以对苯二甲酸及咪唑并[4,5-f][1,10]邻菲咯啉为配体的镉ギ、锌ギ配合物的构筑及晶体结构
车广波 李微微 王珊珊 王 丹 赵 晗 刘春波1,
(1吉林师范大学环境友好材料制备与应用教育部重点实验室,四平 136000)
(2吉林师范大学化学学院,四平 136000)
(3华南理工大学发光材料与器件国家重点实验室,广州 510640)
Metal-organic frameworks (MOFs),an emerging class of functional solid state materials,have provoked significant interest owing to their enormous variety of intriguing structural topologies as well as great potential applications as microporous,magnetic,nonlinear optical and fluorescent materials[1-4]. Although the design and synthesis of such materials are highly influenced by many factors,such as the coordination trend of metal centers,the nature of ligands used,metal-ligand ratio and reaction conditions etc.[5-8],the judicious selection for multifunctional organic ligands is still an effective approach to construct MOFs with unique structures and properties[9-17].Our group has explored a series of 1,10-phenanthrolines derivatives ligands,e.g.Pyphen,TTBT and dppz as rigid ligands for the construction of MOFs.In this contribution,we selected imidazo[4,5-f][1,10]phenanthroline(Imphen)as the neutral chelating ligand and 1,4-benzenedicarboxylic acid (H2bdc)as directional bridging ligand which possesses different coordination modes[18].Two complexes[Cd(bdc)(Imphen)(H2O)]n·nH2O(1)and[Zn(bdc)(Imphen)(H2O)]n·nH2O(2)have been synthesized under hydrothermal conditions.The thermal behaviors and luminescent properties of 1 and 2 have been investigated in detail.
1 Experimental
1.1 Chemicals and general methods
The ligands Imphen was synthesized according to literature method[19].All other analytical grade chemicals and solvent were purchased commercially and used without further purification.Elemental analyses of carbon,hydrogen and nitrogen were performed on a Perkin-Elmer 240C element analyzer.Thermogravimetric analysis (TGA)was performed on a TA Instruments with a heating rate of 10℃·min-1under air atmosphere.The photoluminescent behaviors of the complexes were studied using a Perkin-Elmer LS55 spectrometer.
1.2 Syntheses of complexes
Complex 1:A mixture of Cd(NO3)2·4H2O(0.154 g,0.5 mmol),H2bdc (0.083 g,0.5 mmol),Imphen(0.110 g,0.5 mmol)and water(15 mL)was sealed in a 23 mL Teflon-lined stainless autoclave,and heated at 145℃for 3 d.After cooling to room temperature,a mixture of yellow block crystals and powder were obtained.The crystals of 1 are picked out from the solid mixture in 43%yield based on Cd(NO3)2·4H2O.Anal.calcd.for 1 C21H16N4O6Cd(%):C,47.34;H,3.03;N,10.52.Found(%):C,47.45;H,3.27;N,10.48.
Complex 2:An identical procedure with 1 was followed to prepare 2 except Cd(NO3)2·4H2O was replaced by Zn(NO3)2·6H2O.Yellow crystals of complex 2 suitable for X-ray single-crystal diffraction analysis were picked out from the solid mixture in 51%yield based on Zn(NO3)2·6H2O.Anal.Calcd.for 2 C21H16N4O6Zn(%):C,51.92;H,3.32;N,11.53.Found(%):C,51.99;H,3.40;N,11.47.
1.3 Structure determination
Crystallographic data of two complexes were collected at room temperature on a Bruker SMART APEX CCD diffractometer equipped with equipped with a graphite-monochromatized Mo Kα (λ=0.071 073 nm)radiation by using an ω-2θ scan method at 292(2)K.All the structures were solved by direct methods with SHELXS-97 program[20]and refined by full-matrix least-squares techniques on F2with SHELXL 97[21].All non-hydrogen atoms were refined anisotropically and hydrogen atomsisotropically.Thecrystallographic data of the complexes are summarized in Table 1,and selected bond lengths and angles of 1 and 2 are listed in Table 2.
