970).Though not all the predictions of the auxin canalization hypothesis are necessarily intuitive,they have been rigorously tested and are supported by computer simulation of mathematical models(Mitchison 1980b,1981;Rolland‐Lagan and Prusinkiewicz 2005).
The localization of the five plasma‐membrane‐localized members of the PIN‐FORMED(PIN)family of auxin efflux proteins of Arabidopsis thaliana marks the presumed auxin‐efflux side of cells(Petrasek et al.2006;Wisniewska et al.2006).Thus,the polarity of auxin transport can be inferred from the localization of PIN proteins at the plasma membrane.Local application of auxin to mature tissue induces PIN1 expression in broad domains that connect the applied auxin to the pre‐existing vasculature(Sauer et al.2006).In these domains,PIN1 localization is initially apolar but over time becomes polarized to suggest auxin transport away from the site of auxin application and toward the pre‐existing vasculature basal to the site of auxin application—observations that are all consistent with predictions of the auxin canalization hypothesis.But these studies have also captured aspects of the auxin‐induced vascular differentiation not necessarily implied by the original hypothesis,such as the gradual increase in PIN1 expression in the cells selected for vascular differentiation,and the decline and eventual termination of expression in the cells not selected for vascular differentiation;the underlying mechanism is unknown,but responsiveness of PIN gene expression to auxin levels(Heisler et al.2005;Vieten et al.2005)could be at its basis.
Vascular Differentiation in Callus
Interruption of vascular strand continuity by wounding presumably interrupts polar auxin transport and concentrates auxin in mature tissue near the wound.The disruption of auxin distribution induced by wounding can be imitated in tissue culture—where auxin is continuously supplied through the culture medium(Gautheret 1939;Nobécourt 1939;White 1939)—or in tumors—where auxin is continuously produced by the tissue itself(Henderson and Bonner 1952).Whether because of wounding,tissue culture,or tumor,the resulting disruption of auxin distribution can induce division of vascular‐strand‐associated cells to give rise to a shapeless mass of cells known as callus(Simon 1908;Sugimoto et al.2010).
It is often assumed that callus consists of a homogeneous population of undifferentiated cells;instead,differentiation of vascular cells is very common in callus(Simon 1908).In sections,these vascular cells may appear disconnected,an observation in apparent conflict with a control mechanism that requires continuous cell‐to‐cell transport of an inductive signal;however,in whole‐mount preparations of callus tissue,vascular cells are clearly arranged in continuous strands(Aloni et al.1995),suggesting that the objection is unjustified.
A more serious objection seems to be whether these vascular strands can still be considered expression of a polar control mechanism.Available evidence suggests that they can:when callus forms on both sides of a wound that interrupts the connection of shoot with root,the structure of the callus formed on one side of the wound is different from the structure of the callus formed on the opposite side of the wound(Simon 1908)(Figure 3).The callus that is connected with the shoot includes roots and vascular strands with meandering axes,which is the vascular organization that is expected when there is excess auxin that has no uniform polar outlet.On the other hand,the callus that is connected with the root includes shoots and vascular strands oriented along the shoot‐root axis,suggesting that this callus is a source of auxin that is readily drained toward the root.By acting as partial replacement of either shoot or root,callus formation can thus be considered an attempt to re‐establish the polarity of the vascular strands that connect the different parts of the wounded plant.

Figure 3.Polarity of callus vascular strands.As expressed in the axes of the vascular strands(blue)and in the formation of roots(white)and shoots(green fill),the callus(gray)that forms on the side of the wound that contacts shoot tissue partially replaces the root,while the callus that forms on the side of the wound that contacts root tissue partially replaces the shoot.Callus tissue thus re‐establishes the polarity of vascular strands and the connection of shoot organs with roots.After(Sachs 1991a,1991b).
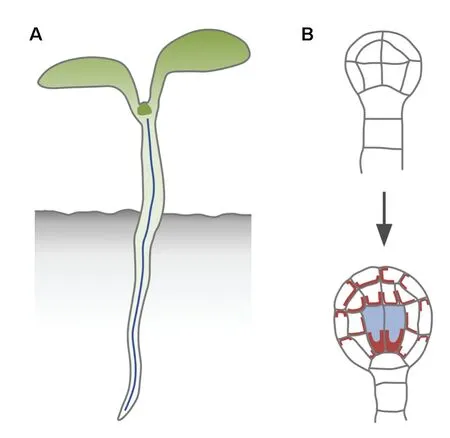
Figure 4.The first vascular strand and its formation.(A)Most of the seedling body is a cylinder with a central vascular strand(blue line).(B)The central vascular strand of the seedling derives from the division of the vascular cells(blue fill)of the globular embryo(bottom);these cells are characterized by strong,polarized expression of PIN1(brown)and arise from the division of the inner cells of the dermatogen embryo(top),a division that is aligned along the future shoot‐root axis of the embryo.
