手性配体2,5-二(4,5-蒎烯-2-吡啶)吡嗪及其铼配合物的合成与表征
李书静 张小朋 刘 建 郑 玮 李承辉*,
(1南京大学化学化工学院,配位化学国家重点实验室,南京微结构国家实验室,南京 210093)(2周口师范学院化学系,周口 466000)
Recently,the investigation of chiral coordination compounds has been a popular topic owing to their intriguing architectures and topologies,as well as potential applications in asymmetric catalysis,enantioselective separations,biomimetic chemistry,nonlinear optical materials,and ferroelectric materials[1-7].Compared to chiral organic compounds which have been intensively investigated several decades ago,the chirality of coordination compounds is much more complex due to the various possible coordination geometries of metal centers,which lead to an enormous number of possible configurations in coordination complexes and make stereoselective synthesis of coordination species a formidable task.The classical method for the preparation of an enantiopure substance is the separation of a racemate.Obviously,this method is only practicable if the number of isomers is rather less,and even then it is often of limited efficiency,and less yield.A more straightforward and effective strategy to construct chiral coordination complexes is using an optically pure chiral organic ligand as a linker to connect adjacent metal centers. In this way, a predetermination of the absolute configuration at the metal center can be reached[8-11].
Pinene is a rigid,bulky group and has been widely used as chiral source in synthesizing chiral compounds[12-14].Meanwhile,the combination of pinene with pyridine moiety leads to ideal binding ligands toward various metals.Some of them have been widely used in self-assembly and stepwise complexation reactions,giving rise to racemic or configurationally undefined products.For example,Rich etal[15]described a series of mononuclear and dinuclear chiral manganese(Ⅱ)complexes containing the neutral bidentate chiral nitrogen ligand (-)-pinene[5,6]bipyridine,which showed good selectivities and moderate enantioselectivities in the epoxidation of aromatic alkenes with peracetic acid.Mamula and coworks[16]reported a trinuclear Euバcomplex formed by chiral bipyridine-carboxylate ligand,which displayed a very interesting mode of helical chirality that originated from the propeller-like arrangement of the ligands around the trinuclear metal core.
Due to the tunable photophysical properities and good chemical stability,Reガcomplexes have attracted more and more attention,and some of them have been used for solar-energy conversion,luminescent biolabeling,and DNA probes.Yi et al[17]studied the photophysical properties of rheniumガ tricarbonyl polypyridine complexes and observed an upconversion quantum yield up to 17.0%.Oriskovich et al[18]reported that the complex Re(bpy)(CO)3(EtG)+could be used as luminescent labels for purine nucleobases.Esteves et al[19]investigated the interaction of tricarbonyl rheniumガcomplexes with calf thymus DNA(CTDNA).However,the investigation of structures and properties of chiral rheniumガ complexes is still limited.Yeung et al[20]synthesized a series of singlestranded helical Reガ complexes by reacting[Re(CO)5Br]with chiral pinene-containing quaterpyridine ligands,in which the chiral information was transmitted from the ligand,through the metal,to the helix,just like in many biomolecules such as R-helix and single-stranded RNA.In this work,we used the 2,5-bis(4,5-pinene-2-pyridyl)pyrazine(L)to construct the organic-inorganic compound[Re(CO)3Cl(L)]·DMF(Scheme 1).The structure of the Reガ complex was characterized by single crystal X-ray diffraction.The UV,PL,CD and VCD spectra,and non-linear optical properties of the ligand and the Reガcomplex were investigated and compared.
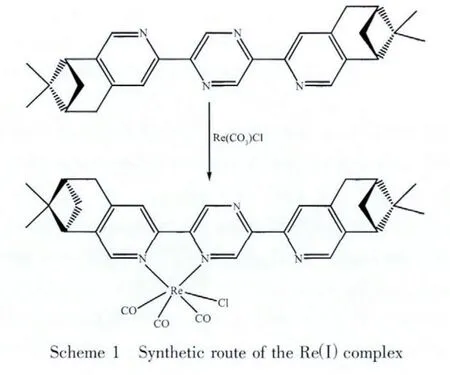
1 Experimental
1.1 General
Organic reagents and Re(CO)3Cl were purchased from Sigma-Aldrich and used as received.Elemental analysis for C,H and N were performed on a Perkin-Elmer 240C analyzer.Solid infrared spectra were recorded on a Vector22 Bruker Spectrophotometer with KBr pellets in the 400~4 000 cm-1region.UVVis absorption spectra were recorded on Shimadzu UV-2700 Spectrophotometer using dichloromethane solutions.Luminescence spectra were recorded in dichloromethane solution on HITACHI F-4600 Fluorescence Spectrophotometer.
