Clinicopathologic characteristics and prognostic factors of 63 gastric cancer patients with metachronous ovarian metastasis
Qiang Feng, Wei Pei, Zhao-Xu Zheng, Jian-Jun Bi, Xing-Hua Yuan
Department of Abdominal Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100021, China
Introduction
Gastric cancer is one of the most common malignant neoplasms.Although the diagnosis and treatment have improved significantly in recent years, about 50% of the patients recrudesce and develop distant metastasis after treatment.Ovaries are the common metastasis site for many malignant neoplasms.The incidence rate of postoperative ovarian metastasis of female gastric cancer patients ranges from 2.7% to 6.7%1,2.However, autopsy results show that the incidence rate of ovarian metastasis ranges from 33% to 41%3,4.Currently, effective treatment methods for gastric cancer ovarian metastasis are lacking.In addition,research on metachronous ovarian metastasis after gastric cancer treatment is insufficient.This study aims to discuss further the clinicopathologic characteristics, treatment methods, and prognosis through retrospective analysis of the clinical data of gastric cancer patients with ovarian metastasis treated in our hospital.
Patients and methods
Clinical data
From January 1999 to December 2011, 1,856 female gastric cancer patients were admitted and treated at the Chinese Academy of Medical Science Tumor Hospital.Among these patients, 63 patients 21 to 70 years old (mean age: 45 years old)were histopathologically proven to have metachronous ovarian metastasis by secondary surgery.Among these 63 patients, 9 were primary gastric cancer patients that had excision in other hospitals with the consultation result borrowed from other hospitals’ tissue sample as histopathologic files, and 54 had their histopathologic diagnosis of surgical samples conducted in our hospital.These patients were grouped according to primary gastric cancer lesion:gastric sinus (40 cases), gastric body (18 cases), and cardia and fundus (5 cases).They were also classified according to their ovarian metastasis neoplasms: unilateral (9 cases) and bilateral(54 cases).The common clinical manifestations were hypogastric pain, abdominal mass, irregular vaginal bleeding, and so on.
Diagnostic methods
The main diagnostic methods include gastroscopy, B ultrasound,computed tomography (CT), nuclear magnetic resonance(NMR), and so on.The tumor marker examination for neoplasms includes CEA, CA199, CA724, and CA125.All patients of this group received treatment for gastric cancer and ovarian metastasis.For primary cancer, 43 cases underwent distal subtotal gastrectomy, 4 underwent proximal subtotal gastrectomy, and 16 underwent total gastrectomy.Ovarian metastatic neoplasms: 20 cases underwent total lesion excision as per the extent of disease and 43 cases underwent palliative excision because of the wide metastasis in peritoneal membrane.Among the cases, 9 cases underwent unilateral excision, 54 cases underwent bilateral excision, 21 cases underwent uterus combination excision, and 3 cases underwent limited peritoneal membrane metastasis combination excision.Auxiliary treatment includes chemotherapy after ovarian metastasis cancer surgery.The chemotherapy medicine mainly includes fluorouracil,oxaliplatin/cisplatin, calcium folinate, docetaxel, and so on.
Follow-up
All the cases in this group underwent follow-up visits mainly via outpatient review, telephone, follow-up mail, and so on.The recorded survival time is from diagnosed ovarian metastasis until death or until final follow-up.
Statistical analysis
All data were statistically analyzed with SPSS 17.0 software statistical package.The survival rate was calculated with the Kaplan-Meier method, and the Log-rank test was conducted to analyze survival rate difference among groups.Cox regression model was applied for multi-factor prognosis analysis.Differences with P<0.05 were considered statistically significant.
Results
Clinical and histopathologic characteristics
In this study, 1,856 female gastric cancer patients underwent excision, among which 63 (3.4%) developed metachronous ovarian metastasis.The interval to metastasis was 2 months to 20 years after gastric cancer surgery, with an average of 16 months.Up to 46 patients were in their 50s (73.0%), and 41 patients (65.1%) were pre-menopausal.Ovarian metastasis patients had corresponding symptoms after primary cancer surgery or de finitive diagnosis after regular review with imaging tests.In this group, imaging results showed that 18 cases with metastatic ovarian lesions were cystic or cystic and solid disease, among which 6 (9.5%) were diagnosed as physiologic enlargement by mistake during B ultrasound/CT regular review in the early period after gastric cancer surgery.Up to 39 patients had corresponding serum tumor marker check, among which 7 (18%) were CEA positive, 15 (38.5%) were CA199 positive,9 (23.1%) were CA724 positive, and 24 (61.5%) were CA125 positive.Any one of the previous three markers was increased at the same time with CA125 in 20 cases (51.3%).A total of 11 cases (28.2%) were negative for all markers.
