一个手性双核镍配合物的合成、结构和CD 光谱
向 华 姜 隆 李焕勇 郑晓丹 黎 彧
(1 广东轻工职业技术学院传播工程系,广州 510300)
(2 中山大学化学与化学工程学院,广州 510275)
(3 广东轻工职业技术学院轻化工程系,广州 510300)
0 Introduction
Interest in chiral polynuclear complexes has been boosted because of their useful properties, such as ferroelectric and nonlinear optical properties[1-4].Generally, the most effective way to prepare such complexes to ensure the chirality of the final products is to use chiral ligands[5-7]. Schiff-base ligands are good candidates because they are easy to possess chirality by the condensation of aldehyde molecules with chiral amine molecules or chiral aldehyde molecules with amine molecules[8-10]. We are interested in chiral polynuclear chemistry. Very recently, we have found that chiral Schiff-base type ligand L-H2vap, condensed from o-vanillin and L-2-amino-3-phenyl-1-propanol, is a good ligand to constrcut chiral polynuclears[11]. As a follow up work, we prepared a new dinulcear nickel complex L-[Ni2(van)(Hvap)2(MeOH)2]ClO4·2.5MeOH·H2O (1) by the reaction of Ni(ClO4)2·H2O with ovanillin and L-2-amino-3-phenyl-1-propanol in the presence of triethylamine at the room temperature.Herein, we report the synthesis, elemental analysis,IR, TGA and solid-state CD spectra of complex 1.
1 Experimental
1.1 General procedures
All the chemicals were commercially available and used without further purification. All the reactions were carried out under aerobic conditions. Elemental analysis was performed using Elementar Vario EL elemental analyzer. The IR spectra were recorded in the 4 000~400 cm-1region using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermal gravimetric analysis (TGA) data were collected on a Netzsch TG-209 instrument under nitrogen atmosphere in the temperature range of 20~800 ℃with a heating rate of 10 ℃·min-1. The solid-state circular dichroism (CD)spectra were recorded with KCl pellets on a JASCO J-810 spectropolarimeter
1.2 Syntheses of L-[Ni2(o-van)(Hvap)2(MeOH)2]ClO4·2.5MeOH·H2O (1)
Caution! Perchlorate salts of metal complexes with organic ligands are potentially explosive. They should be handled with care and prepared only in small quantities.
The addition of Ni(ClO4)2·H2O (366 mg, 1.0 mmol) to a stirred solution of o-vanillin (2-hydroxy-3-methoxybenzaldehyde) (152 mg, 1.0 mmol), L-2-amino-3-phenyl-1-propanol (152 mg, 1.0 mmol) and triethylamine (101 mg, 1.0 mmol) in methanol (40 mL)produced a dark green solution. After stirring at room temperature for 40 minutes, the solution was filtered and the filtrate was evaporated slowly at room temperature. After two weeks, green block crystals of 1 (185 mg, 35%) was isolated from the solution. Anal.Calcd. for Ni2C46.5H65N2O19.5Cl (%, taking in H2O, 1·H2O, Mr=1 116.86): C, 50.01; H, 5.87; N, 2.51. Found(%): C, 49.57; H, 5.38; N, 2.63. IR(KBr, cm-1): 3 595,3 401,2 947,2 837,1 637,1 602,1 552,1 471, 1 447,1 418, 1 403, 1 304, 1 244, 1 214, 1 082, 1 033, 970,954, 854, 750, 727, 704, 653, 623, 602, 576, 512,474 and 443.
1.3 Crystal structure determination
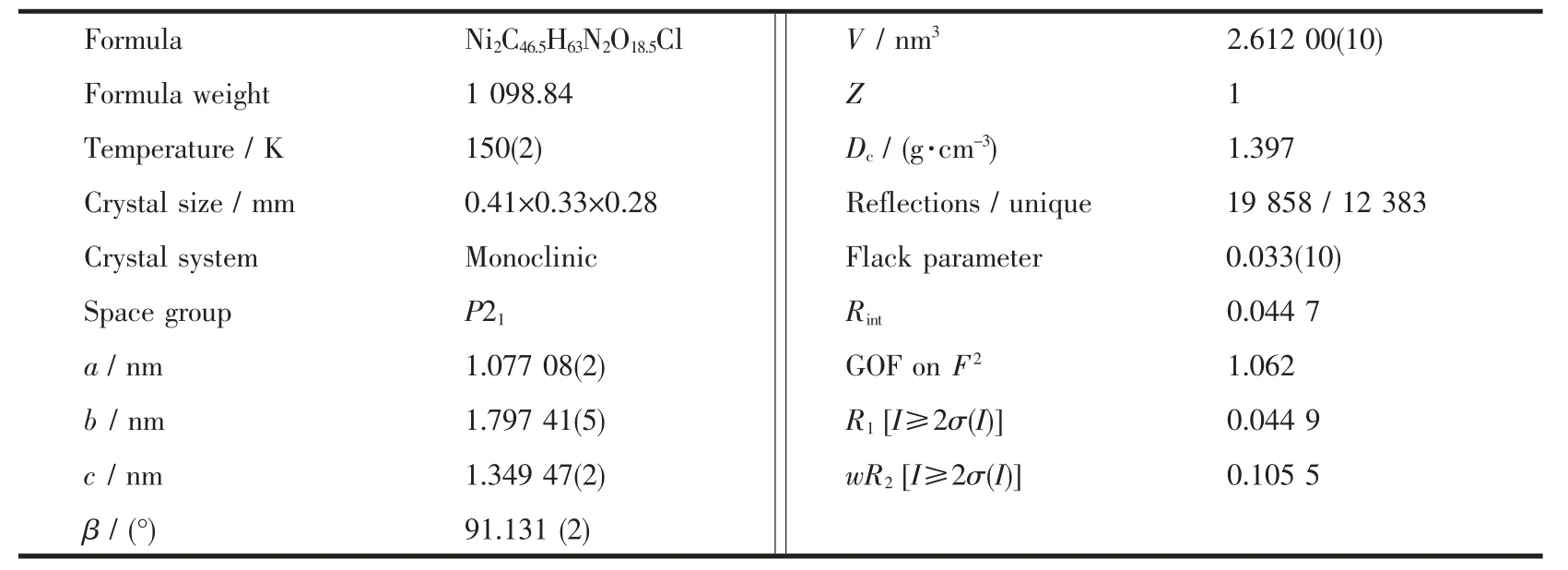
Table 1 Crystal data and structure refinements for complex 1