1.2 Synthesis
The ligand 2,5-bis(4,5-pinene-2-pyridyl)pyrazine was prepared according to the literature method[12],and red crystals suitable for X-ray structure analysis were obtained by the recrystallization in acetonitrile and dimethylformamide.IR (KBr)/cm-1:3 432(m),2 929(s),1 600(m),1 556(m),1 466(s),1 426(m),1 385(m),1 325(m),1 255(m),1 168(m),1 068(m),1 020(m),947(m),910(m),880(m).
The rheniumガcomplex was prepared by addition of the ligand 2,5-bis(4,5-pinene-2-pyridyl)pyrazine to[Re(CO)3Cl]in a 1.5∶1 molar ratio in methanol under reflux conditions for 12 h.Following removal of the solvent in vacuo,the residue was extracted with dichloromethane and purified by chromatography(silica gel,ethyl acrylate-dichloromethane 1∶1).Orange rod-shaped crystalssuitable for X-ray structure analysis were obtained by evaporation of a concentrated solution of the complex in dichloromethane and dimethylformamide.Anal.calcd for C34H37N5O4ClRe(%):C,50.96;H,4.65;N,8.74.Found(%):C,51.01;H,4.77;N,8.69.IR(KBr)/cm-1:3 447(m),2 933(m),2 020(s),1 918(s),1 895(s),1 468(m),1 177(m).
1.3 X-ray crystallography
The crystal structures of the ligand 2,5-bis(4,5-pinene-2-pyridyl)pyrazine and Reガcomplex have been solved by X-ray diffraction analysis.Their main crystallographic data are summarized in Table 1.Intensity data were collected on a Bruker SMART CCD diffractometer using monochromated Mo Kα radiation(λ=0.071 073 nm)at room temperature.The collected frames were processed with the software SAINT[21].The structures were solved by the direct method and refined by full-matrix least-squares on F2using the program SHELXTL-97[22].Non-hydrogen atoms were refined anisotropically.Hydrogen atoms were placed in calculated position or found in the difference Fourier maps.The ligand contains only light scatteringatoms(C,H and N),and the anomalous scattering is not obvious whether using Cu or Mo target,so the absolute structure of it can not be determined reliably and the Flack parameter is meaningless.

Table 1 Crystallographic data for the ligand and Reガcomplex
CCDC:921976,complex;921977,ligand.
1.4 CD and VCD spectra measurements
CD spectra were recorded on a Jasco J-810 spectropolarimeter by using 1 cm quartz cell.Conditions of measurements included a scanning speed of 200 nm·min-1,a step size of 0.5 nm,a bandwidth of 2 nm,a response time of 0.5 s,standard sensitivity setting,and an accumulation of 5 scans at room temperature.The baseline was corrected by subtracting the signal of blank solution(dichloromethane)under the same condition.The solution IR and VCD spectra were recorded on a VERTEX 80v Fourier transform infrared spectrometer equipped with a PMA 50 VCD/IRRAS module (Bruker,Germany)in the region of 1 800~800 cm-1[23-24].The photo elastic modulator (PEM)was set to 1 500 cm-1,the spectral resolution was 4 cm-1,and the zero filling factor was 4.For each set of measurements,a multiple-wave plate(CdS)combined with the second wire grid linear polarizer was employed to calibrate the phase of the lock-in amplifier.A demountable cuvette A145 with BaF2with 0.10 mm Teflon spacer was used.All solution samples were dissolved in deuterated chloroform (CDCl3).The concentration of the ligand was 0.35 mol·L-1,while the concentration of the complex was 0.25 mol·L-1.All VCD measurements were collected for 4 h composed of 12 blocks,each consisting of 1420 scans accumulated for 20 min.Baseline correction was performed with the spectra of deuterated solvent usingthesamemeasurement setup asfor VCD.
1.5 Measurement of SHG responses
Powders of samples were graded by using standard sieves to particle sizes with diameters of 80~150μm (checked by standard optical microscopy technique)and were placed on a microscope slide and held in place with transparent tape.The second-order NLO intensities were estimated by the method previously reported by Kurtz and Perry[25],with the use of a pulsed Q-switched Nd∶YAG laser(λ=1 064 nm)to generate the SHG signal.The backward scattered SHG light was collected by employing a spherical concave mirror and passed through a filter that transmits only 532-nm radiation.The SHG efficiencies of the samples were estimated by comparison with that obtained for urea.
2 Results and discussion
2.1 X-ray structure
Fig.1 shows the crystal structure of the ligand 2,5-bis(4,5-pinene-2-pyridyl)pyrazine.Selected bond lengths and angles are listed in Table 2.The nitrogen atoms are distributed alternatively on the two side of the molecular axis.The pyridine rings on both ends are non-coplanar to the central pyrazine ring(Fig.1),with the dihedral angles of 21.709°and 10.209°,respectively.