The histopathologic result showed that primary gastric cancer was mainly poorly differentiated.Among the 63 patients,53 (84.1%) had primary gastric carcinoma, 55 (87.3%) had invaded serosa, and 43 (63.8%) had N2-3lymph node metastasis.The patients showed different histopathologic types of ovarian metastasis: gastric signet ring cell carcinoma (38 cases), tubular adenocarcinoma (21 cases), and mucinous adenocarcinoma(4 cases).Unilateral metastasis was found in 9 cases, and bilateral metastasis was found in 54 cases.The diameter of neoplasms ranged from 2 to 20 cm, with an average diameter of 8.4 cm.Up to 19 patients had neoplasm diameter >10 cm.Vascular tumors were found in 28 cases, and metastatic peritoneal seeding was found in 46 cases.
Follow-up
The follow-up period was from January 1999 to October 2012.The overall median survival period was 13.6 months, with 49 mortalities.The 1-, 2-, and 3-year survival rates were 52.5%,22.0%, and 9.8%, respectively.The 5-year survival rate was zero.
In fluence on prognostic factors
The single-factor survival analysis showed that peritoneal seeding,metastatic lesion, vascular tumor emboli, lesion excision range,and the treatment mode of auxiliary chemotherapy influenced patient prognosis (P<0.05).Age, menstrual history, gastric cancer lymph node metastasis, the interval between metastasis lesion and primary lesion, size of metastasis, metastasis site, and metastatic histopathologic type had no significant relationship with prognosis (Table 1).The Cox regression model multi-factor analysis result showed that metastatic peritoneal seeding was the determining factor that influenced the prognosis of gastric cancer patients with ovarian metastasis.Compared with gastric cancer ovarian metastasis patients with metastatic peritoneal seeding,those without metastatic peritoneal seeding had significant extended survival period (P<0.01, Table 2 and Figure 1).
Discussion
The ovarian metastatic tumor from gastric cancer is also called Krukenberg tumor, which was first reported by the German pathologist Friedrich Ernst Krukenberg in 1896.The reports on the incidence of gastric cancer ovarian metastasis are quite different.For 12 years, the incidence rate of metachronous ovarian metastasis was 3.4% in this research.Given that the research excluded from synchronous metastasis and non-surgical cases, the actual incidence of ovarian metastasis should be higher than the statistical result.
The pathogenesis of ovarian metastasis is unclear.Generally,there are three possible mechanisms: lymph node metastasis,hematogenous metastasis, and seeding metastasis.According to research, the ovarian reticular lymphatic tissue is rich and the cancer cells can pass to the waist lymph node through the retroperitoneal lymph node and paradoxic metastasis to the ovary, which is considered the most likely transfer method5.Kim et al.2performed a multi-factor analysis on 690 female gastric cancer patients and showed that the incidence of ovarian metastasis is closely related to the extent of gastric cancer lymph node metastasis.Patients with metastases to more than six lymph nodes are more likely to have ovarian metastasis.In this group, the number of patients with N2-3primary tumor lymph node metastasis (68.3%) was significantly higher than those without lymph node metastasis.Yamanishi et al.6applied immunohistochemical staining and found that 57.0% of patients with ovarian metastasis have lymphatic involvement,which proves that ovarian metastases occur through lymph.Recently, hematogenous metastasis is receiving increasing support.Active premenopausal ovaries provide a more suitable growing environment for metastatic tumors because of their high hormone levels and rich blood supply.Therefore,young premenopausal patients are more likely to have ovarian metastasis7.When gastric cancer invades serous membranes, the cancer cells enter into the abdominal cavity and into the ovaries through the split holes formed during ovulation, becoming seeding metastasis.Seeding metastasis often spreads widely on the peritoneum and leads to unfavorable prognosis8.Most of the patients developed ovarian metastasis via this mechanism.Combination peritoneal seeding spread was observed in 73.0%of the patients.This finding was also the main reason for the unfavorable prognosis of this group.In fact, multiple metastases might coexist because the primary gastric cancers are mostly in the progressive stage.