CCDC:947287,1;947288,2.
2 Results and discussion
2.1 Description of the crystal structure
2.1.1 Crystal structure of[Cd(bdc)(Imphen)(H2O)]n·nH2O(1)
There are one Cd2+cation,one bdc2-ligand,one Imphen ligand,one coordinated water molecule and one free water molecule in the asymmetric unit.The Cd center is six-coordinate via two nitrogen atoms(Cd-N1=0.230 5(2)nm and Cd-N2=0.231 99(19)nm)derived from a Imphen ligand and three oxygen atoms(Cd-O1=0.237 2(2)nm,Cd-O2=0.237 39(17)nm,and Cd-O3=0.219 50(17)nm) from two different bdc2-ligands,and one oxygen atom (Cd-O1W=0.228 2(2)nm)from a coordinated water molecule in a slightly distorted octahedral coordination geometry(Fig.1).Adjacent Cd atoms are bridged alternatively by one bidentate chelating bdc2-ligand (Cd-Cd 1.118 7 nm)and one monodentate bdc2-ligand(Cd-Cd 1.156 8 nm)to form a single-chain structure(Fig.2,Scheme 1a and 1b),and the Imphen ligands are attached on both sides of the 1D chain.Notably,the adjacent chains are linked through aromatic π-π interactions between Imphen ligands(six-member-ring:C4-C5-C7-C8-C12-C13 and six-member-ring:C4i-C5i-C7i-C8i-C12i-C13i,centroid-to-centroid distance ca.0.346 6 nm,i1-x,1-y,1-z,centroid coordinates-0.150 02,0.525 04,0.036 62 and 0.150 02,0.474 96,-0.036 62,dihedral angle between two planes:0.000°),and between bdc2-ligands and Imphen ligands (six-member-ring:C19-C20-C21-C19ii-C20ii-C21iiand six-member-ring:C4-C5-C7-C8-C12-C13,centroid-to-centroid distance ca.0.368 7 nm,ii2-x,1-y,1-z,centroid coordinates-0.000 00,1.000 00,1/2 and-0.349 98,1.025 04,0.463 38,dihedral angle between two planes:8.716°)(Fig.3).In addition,the O-H … O and N-H … O hydrogen bonds (Table 3)involving the water
molecules,the carboxylic groups of bdc2-anions and Imphen ligands further extend the 1D chains into an interesting 3D supramolecular structure.
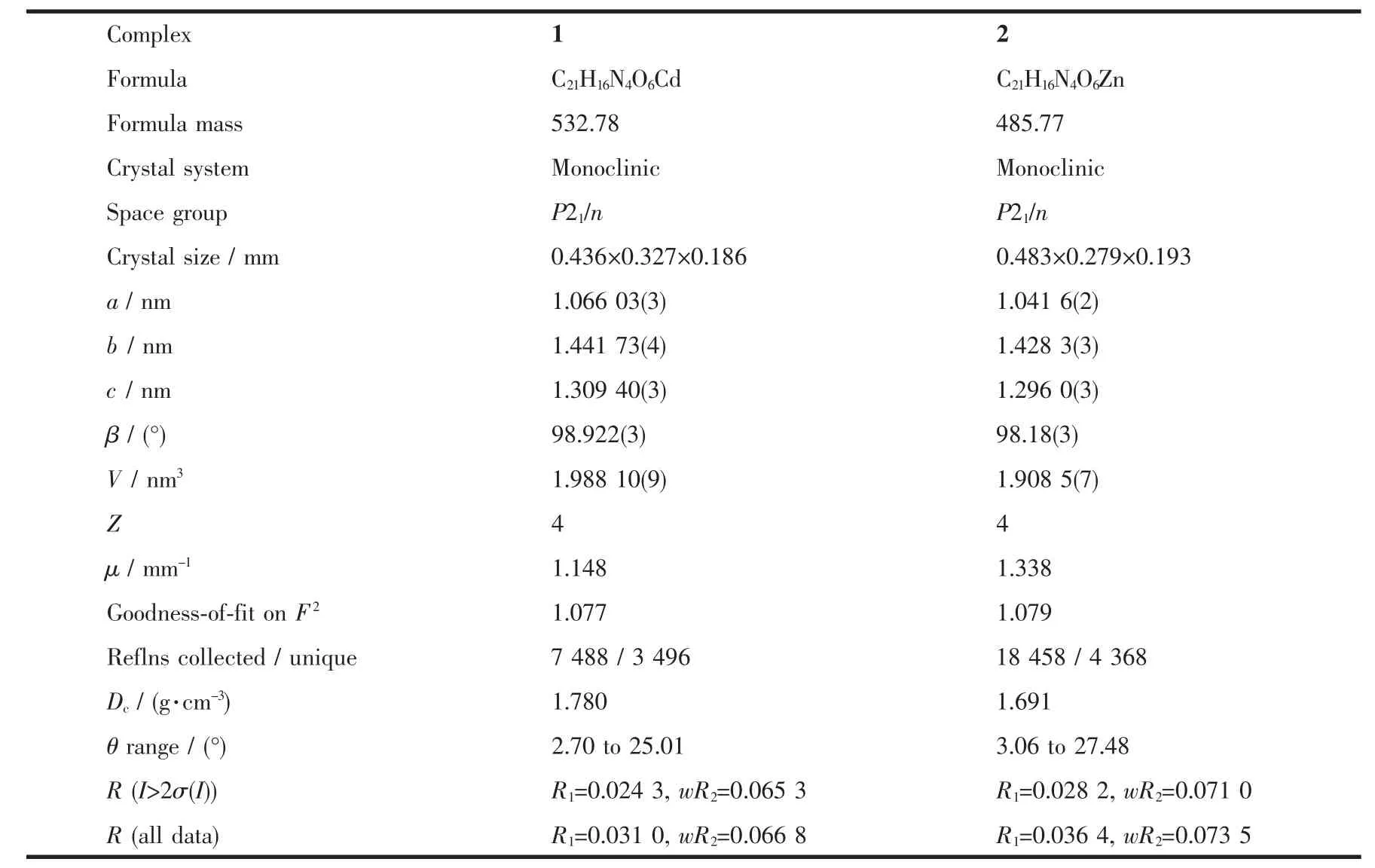
Table 1 Crystallographic data for 1 and 2