Table 2 Select bond lengths(nm)and angles(°)for the ligand and complex

Fig.2 shows the crystal structure of the Reガcomplex.The asymmetric unit consists of a [Re(CO)3Cl(L)]complex and a free solvent DMF molecule.The 4,5-pinene-2-pyridine turns around the C-C bond,thus providing two ipsi-lateral nitrogen atoms to bind the Reガcenter.The 2,5-bis(2-pyridyl)pyrazine moiety islessdistorted fromco-planarity(the dihedral angles of the pyridine ring to the pyrazine ring are 23.700°and 6.267°,respectively).Due to the turnover of the 4,5-pinene-2-pyridine groups,the coplanarity(vide infra)further decreases.The Reガis surrounded by an approximated octahedral arrange-ment of two nitrogen atoms(N1,N2)from the chiral ligand,three carbon atoms (C1,C2 and C3)from CO,and one chlorine atom (Cl1).The mean Re-N and Re-C bond lengths are 0.216 8 and 0.194 1 nm respectively,and the Re-Cl bond length is larger with the value 0.242 07 nm (Table 2).The three CO ligands are in end-on coordination,and they are in close proximity with the ligand backbone.
2.2 Absorption and emission spectra
Fig.3 presents the absorption spectra of the ligand and Reガ complex in dichloromethane solutions with a concentration of 1 ×10-5mol·L-1.Absorption spectra of the ligand and Reガcomplex are very similar.The ligand shows lower intensity UV band with three peaks at 225,264 and 333 nm,which can be attributed toπ→π*interligand transitions(K band)[26].In the Reガcomplex,the bands exhibit red shift to 255,307 and 376 nm.The moderately intense,poorly distinguished absorption band extending into the visible region from ca.400 to 600 nm was tentatively assigned to an admixture of metal-to-ligand charge transfer states dπ(Re)→π*(L).

When excited in dichloromethane solution at room temperature within maximum excitation wavelength,the ligand and Reガcomplex exhibit maximum emission at 420 and 650 nm,respectively(Fig.4).The full width at half maximum (FWHM)of the emission spectrum of Reガ complex is~120 nm,which is typical for MLCT-based luminescence in this type of complex[27-28].There is little overlap between the absorption and emission spectra of the Reガcomplex.The Stokes shift is as large as about 270 nm,which should be caused by significant structural differences between the ground state and excited state upon photo excitation[29].In the experiment,we also observed that the kind of solvents can influence the fluorescence,when using polarity stronger acetonitrile or methanol as solvents will induce fluorescent quenching.

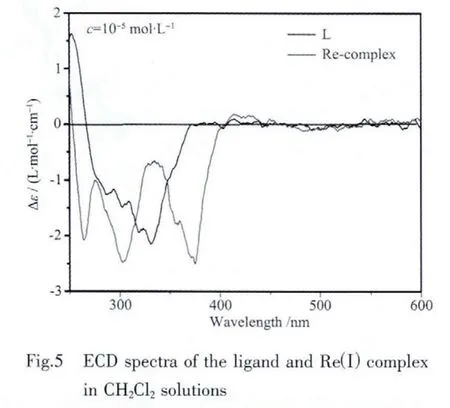
2.3 ECD and VCD spectra
Fig.5 presents the ECD spectra of the ligand and Reガcomplex in dichloromethane solutions with a concentration of 5×10-5mol·L-1.The ECD spectrum of the ligand shows a vibronic negative Cotton effects at 331 nm with a shoulder at 276 nm.The Reガ complex exhibits three negative Cotton effects at 263,302,and 375 nm,respectively.All these features are in good agreement with the UV-Vis spectra.
The IR and VCD spectra of the ligand and Reガcomplex in CDCl3solutions are shown in Fig.6.The IR spectrum of the Reガcomplex is similar to that of the bare ligand,in spite that some of the vibration energies are shifted due to coordination.Distinct bisignate signals at 1 450~1 490 cm-1,corresponding to the most prominent peaks (1 461 cm-1for the ligand and 1 468 cm-1for the Reガcomplex)in the IR spectra,were observed in the VCD spectra.These signals can be ascribed to the vibration of C-H bond in the pinene groups.Interestingly,the intensity of the bisignate signals are significantly enhanced in the Reガ complex.This is in agreement with the X-ray crystallographic results that the ligand forms singlehelical structure in the Reガ complex.