Formation of the First Vascular Strand
Most of the body of the seedling of a seed plant can be formalized as a cylinder with a vascular strand in its center(Figure 4A).The formation of this body axis in the globular embryo is associated with the formation of the first vascular cells,whose axes are aligned along the embryo axis(Mansfield and Briarty 1991;Gillmor et al.2010)(Figure 4B).The embryo axis first becomes evident from the division of the inner cells of the dermatogen‐stage embryo(Figure 4B),a division that occurs along a single axis.The resulting globular embryo is no longer radially symmetrical but is comprised of concentric cylinders,though its overall shape is still spherical(Figure 4B).
At the molecular level,embryo axis formation in the globular embryo is associated with polar localization of PIN1 at the basal end of the inner cells(Steinmann et al.1999)(Figure 4B).Consistent with predictions of the auxin canalization hypothesis,polarization of PIN1 localization is particularly pronounced in the first vascular cells(Figure 4B),which are thus molecularly polar.But these cells are also morphologically polar,as their apical end connects to the upper tier of cells and their basal end to the uppermost cell of the extra‐embryonic suspensor,the hypophysis(Figure 4B).The following divisions will extend the individual cell files and elaborate the poles of the embryo axis,using this axis as a positional reference(Berleth 2001).
Available evidence suggests that the formation of the embryo axis and of the vascular strand in its center depend on polar auxin transport and signaling.Development of embryos in the presence of auxin transport inhibitors occasionally results in nearly spherical,apparently apolar,embryos,and seedlings(Schiavone and Cooke 1987;Hadfi et al.1998).Similar defects seem to appear in the most extreme examples of mutants in multiple PIN genes(Friml et al.2003)but can also be induced by mutation of a single gene of Arabidopsis:EMBRYO DEFECTIVE30/GNOM(EMB30/GN;GN hereafter)(Mayer et al.1993).The GN protein is a guanine nucleotide exchange factor required to transport PIN proteins to their proper location at the plasma membrane(Steinmann et al.1999;Geldner et al.2003;Kleine‐Vehn et al.2008).However,only a small fraction of gn embryos develop into nearly spherical seedlings;most of them develop into seedlings in which the embryo axis is replaced by a conical structure composed of morphologically indistinct cells(Mayer et al.1993),a defect that also appears in embryos treated with auxin antagonists and in mutants in auxin production,perception,or response(Hadfi et al.1998;Hardtke and Berleth 1998;Hamann et al.2002;Dharmasiri et al.2003,2005,2007;Hellmann et al.2003;Cheng et al.2007;Stepanova et al.2008;Thomas et al.2009).Among them,embryo axis defects are most pronounced in mutants of the Arabidopsis gene MONOPTEROS/AUXIN RESPONSE FACTOR5(MP/ARF5;MP hereafter),which encodes a transcription factor that regulates auxin‐responsive gene expression(Berleth and Jürgens 1993;Hardtke and Berleth 1998;Mattsson et al.2003).Defects in gn and mp have been traced back to similar abnormal divisions in early embryogenesis(Mayer et al.1993;Hamann et al.1999),but these are likely to be the consequence of rather than the cause of the embryo axis defects,as randomization of orientation of cell division does not lead to embryo axis defects(e.g.,Torres‐Ruiz and Jürgens 1994;Lukowitz et al.1996;Strompen et al.2002).
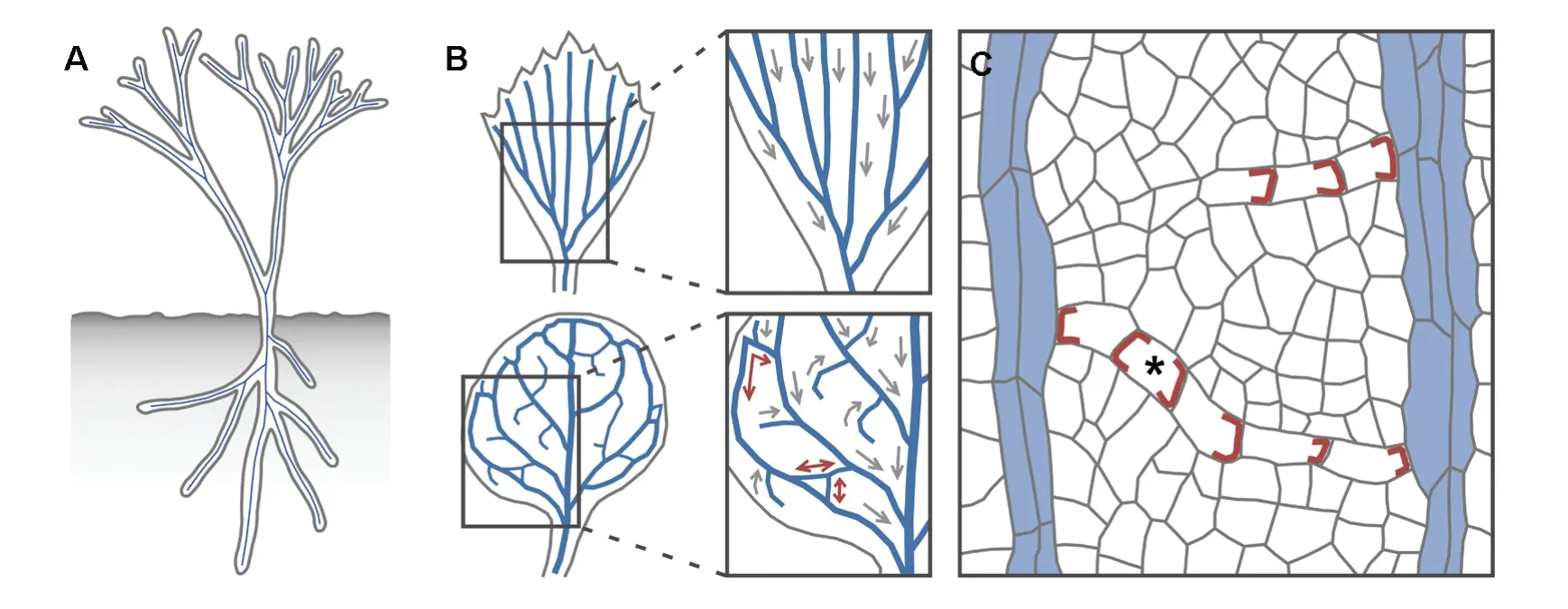