Research has shown that metastatic tumors are often large,about 9 cm in diameter on average, whereas primary ovarian tumors are usually small9.The average tumor diameter among the patients was 8.4 cm, with 30.2% (19/63) larger than 10 cm.This result demonstrates that early ovarian metastasis is complicated.Koyama et al.10reported that B ultrasound,CT, MR, and other examinations could reveal asymptomatic metastatic lesions during the early stage.For solid, lobulated, and contrast-enhanced ovarian lesions, the possibility for metastasis should always be considered.However, 18 (28.6%) of the 63 patients of this group had cystic or solid and cystic disease, as determined via B ultrasound and CT examination.Six cases were erroneously diagnosed as physiologic enlargement of the ovaries during the early stage of metastasis; these patients did not receive treatment in time.Therefore, we should pay attention to abnormal ovarian enlargement regardless of whether they are cystic or solid.Yada-Hashimoto et al.4believed that a rapid increase in CA125 could be considered an auxiliary marker of ovarian tumors and Krukenberg tumors in the early stage.In this group, most patients were only tested for gastrointestinal tumor markers (CEA, CA199, or CA724) after gastric cancer surgery.Up to 39 cases were tested for CA125 when the ovarian lesion was found, and the results showed that CA125 increased to 61.5%, higher than the positive rate of any one marker.The CA125 test was indicative of Krukenberg tumor.Therefore, thesurgeons should routinely test CA125 during the postoperative review of female gastric cancer patients.
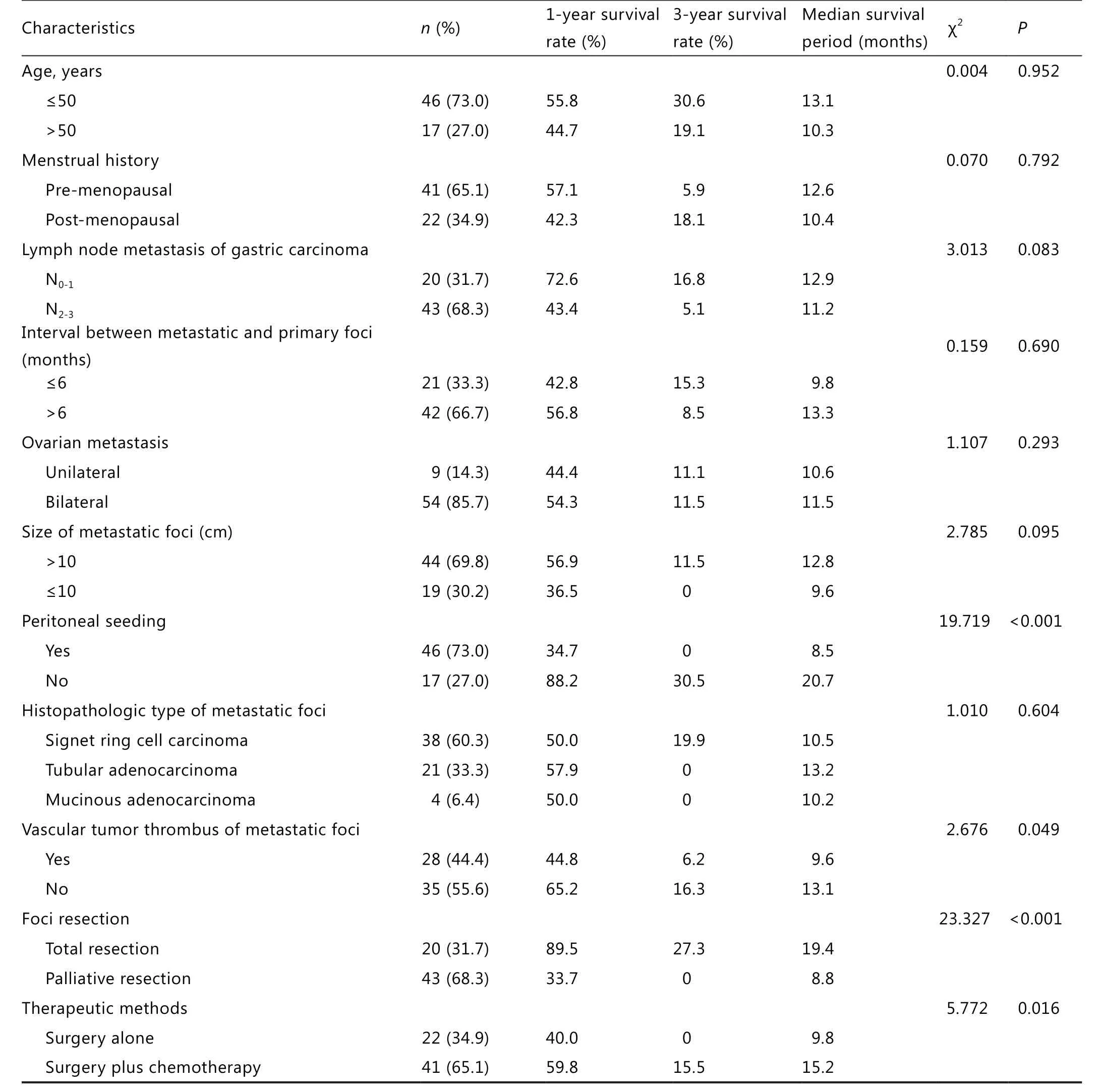
Table 1 Univariate analysis of prognostic factors for gastric cancer patients with ovarian metastasis
The ovarian metastasis of gastric cancer is in the IV period,with unfavorable prognosis.Chemotherapy was previously recommended over surgical treatment.One of the main reasons for the unfavorable prognosis is the wide seeding metastasis of abdominal and pelvic peritoneum11,12.In recent years, with the improvement of diagnostic technology and the perfection of postoperative follow-up examination systems, some patients have been diagnosed during the early stage of ovarian metastasis,thereby extending the survival period through tumor excision.Jun et al.13showed that the average survival period of gastriccancer patients with ovarian metastasis after secondary surgery is 18.8 months, among which the survival period of patients who underwent total excision of the metastatic lesion can reach 23.7 months.Early diagnosis and complete metastatic lesion excision can improve the