2.4 Second-order NLO properties
Both the ligand and Reガcomplex show secondorder NLOproperties.The second harmonic generation(SHG)responses of the ligand and Reガcomplex estimated using the Kurtz powder method[25]according to the literature procedure are ~0.4 and ~0.3 times that of urea,respectively.The SHG response of the ligand is higher than that of the Reガcomplex,in spite that the chirality is increased in the Reガcomplex as revealed by X-ray crystallography and VCD spectroscopic study.This is due to that the ligand crystallizes in the polar P21space group.However,the Reガcomplex crystallizes in the nonpolar P212121space group.The three orthogonal twofold screw axes in P212121space group will cancel the molecular dipoles and collectively reduce the net dipole moment and hyperpolarizability[30].
3 Conclusions
In this paper,a chiral ligand 2,5-bis(4,5-pinene-2-pyridyl)pyrazine (L)and its rheniumガcomplex[Re(CO)3Cl(L)]·DMF were synthesized and characterized.Both ligand and[Re(CO)3Cl(L)]·DMFshow optical activity as manifested by the ECD and VCD spectra.Upon excitation,the ligand and Reガcomplex emit at 420 nm and 650 nm,respectively.X-ray crystallographic analysisshowsthat theligand crystallizesin monoclinic P21space group while[Re(CO)3Cl(L)]·DMF crystallizes in orthorhombic P212121space group.Both the ligand and Reガcomplex show second-order nonlinear optic (NLO)properties as they crystallize in non-centric space groups.
[1]Amouri H,Gruselle M.Chirality in Transition Metal Chemistry:Molecules,Supramolecular Assemblies and Materials.Chichester:John Wiley&Sons,2008.
[2]von Zelewsky A.Stereochemistry of Coordination Compounds.Chichester:John Wiley&Sons,1996.
[3]Liu J,Zhang X P,Wu T,et al.Inorg.Chem.,2012,51:8649-8651
[4]Zhang J,Gao S,Zhang X X,et al.Dalton Trans.,2012,41:2626-2631
[5]Mutti F G,Zoppellaro G,Gullotti M,et al.Eur.J.Inorg.Chem.,2009,4:554-566
[6]Mejia E,Aardoom R,Togni A.Eur.J.Inorg.Chem.,2012,31:5021-5032
[7]Chen Q,Zhou J,Han Q,et al.J.Solid State Electrochem.,2012,16:2481-2485
[8]Gong L,Mulcahy S P,Harms K,et al.J.Am.Chem.Soc.,2009,131:9602-9603
[9]Pradeep C P,Zacharias P S,Das SK.Eur.J.Inorg.Chem.,2007,34:5377-5389
[10]Hamann C,von Zelewsky A,Neels A,et al.Dalton Trans.,2004,3:402-406
[11]Knof U,von Zelewsky A.Angew.Chem.Int.Ed.,1999,38:302-322
[12]Bark T,Stoeckli-Evans H,von Zelewsky A.J.Chem.Soc.Perkin Trans.,2002,16:1881-1886
[13]Malkov AV,Stewart-Liddon AJP,Teply F,etal.Tetrahedron,2008,64:4011-4025
[14]Malkov A V,Bella M,Langer V,et al.Org.Lett.,2000,2:3047-3049
[15]Rich J,Rodriguez M,Romero I,et al.Dalton Trans.,2009,38:8117-8126
[16]Mamula O,Lama M,Telfer S G,et al.Angew.Chem.Int.Ed.,2005,44:2527-2531
[17]Yi X Y,Zhao JZ,Wu W H,et al.Dalton Trans.,2012,41:8931-8940
[18]Oriskovich T A,White PS,Thorp H H.Inorg.Chem.,1995,34:1629-1631
[19]Esteves T,Xavier C,Gama S,et al.Org.Biomol.Chem.,2010,8:4104-4116
[20]Yeung H L,Wong W Y,Wong C Y,et al.Inorg.Chem.,2009,48:4108-4117
[21]SAINT,Area Detector Control and Integration Software,Siemens Analytiacl X-ray instruments Inc.,Madison,WI,USA,1996.
[22]Sheldrick G M.SHELXTL-97,Program for Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[23]Wu T,Li C H,Li Y Z,et al.Dalton Trans.,2010,39:3227-3232
[24]Wu T,Zhang X P,Li C H,et al.Chirality,2012,24:451-458
[25]Kurtz SK,Perry T T.J.Appl.Phys.,1968,39:3798-3813
[26]Lama M,Mamula O,Kottas G S,et al.Inorg.Chem.,2008,47:8000-8015
[27]Uddin M J,Dicesare N,Lakowicz J R.Inorg.Chim.Acta,2012,381:104-110
[28]Waterland M R,Simpson T J,Gordon K C,et al.J.Chem.Soc.,Dalton Trans.,1998,1:185-192
[29]Li X,Zhang D Y,Lu G H,et al.J.Photochem.Photobio.A,2012,214:1-7
[30]Li D P,Li C H,Wang J,et al.Eur.J.Inorg.Chem.,2009,32:4844-4849