Figure 5.Networks of polar vascular strands.(A)The body of early,leafless plants was a system of branching cylinders with a vascular strand(blue line)in their center.(B)Extant plants bear leaves with open(top)or closed(bottom)networks of vascular strands.A unique shoot‐to‐root polarity(gray arrow)can be assigned to each vascular strand in open networks;attempts to assign shoot‐to‐root polarity to individual strands in closed networks lead to strands with ambiguous polarity(brown double‐headed arrows).(C)Polar localization of PIN1(brown)in files of vascular cells suggests auxin transport toward pre‐existing vasculature(blue fill;for simplicity,PIN1 expression in pre‐existing vasculature is not shown).Thus,in unilaterally connected vascular strands,a single auxin transport polarity exists;in bilaterally connected strands,the two opposite polarities are integrated by a cell with PIN1 at both ends(asterisk).
Formation of Closed Vascular Networks
A cylindrical structure with a vascular strand in its center is not only the base unit of the embryo axis but of the whole body of early land plants(Fairon‐Demaret and Li 1993).These leafless plants can in fact be described as two systems of branching cylindrical organs—one above ground and one below ground—with a vascular strand in the center of each cylinder(Figure 5A).Most extant plants bear flat organs such as leaves and thus deviate from the basic cylindrical structure;however,this basic structure can still be recognized at early stages of development of flat organs,when these organs appear as cylindrical primordia with a vascular strand in their center(Mattsson et al.1999;Kang and Dengler 2004;Scarpella et al.2004).The cylindrical shape is soon lost,and the organs acquire their distinctive flattened shape,a process that coincides with the formation of branching systems of vascular strands.These vascular networks are said to be “open,”if each vascular strand ends freely at one end and contacts another strand at the other end,and “closed,”if at least some vascular strands contact other strands at both ends(Roth‐Nebelsick et al.2001)(Figure 5B).
A unique shoot‐to‐root polarity can be assigned to all vascular strands in open networks,but in closed networks there are strands whose polarity is ambiguous(Sachs 1975)(Figure 5B).Thus closed networks seem incompatible with a control mechanism that relies on polar transport of auxin;however,the dynamics of PIN1 expression during the formation of closed vascular networks suggest that the incompatibility is only apparent(Scarpella et al.2006;Wenzel et al.2007;Sawchuk et al.2013)(Figure 5C).During formation of all veins,weakly polar—or altogether apolar—PIN1 expression is initiated in broad domains in continuity with pre‐existing vasculature.Over time,the broad PIN1‐expression domains narrow to sites of vascular strand formation,and PIN1 localization becomes polarized toward pre‐existing vasculature;both processes initiate and proceed away from pre‐existing vasculature.As a unilaterally connected PIN1 expression domain with uniform auxin‐transport polarity toward pre‐existing vasculature becomes connected at both ends,or merges with another unilaterally connected domain,a single cell in which PIN1 localizes at both ends—a “bipolar”cell—appears along the now bilaterally connected PIN1 expression domain;this bipolar cell bridges the two,opposite auxin‐transport polarities—each toward pre‐existing vasculature—that now exist in the bilaterally connected vascular strand.
Like auxin application to other dividing tissues(Kirschner et al.1971),auxin application to developing leaves induces formation of vascular strands in which cells are aligned along the axis of the strand(Scarpella et al.2006;Sawchuk et al.2007);however,vascular cell alignment is lost in wild‐type leaves developed in the presence of auxin transport inhibitors(Mattsson et al.1999;Sieburth 1999)and in leaves of severe auxin‐response mutants(Przemeck et al.1996;Mattsson et al.1999),suggesting that the orienting effect of auxin on cell alignment within vascular strands depends on both polar auxin signaling and the cell division capability of the tissue.