survival rate of these patients.Cheong et al.14analyzed clinical data from 34 ovarian metastasis cases.The result showed that patients who underwent total excision of the metastatic lesion had better prognosis than patients with residual tumors.Single-factor analysis of this group showed that patients who underwent total lesion excision had significantly longer median survival time compared with patients who underwent palliative lesion excision.This result was related with prognosis.However, multi-factor analysis showed that the lesion excision range did not influence the independent risk factors of prognosis.

Table 2 Multi-factorial analysis of prognostic factors for gastric cancer patients with ovarian metastasis

Figure 1 Survival curves for patients with/without metastatic peritoneal seeding.
The survival period of the patients in this group was 13.6 months.The 1-, 2-, and 3-year survival rates were 52.5%, 22.0%,and 9.8%, respectively, and the prognosis was unfavorable.The main reason was that most patients also developed metastatic peritoneal seeding, and only 32% of the patients underwent complete lesion excision.Multi-factor analysis showed that metastatic peritoneal seeding is the only independent risk factor that influenced patient prognosis in this group.Most studies7,12showed that the tumor metastatic range and whether the tumor was resectable via radical excision were the main factors that influence prognosis.This report indicates that if the patients developed widespread peritoneal seeding metastasis simultaneously, surgery would not improve the prognosis.Jacquet et al.15found that the accuracy of CT for the preoperative diagnosis of metastatic peritoneal seeding was only 50%.In recent years, laparoscopic exploration has been widely used in evaluating tumor resectability during the progressive stage.Performing ancillary imaging tests will more accurately identify ovarian metastasis patients12,16who are suitable for surgical excision.At present, effective treatment methods for metastatic peritoneal spread are lacking.More reports about cytoreductive surgery for colorectal cancer patients have proven that it can effectively extend the survival time.However, reports about cytoreductive surgery for gastric cancer patients are insufficient.Bozzetti et al.17showed that the median survival period was only 8 months to 11 months, with a surgical death rate of 2% to 7.1%.Recent studies18have shown that hyperthermic intraperitoneal chemotherapy can treat metastatic peritoneal seeding and that its combination with cytoreductive surgery improves the survival rate of some peritoneal metastasis patients.