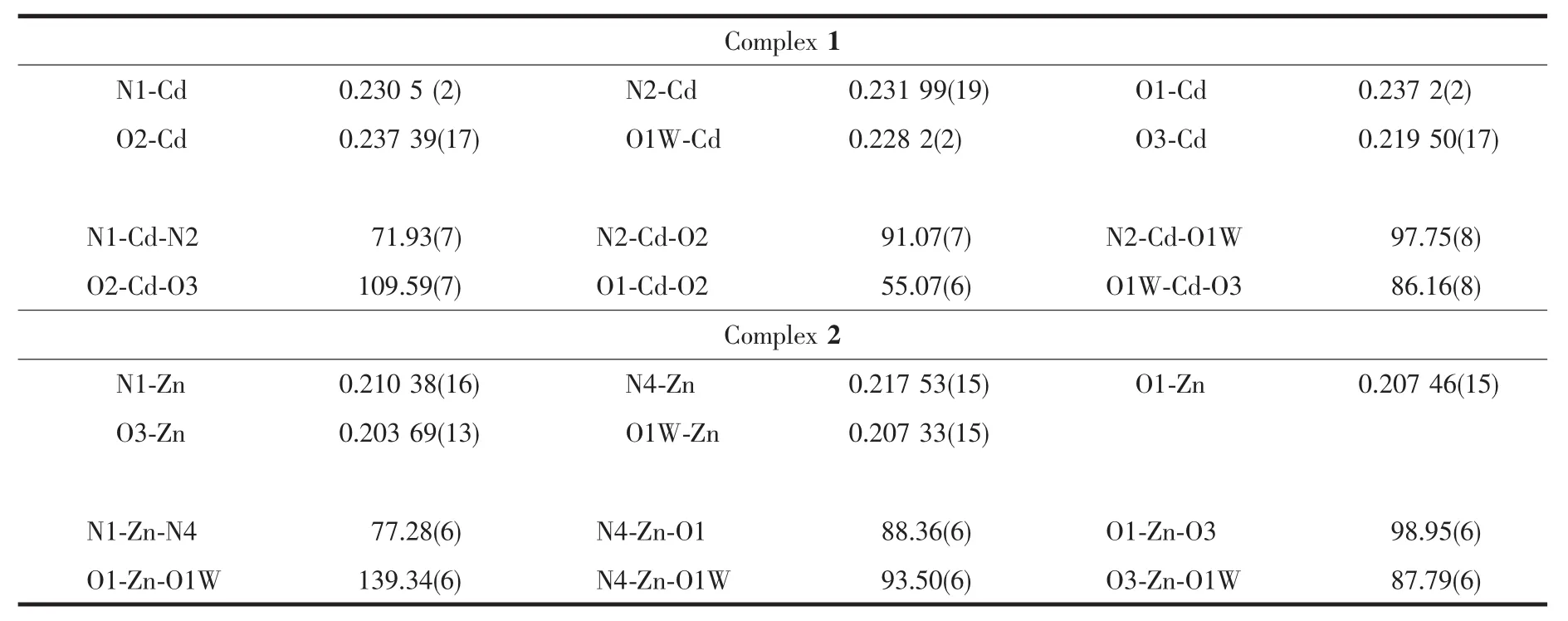
Table 2 Selected bond lengths(nm)and bond angles(°)
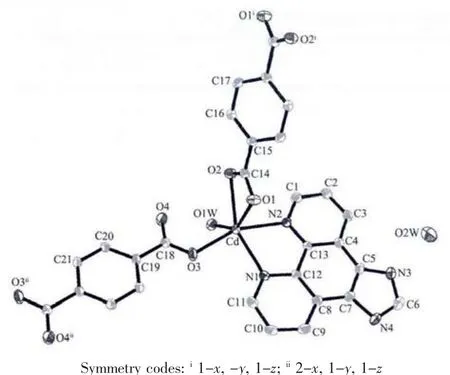
Fig.1 Crystal structure of complex 1
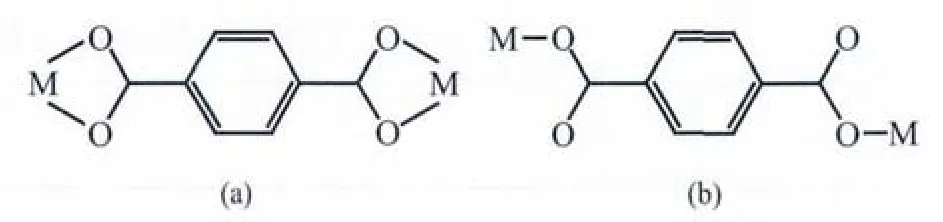
Scheme 1 Coordination modes of H2bdc ligand in complexes 1 and 2
2.1.2 Crystal structure of[Zn(bdc)(Imphen)(H2O)]n·nH2O(2)
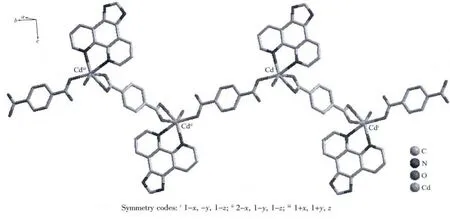
Fig.2 One-dimensional chain structure of 1
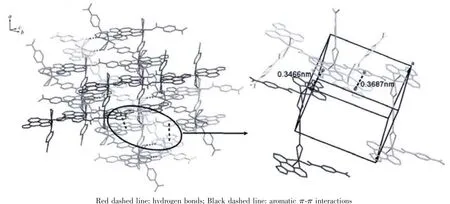
Fig.3 3D supramolecular network of 1
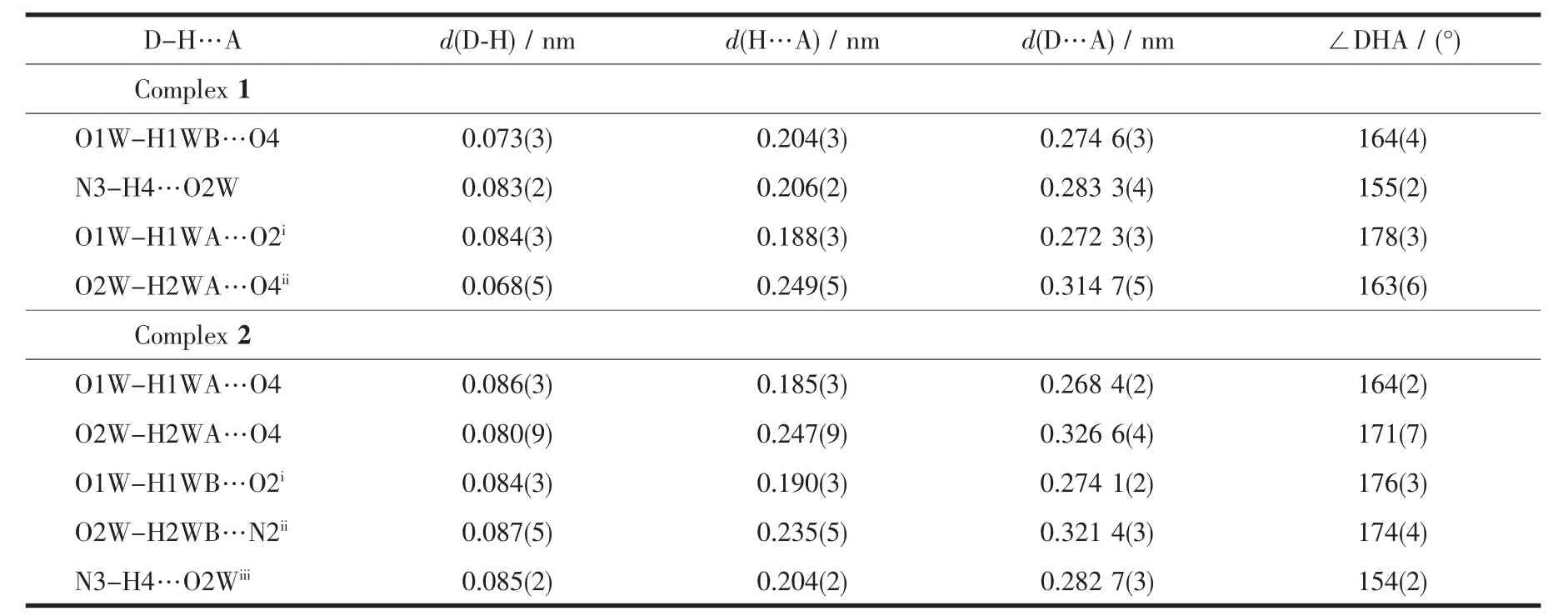
Table 3 Hydrogen bond lengths and bond angles
X-ray analysis revealed that the asymmetric unit of 2 contains one Zn atom,one bdc2-ligand,one Imphen ligand,one coordinated water molecule and one free water molecule.As is shown in Fig.4,each Zn atom is five coordinated with two nitrogen atoms of one Imphen ligand(Zn-N1 0.210 38(16)nm and Zn-N4 0.217 53(15)nm),two oxygen atoms from two bdc2-ligands (Zn-O1=0.207 46(15)nm and Zn-O3 0.203 