Continuous Vascular Differentiation
A control mechanism that relies on continuous,cell‐to‐cell transport of auxin predicts that vascular strands should form without interruptions;yet interruptions have been observed in vascular strands of wild‐type and mutant leaves(Pray 1955a,1955b;Lersten 1965;Herbst 1971;Berleth and Jürgens 1993;Carland et al.1999;Deyholos et al.2000;Koizumi et al.2000;Steynen and Schultz 2003;Sawa et al.2005).Further scrutiny,however,suggests that some of these interrupted vascular strands are composed of stretches of mature vascular cells connected by stretches of immature vascular cells(Pray 1955a,1955b;Lersten 1965;Herbst 1972;Przemeck et al.1996);because the identification of immature vascular cells can be problematic(Esau 1943),these strands have been interpreted as interrupted when they really are continuous,though only partly differentiated.By contrast,in other interrupted vascular strands,stretches of mature vascular cells are separated by mature nonvascular tissue(Herbst 1972;Carland et al.1999;Deyholos et al.2000).However,these strands emerge as continuous files of immature vascular cells that over time break down into fragments(Herbst 1972;Scarpella et al.2006;Naramoto et al.2009);this is reflected in the breaking down of initially continuous PIN1 expression domains(Scarpella et al.2006;Naramoto et al.2009),suggesting that the interrupted strands are the outcome of defective maintenance of normally established,continuous auxin transport.All these “interrupted”strands are thus continuous,at least at formative stages,and are thus compatible with an auxin‐transport‐dependent control mechanism.An observation that is instead more difficult to reconcile with such mechanism is the presence of seemingly isolated,randomly oriented,mature vascular cells in gn cotyledons(Mayer et al.1993);however,it is unknown whether these cells are ever connected by immature vascular cells and,if so,what the axis of the resulting strand would be.
Continuity of vascular strands is a stringent requirement for a control mechanism that relies on continuous auxin transport but also for transport of water and nutrients,a complex function supported by the complex ultrastructure of vascular cells(Scott et al.1960).Aspects of this ultrastructure are shared by isolated cells with no defined axis(Solereder 1908).Because these cells store—rather than transport—water and nutrients(Foster 1956),they cannot be considered vascular cells and are thus not an objection to a control mechanism that depends on continuous auxin transport;rather,they suggest that the same cellular differentiation pathway can be recruited to support different,though related,functions.
Conclusions
The discussion here focused on evidence in support of and objections against mechanisms proposed to control the formation of polar,continuous,and aligned vascular strands.One such mechanism had been hypothesized to account for the polar and continuous—though not necessarily aligned—vascular strands that form in mature tissue in response to auxin application.However,the auxin canalization hypothesis and its predictions have also turned out to be consistent with the molecular genetics and cell biology of embryo axis formation and shoot organ development.Objections to the hypothesis include claims of apolar or discontinuous vascular strands in callus and leaves;however,the evidence does not seem to support the claims,and thus the objections seem unjustified.Nevertheless,major questions remain unanswered.
The auxin canalization hypothesis seems to imply that cells can sense auxin flux—that is,the amount of auxin that flows through a cell over time.Though the positive effect of auxin on its own transport is experimentally well supported(Rayle et al.1969;Paciorek et al.2005),whether this effect is at the basis of an auxin‐flux‐sensing mechanism remains unclear.Alternatives to a “flux sensor”have been proposed(Mitchison 1981;Kramer 2009;Wabnik et al.2010),but all of them make assumptions awaiting experimental support and reproduce only some aspects of vascular strand formation.
The auxin canalization hypothesis also predicts low amounts of auxin in vascular strands,which seems in conflict with experimental evidence(Mattsson et al.2003);solutions to this conflict have been proposed(Kramer 2004,2009;Feugier et al.2005;Bayer et al.2009;Wabnik et al.2010),but whether the effects of experimentally interfering with the additional assumptions are consistent with the predicted outcomes remains unknown.
However successful the attempts to reconcile hypothesis and evidence may be,it would seem naïve to expect that a single mechanism can account for all the properties of a complex process such as vascular strand formation;instead,it would seem likely that at least some of these properties can be controlled by other,unidentified mechanisms.One of these properties seems to be vascular strand alignment:though necessary,polar auxin transport can in fact only promote the oriented divisions required for such alignment in tissue that has retained the capability to divide and can thus respond to the orienting signal.
In the end,however,the most surprising finding is perhaps that so few objections to the auxin canalization hypothesis have been raised.This may simply reflect the few,possibly exceptional,contexts in which the hypothesis has been tested,and as experimental evidence catches up with intuitive concepts we should expect many more inconsistencies to be exposed.Nevertheless,it seems justified to suggest that the consistencies outlined above will provide an entry point into dissecting the complexity of vascular strand formation.
Acknowledgements
We dedicate this manuscript to Erin Leigh Sawchuk,who passed away on 5 May 2013.We apologize to colleagues whose results could not be included in the available space.The authors’vascular research is supported by Discovery Grants of the Natural Sciences and Engineering Research Council of Canada(NSERC).M.G.S.was supported by an NSERC CGS‐M Scholarship and an NSERC CGS‐D Scholarship.
Aloni R(2001)Foliar and axial aspects of vascular differentiation:Hypotheses and evidence.J.Plant Growth Regul.20,22–34.
Aloni R(2013)The role of hormones in controlling vascular differentiation.In:Fromm J,ed.Cellular Aspects of Wood Formation.Springer‐Verlag,Berlin,Heidelberg.pp.99–139.