In summary, premenopausal female patients with gastric cancer should be vigilant about recurrent ovarian metastasis after surgery.Besides imaging tests, CA125 should be considered a routine marker after surgery for early diagnosis and subsequent treatment.In addition, prevention and effective treatment of widespread metastatic peritoneal seeding are the key points for improving patient survival.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Kobayashi O, Sugiyama Y, Cho H, Tsuburaya A, Sairenji M,Motohashi H, et al.Clinical and pathological study of gastric cancer with ovarian metastasis.Int J Clin Oncol 2003;8:67-71.
2.Kim NK, Kim HK, Park BJ, Kim MS, Kim YI, Heo DS, et al.Risk factors for ovarian metastasis following curative resection of gastric adenocarcinoma.Cancer 1999;85:1490-1499.
3.Wang J, Shi YK, Wu LY, Wang JW, Yang S, Yang JL, et al.Prognostic factors for ovarian metastases from primary gastric cancer.Int J Gynecol Cancer 2008;18:825-832.
4.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H,Sawai K, et al.Metastatic ovarian tumors: a review of 64 cases.Gynecol Oncol 2003;89:314-317.
5.Al-Agha OM, Nicastri AD.An in-depth look at Krukenberg tumor:an overview.Arch Pathol Lab Med 2006;130:1725-1730.
6.Yamanishi Y, Koshiyama M, Ohnaka M, Ueda M, Ukita S,Hishikawa K, et al.Pathways of metas- tases from primary organs to the ovaries.Obstet Gynecol Int 2011;2011:612817.
7.Yook JH, Oh ST, Kim BS.Clinical prognostic factors for ovarian metastasis in women with gastric cancer.Hepatogastroenterology 2007;54:955-959.
8.Sodek KL, Murphy KJ, Brown TJ, Ringuette MJ.Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis.Cancer Metastasis Rev 2012;31:397-414.
9.Kobayashi O, Sugiyama Y, Cho H, Tsuburaya A, Sairenji M,Motohashi H, et al.Clinical and pathological study of gastric cancer with ovarian metastasis.Int J Clin Oncol 2003;8:67-71.
10.Koyama T, Mikami Y, Saga T, Tamai K, Togashi K.Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation.Abdom Imaging 2007;32:784-795.
11.Sharma K, Rathi AK, Sood G, Malhotra A, Kumar V, Bahadur AK,et al.Metastatic ovarian tumour in patient of carcinoma stomach.Trop Gastroenterol 2008;29:112-113.
12.Jiang R, Tang J, Cheng X, Zang RY.Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors.Eur J Surg Oncol 2009;35:92-97.
13.Jun SY, Park JK.Metachronous ovarian metastases following resection of the primary gastric cancer.J Gastric Cancer 2011;11:31-37.
14.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH.Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer.J Surg Oncol 2004;87:39-45.
15.Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH.Evaluation of computed tomography in patients with peritoneal carcinomatosis.Cancer 1993;72:1631-1636.
16.Shim JH, Yoo HM, Lee HH, Kim JG, Jeon HM, Song KY, et al.Use of laparoscopy as an alternative to computed tomography (CT)and positron emission tomography (PET) scans for the detection of recurrence in patients with gastric cancer: a pilot study.Surg Endosc 2011;25:3338-3344.
17.Bozzetti F, Yu W, Baratti D, Kusamura S, Deraco M.Locoregional treatment of peritoneal carcinomatosis from gastric cancer.J Surg Oncol 2008;98:273-276.
18.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al.Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgerycombined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients.Cancer 2010;116:5608-5618.
 Cancer Biology & Medicine2013年2期
Cancer Biology & Medicine2013年2期
- Cancer Biology & Medicine的其它文章
- Modi fied frontolateral partial laryngectomy operation:combined muscle-pedicle hyoid bone and thyrohyoid membrane flap in laryngeal reconstruction
- A survey and evaluation of population-based screening for gastric cancer
- Research progress in the radioprotective effect of the canonical Wnt pathway
- Preoperative intestinal stent decompression with primary laparoscopic surgery to treat left-sided colorectal cancer with obstruction: a report of 21 cases
- Erratum to research development of the relationship between thymidine phosphorylase expression and colorectal carcinoma