69(13)nm)and one oxygen atom from water molecule(Zn-O1W 0.207 33(15)nm).The Zn atoms are linked together by monodentate bdc2-ligands to form a one-dimensional single chain structure(Fig.5,Scheme 1b).One-dimensional chains were connected together by two types of π-π interactions:one is between Imphen ligands(six-member-ring:C4-C5-C7-C8-C12-C13 and six-member-ring:C4i-C5i-C7i-C8i-C12i-C13i,centroid-to-centroid distance ca.0.346 8 nm,i-x,1-y,-z,centroid coordinates-0.150 02,0.525 04,0.036 62 and 0.150 02,0.474 96,-0.036 62,dihedral angle between two planes:0.000°),and another is between bdc2-and Imphen ligand(six-member-ring:C19-C20-C21-C19ii-C20ii-C21iiand six-member-ring:C4-C5-C7-C8-C12-C13,centroid-to-centroid distance ca.0.362 6 nm,ii-1+x,y,-1+z,centroid coordinates-0.000 00,1.000 00,1/2 and-0.349 98,1.025 04,0.463 38,dihedral angle between two planes:8.530°),as shown in Fig.6.Furthermore,the O-H…O,O-H…N and N-H…O hydrogen-bond interactions between the coordinated water molecules,free water molecules or-NH-groups and carboxylate O atoms or N atoms of Imphen ligands lead to the construction of a stable 3D network(Table 3).
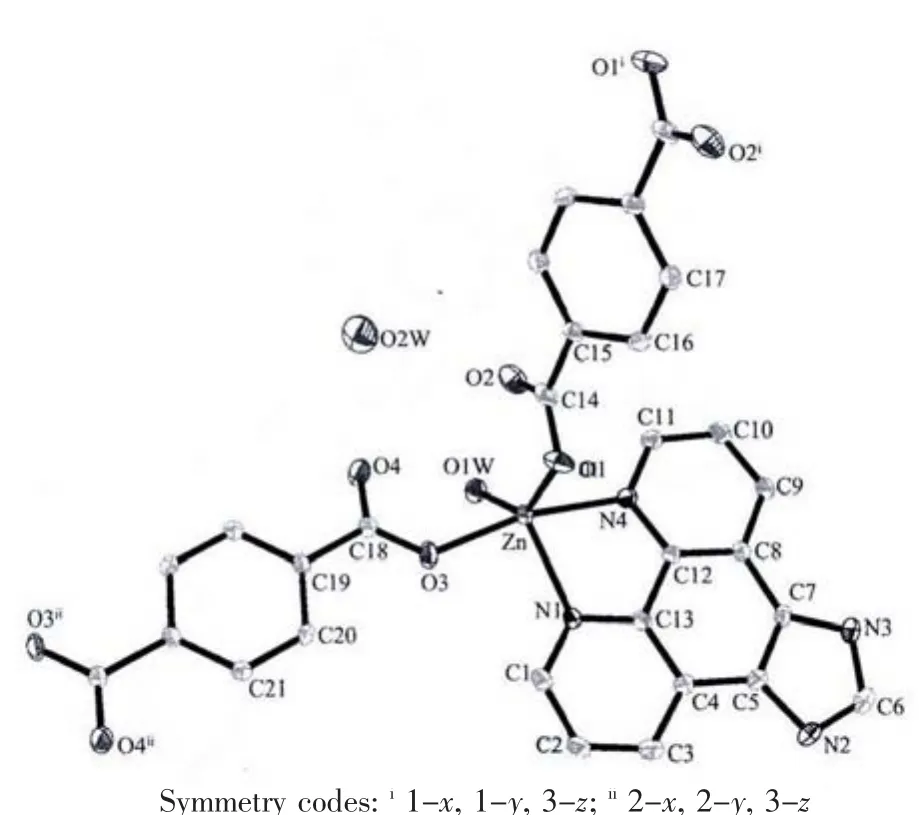
Fig.4 Crystal structure of complex 2
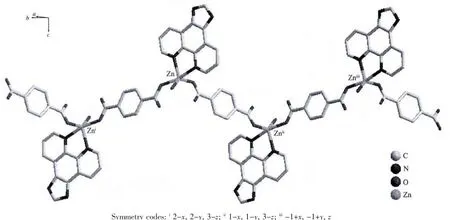
Fig.5 One-dimensional chain structure of complex 2
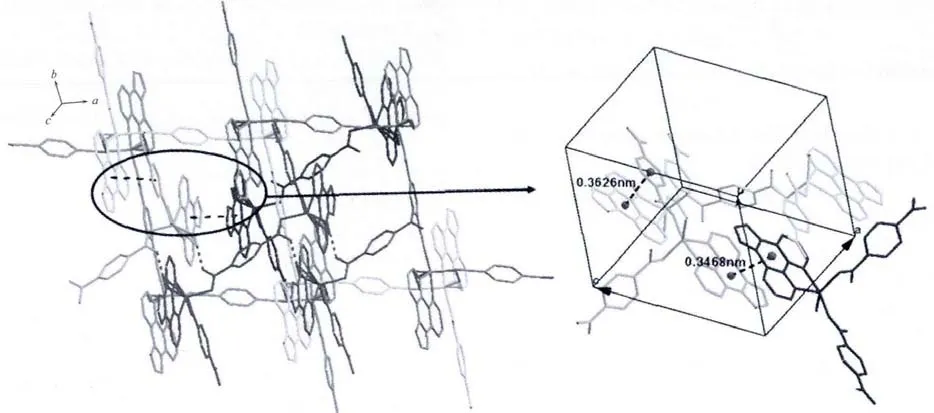
Fig.6 3D supramolecular network of 2
2.2 TGA
Although the coordination models of two complexes are slightly different,TGA curves of 1 and 2 are similar.So 1 represents 2 for detailed TGA.Three distinct weight losses were observed for 1.The first weight loss of 3.40%is in the range 80~110 ℃,assigned to the release of the free water molecules(Calcd.3.38%).The second weight loss of 3.45%is in the range 120~220 ℃,assigned to the loss of the coordinated water molecules(Calcd.3.38%).The third sharp weight loss of 71.80%is ascribable to the decomposition of organic ligands bdc2-and Imphen(Calcd.72.14%).The final product may be CdO.
2.3 Photoluminescence properties
It is well known that the d10metal coordination polymers have interesting photoluminescent properties.The solid-state luminescence spectra of 1 and 2 at room temperature are determined (Fig.7).Complex 1 and 2 exhibit strong luminescence peaks at 506 and 531 nm upon excitation at 365 nm,respectively.In order to understand the nature of these emission bands,the luminescent properties of free ligands H2bdc and Imphen were analyzed,showing they exhibit the strongest emission peaks at about 390[22]and 460 nm[23]from 300 to 720 nm,respectively.Compared with the luminescent spectra of free ligand,the emission spectra of two complexes are obviously similar to that of Imphen,which might be ascribable to chelating coordination of the planar Imphen ligand in both complexes.The photoluminescent emissions of 1 and 2 also mainly originate from the intraligand fluorescent emissions of Imphen[24].