Aloni R,Pradel K,Ullrich C(1995)The three‐dimensional structure of vascular tissues in Agrobacterium tumefaciens‐induced crown galls and in the host stems of Ricinus communis L.Planta 196,597–605.
Avery GS Jr.(1935)Differential distribution of a phytohormone in the developing leaf of Nicotiana,and its relation to polarized growth.Bull.Torrey Bot.Club 62,313–330.
Bayer EM,Smith RS,Mandel T,Nakayama N,Sauer M,Prusinkiewicz P,Kuhlemeier C(2009)Integration of transport‐based models for phyllotaxis and midvein formation.Genes Dev.23,373–384.
Berleth T(2000)Plant development:Hidden networks.Curr.Biol.10,R658–R661.
Berleth T(2001)Top‐down and inside‐out:Directionality of signaling in vascular and embryo development.J.Plant Growth Regul.20,14–21.
Berleth T,Jürgens G(1993)The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo.Development 118,575–587.
Berleth T,Mattsson J(2000)Vascular development:Tracing signals along veins.Curr.Opin.Plant Biol.3,406–411.
Berleth T,Sachs T(2001)Plant morphogenesis:Long‐distance coordination and local patterning.Curr.Opin.Plant Biol.4,57–62.
Berleth T,Mattsson J,Hardtke CS(2000)Vascular continuity and auxin signals.Trends Plant Sci.5,387–393.
Berleth T,Scarpella E,Prusinkiewicz P(2007)Towards the systems biology of auxin‐transport‐mediated patterning.Trends Plant Sci.12,151–159.
Cano‐Delgado A,Lee JY,Demura T(2010)Regulatory mechanisms for specification and patterning of plant vascular tissues.Annu.Rev.Cell Dev.Biol.26,605–637.
Carland FM,Berg BL,FitzGerald JN,Jinamornphongs S,Nelson T,Keith B(1999)Genetic regulation of vascular tissue patterning in Arabidopsis.Plant Cell 11,2123–2137.
Cheng Y,Dai X,Zhao Y(2007)Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis.Plant Cell 19,2430–2439.
Dalessandro G,Roberts LW(1971)Induction of xylogenesis in pith parenchyma explants of lactuca.Am.J.Bot.58,378–385.
Dengler NG(2006)The shoot apical meristem and development of vascular architecture.Can.J.Bot.84,1660–1671.
Dengler N,Kang J(2001)Vascular patterning and leaf shape.Curr.Opin.Plant Biol.4,50–56.
Dettmer J,Elo A,Helariutta Y(2009)Hormone interactions during vascular development.Plant Mol.Biol.69,347–360.
Deyholos MK,Cordner G,Beebe D,Sieburth LE(2000)The SCARFACE gene is required for cotyledon and leaf vein patterning.Development 127,3205–3213.
Dharmasiri S,Dharmasiri N,Hellmann H,Estelle M(2003)The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis.EMBO J.22,1762–1770.
Dharmasiri N,Dharmasiri S,Weijers D,Lechner E,Yamada M,Hobbie L,Ehrismann JS,Jürgens G,Estelle M(2005)Plant development is regulated by a family of auxin receptor F Box proteins.Dev.Cell 9,109–119.
Dharmasiri N,Dharmasiri S,Weijers D,Karunarathna N,Jürgens G,Estelle M(2007)AXL and AXR1 have redundant functions in RUB conjugation and growth and development in Arabidopsis.Plant J.52,114–123.
Esau K(1942)Vascular differentiation in the vegetative shoot of linum.I.The procambium.Am.J.Bot.29,738–747.
Esau K(1943)Origin and development of primary vascular tissues in plants.Bot.Rev.9,125–206.
Fairon‐Demaret M,Li C‐S(1993)Lorophyton goense gen.et sp.nov.from the lower givetian of Belgium and a discussion of the middle devonian cladoxylopsida.Rev.Palaeobot.Palyno.77,1–22.
Feugier FG,Mochizuki A,Iwasa Y(2005)Self‐organization of the vascular system in plant leaves:Inter‐dependent dynamics of auxin flux and carrier proteins.J.Theor.Biol.236,366–375.
Foster A(1956)Plant idioblasts:Remarkable examples of cell specialization.Protoplasma 46,184–193.
Friml J,Vieten A,Sauer M,Weijers D,Schwarz H,Hamann T,Offringa R,Jürgens G(2003)Efflux‐dependent auxin gradients establish the apical–basal axis of Arabidopsis.Nature 426,147–153.
Fukuda H(2004)Signals that control plant vascular cell differentiation.Nat.Rev.Mol.Cell Biol.5,379–391.
Gautheret R(1939)Sur la possibilité de réaliser la culture indéfinie des tissus de tubercules de carotte.C.R.Acad.Sci.208,118–130.
Geldner N,Anders N,Wolters H,Keicher J,Kornberger W,Muller P,Delbarre A,Ueda T,Nakano A,Jürgens G(2003)The Arabidopsis GNOM ARF‐GEF mediates endosomal recycling,auxin transport,and auxin‐dependent plant growth.Cell 112,219–230.
Gersani M(1987)The induction of differentiation of organized vessels in a storage organ.Ann.Bot.59,31–34.
Gillmor CS,Park MY,Smith MR,Pepitone R,Kerstetter RA,Poethig RS(2010)The MED12‐MED13 module of mediator regulates the timing of embryo patterning in Arabidopsis.Development 137,113–122.
Groff P,Kaplan D(1988)The relation of root systems to shoot systems in vascular plants.Bot.Rev.54,387–422.
Hadfi K,Speth V,Neuhaus G(1998)Auxin‐induced developmental patterns in Brassica juncea embryos.Development 125,879–887.
Hamann T,Mayer U,Jürgens G(1999)The auxin‐insensitive bodenlos mutation affects primary root formation and apical‐basal patterning in the Arabidopsis embryo.Development 126,1387–1395.
Hamann T,Benkova E,Baurle I,Kientz M,Jürgens G(2002)The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS‐mediated embryo patterning.Genes Dev.16,1610–1615.
Hardtke CS,Berleth T(1998)The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development.EMBO J.17,1405–1411.
Heisler MG,Ohno C,Das P,Sieber P,Reddy GV,Long JA,Meyerowitz EM(2005)Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem.Curr.Biol.15,1899–1911.
Hellmann H,Hobbie L,Chapman A,Dharmasiri S,Dharmasiri N,del Pozo C,Reinhardt D,Estelle M(2003)Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis.EMBO J.22,3314–3325.
Henderson JHM,Bonner J(1952)Auxin metabolism in normal and crown gall tissue of sunflower.Am.J.Bot.39,444–451.
Herbst D(1971)Disjunct foliar veins in Hawaiian Euphorbias.Science 171,1247–1248.
Herbst D(1972)Ontogeny of foliar venation in Euphorbia forbesii.Am.J.Bot.59,843–850.
Jacobs WP(1952)The role of auxin in differentiation of xylem around a wound.Am.J.Bot.39,301–309.
Jost L(1942)über gefässbrücken.Zeitsch.Bot.38,161–215.
Kang J,Dengler N(2004)Vein pattern development in adult leaves of Arabidopsis thaliana.Int.J.Plant Sci.165,231–242.
Kerk NM,Jiang K,Feldman LJ(2000)Auxin metabolism in the root apical meristem.Plant Physiol.122,925–932.
Kirschner H,Sachs T,Fahn A(1971)Secondary xylem reorientation as a special case of vascular tissue differentiation.Israel J.Bot.20,184–198.
Kleine‐Vehn J,Dhonukshe P,Sauer M,Brewer PB,Wisniewska J,Paciorek T,Benková E,Friml J(2008)ARF GEF‐dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis.Curr.Biol.18,526–531.
Koizumi K,Sugiyama M,Fukuda H(2000)A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network:Calling the auxin signal flow canalization hypothesis into question.Development 127,3197–3204.
Kramer EM(2004)PIN and AUX/LAX proteins:Their role in auxin accumulation.Trends Plant Sci.9,578–582.
Kramer EM(2009)Auxin‐regulated cell polarity:An inside job?Trends Plant Sci.14,242–247.
Kraus EJ,Brown NA,Hamner KC(1936)Histological reactions of bean plants to indoleacetic acid.Bot.Gaz.98,370–420.
Lersten N(1965)Histogenesis of leaf venation in Trifolium wormskioldii(Leguminosea).Am.J.Bot.52,767–774.
Lucas WJ,Groover A,Lichtenberger R,Furuta K,Yadav SR,Helariutta Y,He XQ,Fukuda H,Kang J,Brady SM,Patrick JW,Sperry J,Yoshida A,Lopez‐Millan AF,Grusak MA,Kachroo P(2013)The plant vascular system:Evolution,development and functions.J.Integr.Plant Biol.55,294–388.
Lukowitz W,Mayer U,Jürgens G(1996)Cytokinesis in the Arabidopsis embryo involves the syntaxin‐related KNOLLE gene product.Cell 84,61–71.
Mansfield SG,Briarty LG(1991)Early embryogenesis in Arabidopsis thaliana.II.The developing embryo.Can.J.Bot.69,461–476.
Mattsson J,Sung ZR,Berleth T(1999)Responses of plant vascular systems to auxin transport inhibition.Development 126,2979–2991.
Mattsson J,Ckurshumova W,Berleth T(2003)Auxin signaling in Arabidopsis leaf vascular development.Plant Physiol.131,1327–1339.
Mayer U,Buttner G,Jürgens G(1993)Apical‐basal pattern formation in the Arabidopsis embryo:Studies on the role of the gnom gene.Development 117,149–162.
Mitchison GJ(1980a)The dynamics of auxin transport.Proc.R.Soc.Lond.B Biol.Sci.209,489–511.
Mitchison GJ(1980b)A model for vein formation in higher plants.Proc.R.Soc.Lond.B Biol.Sci.207,79–109.
Mitchison GJ(1981)The polar transport of auxin and vein patterns in plants.Philos.Trans.R.Soc.Lond.B Biol.Sci.295,461–471.
Naramoto S,Sawa S,Koizumi K,Uemura T,Ueda T,Friml J,Nakano A,Fukuda H(2009)Phosphoinositide‐dependent regulation of VAN3 ARF‐GAP localization and activity essential for vascular tissue continuity in plants.Development 136,1529–1538.