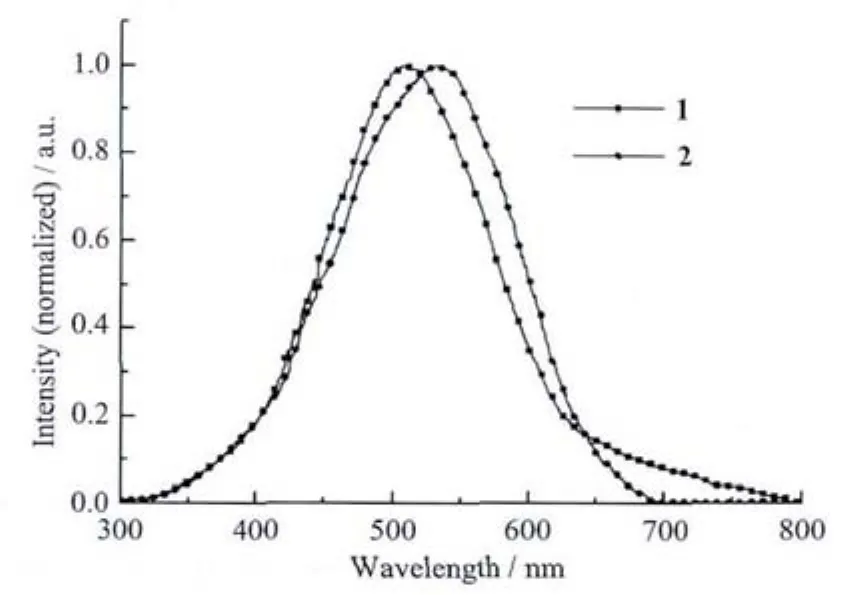
Fig.7 Photoluminescent spectra of complex 1 and 2
[1]Liao P Q,Zhou D D,Zhu A X,et al.J.Am.Chem.Soc.,2012,134(42):17380-17383
[2]Wang G H,Lei Y Q,Wang N,et al.Cryst.Growth Des.,2010,10(2):534-540
[3]Wang Y T,Tang G M,Wei Y Q,et al.Cryst.Growth Des.,2009,10(1):25-28
[4]Wang R M,Zhang J,Li L J.Inorg.Chem.,2009,48(15):7194-7200
[5]Awaleh M O,Badia A,Brisse F.Cryst.Growth Des.,2006,6(12):2674-2685
[6]Xie Y B,Li J R,Zhang C,et al.Cryst.Growth Des.,2005,5(5):1743-1749
[7]Hu S,Meng Z S,Tong M L.Cryst.Growth Des.,2010,10(4):1742-1748
[8]Sun Z H,Zhang G H,Wang X Q,et al.Cryst.Growth Des.,2009,9(7):3251-3259
[9]Che G B,Liu C B,Liu B,et al.CrystEngComm,2008,10:184-191
[10]Zhou Q,Yang F,Liu D,et al.Inorg.Chem.,2012,51(14):7529-7536
[11]Liu C B,Wang S S,Che G B,et al.Inorg.Chem.Commun.,2013,27:69-75
[12]He J,Zhang J X,Tan G P,et al.Cryst.Growth Des.,2007,7:1508-1513
[13]Che G B,Liu C B,Wang L,et al.J.Coord.Chem.,2007,60:1997-2007
[14]Han Y F,Li X Y,Li L Q,et al.Inorg.Chem.,2010,49(23):10781-10787
[15]Li L N,Wang S Y,Chen T L,et al.Cryst.Growth Des.,2012,12(8):4109-4115
[16]Li S L,Lan Y Q,Ma J C,et al.Cryst.Growth Des.,2010,10(3):1161-1170
[17]WANG Qian-Qian(王倩倩),LIU Chun-Bo(刘春波),LI Xiu-Ying(李秀颖),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(3):619-625
[18]Yi X Y,Ying Y,Fang H C,et al.Inorg.Chem.Commun.,2011,14:453-457
[19]Che G B,Li W L,Kong Z G,et al.Synth.Commun.,2006,36:2519-2524
[20]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Göttingen,1997.
[21]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of Göttingen,1997.
[22]Chen W,Wang J Y,Chen C,et al.Inorg.Chem.,2003,42:944-946
[23]Liu J Q,Zhang Y N,Wang Y Y,et al.Dalton Trans.,2009:5365-5378
[24]Li X Y,Liu C B,Che G B,et al.Inorg.Chim.Acta,2010,363:1359-1366