Neeff F(1914)über Zellumlagerung.Ein Beitrag zur experimentellen anatomie.Zeitsch.Bot.6,465–547.
Nelson T,Dengler N(1997)Leaf vascular pattern formation.Plant Cell 9,1121–1135.
Nobécourt P(1939)Sur la pérennité et l’augmentation de volume des cultures de tissus végétaux.C.R.Soc.Biol.130,1270–1271.
Ohashi‐Ito K,Fukuda H(2010)Transcriptional regulation of vascular cell fates.Curr.Opin.Plant Biol.13,670–676.
Paciorek T,Zazimalova E,Ruthardt N,Petrasek J,Stierhof YD,Kleine‐Vehn J,Morris DA,Emans N,Jürgens G,Geldner N,Friml J(2005)Auxin inhibits endocytosis and promotes its own efflux from cells.Nature 435,1251–1256.
Petrasek J,Mravec J,Bouchard R,Blakeslee JJ,Abas M,Seifertova D,Wisniewska J,Tadele Z,Kubes M,Covanova M,Dhonukshe P,Skupa P,Benkova E,Perry L,Krecek P,Lee OR,Fink GR,Geisler M,Murphy AS,Luschnig C,Zazimalova E,Friml J(2006)PIN proteins perform a rate‐limiting function in cellular auxin efflux.Science 312,914–918.
Pray TR(1955a)Foliar venation in angiosperms.II.Histogenesis of the venation of Liriodendron.Am.J.Bot.42,18–27.
Pray TR(1955b)Foliar venation of angiosperms.IV.Histogenesis of the venation of Hosta.Am.J.Bot.42,698–706.
Przemeck GK,Mattsson J,Hardtke CS,Sung ZR,Berleth T(1996)Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization.Planta 200,229–237.
Raven JA(1975)Transport of indole acetic acid in plant cells in relation to pH and electrical potential gradients,and its significance for polar IAA transport.New Phytol.74,163–172.
Rayle DL,Ouitraku R,Hertel R(1969)Effect of auxins on auxin transport system in Coleoptiles.Planta 87,49–53.
Roberts LW,Baba S(1968)IAA‐induced xylem differentiation in the presence of colchicine.Plant Cell Physiol.9,315–321.
Rolland‐Lagan AG(2008)Vein patterning in growing leaves:Axes and polarities.Curr.Opin.Genet.Dev.18,348–353.
Rolland‐Lagan AG,Prusinkiewicz P(2005)Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis.Plant J.44,854–865.
Roth‐Nebelsick A,Uhl D,Mosbrugger V,Kerp H(2001)Evolution and function of leaf venation architecture:A review.Ann.Bot.87,553–566.
Rubery PH,Sheldrake AR(1974)Carrier‐mediated auxin transport.Planta 118,101–121.
Sachs T(1968a)On determination of pattern of vascular tissues in peas.Ann.Bot.32,781–790.
Sachs T(1968b)The role of the root in the induction of xylem differentiation in peas.Ann.Bot.32,391–399.
Sachs T(1970)A control of bud growth by vascular tissue differentiation.Israel J.Bot.19,484–498.
Sachs T(1975)Control of differentiation of vascular networks.Ann.Bot.39,197–204.
Sachs T(1981)The control of the patterned differentiation of vascular tissues.Adv.Bot.Res.9,151–262.
Sachs T(1983)Signal flow as a basis for organized differentiation.In:Oplatka A,Balaban M,eds.Biological Structures and Coupled Flows.Academic Press,New York.pp.457–471.
Sachs T(1984)Axiality and polarity in vascular plants.In:Barlow PW,Carr DJ,eds.Positional Controls in Plant Development.Cambridge University Press,Cambridge.pp.193–224.
Sachs T(1989)The development of vascular networks during leaf development.Curr.Top.Plant Biochem.Physiol.8,168–183.
Sachs T(1991a)Cell polarity and tissue patterning in plants.Development(Suppl 1),83–93.
Sachs T(1991b)Pattern Formation in Plant Tissues.Cambridge University Press,Cambridge.
Sachs T(2000)Integrating cellular and organismic aspects of vascular differentiation.Plant Cell Physiol.41,649–656.
Sachs T(2003)Collective specification of cellular development.BioEssays 25,897–903.
Sachs T(2005)Auxin’s role as an example of the mechanisms of shoot/root relations.Plant Soil 268,13–19.
Sack L,Scoffoni C(2013)Leaf venation:Structure,function,development,evolution,ecology and applications in the past,present and future.New Phytol.198,983–1000.
Sauer M,Balla J,Luschnig C,Wisniewska J,Reinohl V,Friml J,Benkova E(2006)Canalization of auxin flow by Aux/IAA‐ARF‐dependent feedback regulation of PIN polarity.Genes Dev.20,2902–2911.
Sawa S,Koizumi K,Naramoto S,Demura T,Ueda T,Nakano A,Fukuda H(2005)DRP1A is responsible for vascular continuity synergistically working with VAN3 in Arabidopsis.Plant Physiol.138,819–826.
Sawchuk MG,Head P,Donner TJ,Scarpella E(2007)Time‐lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation.New Phytol.176,560–571.
Sawchuk MG,Edgar A,Scarpella E(2013)Patterning of leaf vein networks by convergent auxin transport pathways.PLoS Genet.9,e1003294.
Scarpella E,Francis P,Berleth T(2004)Stage‐specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation.Development 131,3445–3455.
Scarpella E,Marcos D,Friml J,Berleth T(2006)Control of leaf vascular patterning by polar auxin transport.Genes Dev.20,1015–1027.
Schiavone FM,Cooke TJ(1987)Unusual patterns of somatic embryogenesis in the domesticated carrot:Developmental effects of exogenous auxins and auxin transport inhibitors.Cell Differ.21,53–62.
Scott FM,Sjaholm V,Bowler E(1960)Light and electron microscope studies of the primary xylem of Ricinus communis.Am.J.Bot.47,162–173.
Sieburth LE(1999)Auxin is required for leaf vein pattern in Arabidopsis.Plant Physiol.121,1179–1190.
Sieburth LE,Deyholos MK(2006)Vascular development:The long and winding road.Curr.Opin.Plant Biol.9,48–54.
Simon SV(1908)Experimentelle Untersuchungen über die Differenzierungsvorgänge im Callusgewebe von Holzgewächsen.Jb.Wiss.Bot.45,351–478.
Simon SV(1930)Transplantationsvercuche zwischen Solanum melongena und Iresine Lindeni.Jb.Wiss.Bot.72,137–160.
Solereder H(1908)Systematic Anatomy of the Dicotyledons.Clarendon Press,Oxford.
Steinmann T,Geldner N,Grebe M,Mangold S,Jackson CL,Paris S,Galweiler L,Palme K,Jürgens G(1999)Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF.Science 286,316–318.
Stepanova AN,Robertson‐Hoyt J,Yun J,Benavente LM,Xie DY,Dolezal K,Schlereth A,Jürgens G,Alonso JM(2008)TAA1‐mediated auxin biosynthesis is essential for hormone crosstalk and plant development.Cell 133,177–191.
Steynen QJ,Schultz EA(2003)The FORKED genes are essential for distal vein meeting in Arabidopsis.Development 130,4695–4708.
Strompen G,El Kasmi F,Richter S,Lukowitz W,Assaad FF,Jürgens G,Mayer U(2002)The Arabidopsis HINKEL gene encodes a kinesin‐related protein involved in cytokinesis and is expressed in a cell cycle‐dependent manner.Curr.Biol.12,153–158.
Sugimoto K,Jiao Y,Meyerowitz EM(2010)Arabidopsis regeneration from multiple tissues occurs via a root development pathway.Dev.Cell 18,463–471.
Thimann KV,Skoog F(1934)On the inhibition of bud development and other functions of growth substance in Vicia faba.Proc.R.Soc.Lond.B‐Biol.Sci.114,317–339.
Thomas CL,Schmidt D,Bayer EM,Dreos R,Maule AJ(2009)Arabidopsis plant homeodomain finger proteins operate downstream of auxin accumulation in specifying the vasculature and primary root meristem.Plant J.59,426–436.
Torres‐Ruiz RA,Jürgens G(1994)Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development.Development 120,2967–2978.
Turner S,Sieburth LE(2003)Vascular patterning.The Arabidopsis Book,e0073.doi:10.1199/tab.0073
Ullrich CI,Aloni R(2000)Vascularization is a general requirement for growth of plant and animal tumours.J.Exp.Bot.51,1951–1960.
Vieten A,Vanneste S,Wisniewska J,Benkova E,Benjamins R,Beeckman T,Luschnig C,Friml J(2005)Functional redundancy of PIN proteins is accompanied by auxin‐dependent cross‐regulation of PIN expression.Development 132,4521–4531.
Wabnik K,Kleine‐Vehn J,Balla J,Sauer M,Naramoto S,Reinohl V,Merks RM,Govaerts W,Friml J(2010)Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling.Mol.Syst.Biol.6,447.
Wangermann E(1974)The pathway of transport of applied indolyl‐acetic acid through internode segments.New Phytol.73,623–636.
Went FW(1928)Wuchsstoff und Wachstum.Rec.Trav.Bot.Neerland.25,1–116.
Wenzel CL,Schuetz M,Yu Q,Mattsson J(2007)Dynamics of MONOPTEROS and PIN‐FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana.Plant J.49,387–398.
White PR(1939)Potentially unlimited growth of excised plant callus in an artificial nutrient.Am.J.Bot.26,59–64.
Wisniewska J,Xu J,Seifertova D,Brewer PB,Ruzicka K,Blilou I,Rouquie D,Benkova E,Scheres B,Friml J(2006)Polar PIN localization directs auxin flow in plants.Science 312,883.
Ye ZH(2002)Vascular tissue differentiation and pattern formation in plants.Ann.Rev.Plant Biol.53,183–202.
 Journal of Integrative Plant Biology2013年9期
Journal of Integrative Plant Biology2013年9期
- Journal of Integrative Plant Biology的其它文章
- Targeting and Regulation of Cell Wall Synthesis During Tip Growth in Plants
- Cell Polarity and Development