Single-crystal data for 1 were collected at 150(2)K on an Agilent Gemini Ultra diffractometer, with Mo Kα radiation (λ=0.071 073 nm). The empirical absorption corrections were applied using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm[12]. The structures were solved using direct methods, which yield the positions of all nonhydrogen atoms. These were refined first isotropically and then anisotropically.All the hydrogen atoms(except those of water molecules) were placed in calculated positions with fixed isotropic thermal parameters and included in structure factor calculations in the final stage of full-matrix least-squares refinement. All calculations were performed using the SHELXTL-97 system of computer programs[13]. The counterion perchlorate ion is disordered and treated with DFIX and ISOR as normal. The detailed crystallographic data are listed in Table 1 and the selected bond lengths and angles are included in Table 2.

Table 2 Selected bond lengths (nm) and angles (°) for complex 1
CCDC: 928211.
2 Results and discussion
2.1 Preparation chemistry
1 was prepared reproducibly by the reaction of Ni(ClO4)2·H2O (366 mg, 1.0 mmol) with o-vanillin and L-2-amino-3-phenyl-1-propanol in the presence of triethylamine at the room temperature. The chiral Schiff base ligand L-H2vap was in-situ formed. Part of the o-vanillin molecules were deprotonated and acted as a bridge between two nickel(Ⅱ)ions, which is not found in our previous work[11]. It is presumed that the condensation of o-vanillin and L-2-amino-3-phenyl-1-propanol is reversible at room temperature (Scheme 1), while heating at about 50°C leads to the complete formation of L-H2vap[11].
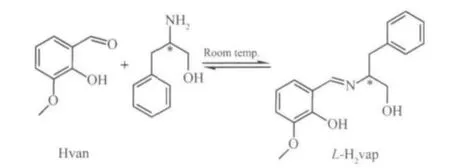
Scheme 1 Synthesis of the Schiff base ligand L-H2vap
2.2 Crystal structure of 1
Single crystal X-ray structural analyses reveal that complex 1 crystallizes in monoclinic, chiral space group P21with Z=1. As shown in Fig.1, the two nickel(Ⅱ)ions are bridged by phenoxide μ2-O2-atom from the deprotonated η2∶η2∶μ2-van-molecule (O8) to form a{Ni2O} dinulcear structure. The coordination modes of the van-molecule and the Schiff-base ligand are shown in Fig.2. Peripheral ligation is provided by two methanol molecules. Ni1/Ni2 is six-coordinated with one oxygen atom from a coordinated methanol molecule, two oxygen atoms from the van-molecule,and one nitrogen atom and two oxygen atoms from the individual Schiff-base ligand, adopting a distorted octahedral coordination sphere. There are intramolecular hydrogen bonding interactions between coordinated methanol molecules, Hvap-anions, perchlorate anions and solvent molecules (Fig.1 and Table 3). Charge balance is afforded by a perchlorate anion in the lattice.
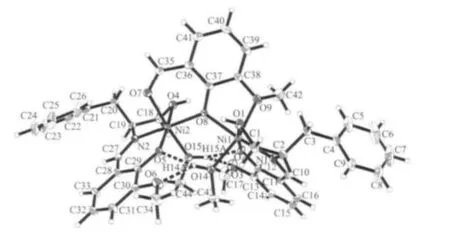
Fig.1 Molecular structure of 1 with the atom-numbering scheme with thermal ellipsoids at the 30%probability level; Dotted lines represent intramolecular hydrogen bonds
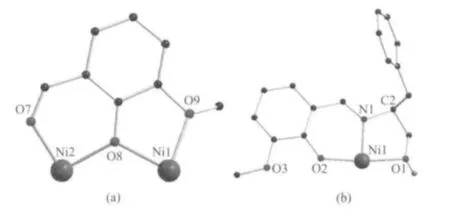
Fig.2 Coordination modes of the van-(a, deprotonated o-vanillin molecule) and Hvap-(b) ligands in 1
As listed in Table 2,the Ni-N distances(0.198 9(2)~0.199 4(3) nm) and the Ni-O distances (0.197 7(3)~0.212 5(2) nm) of Ni1/Ni2 ion in the normal range.The Ni…Ni distance is 0.364 38(5) nm.
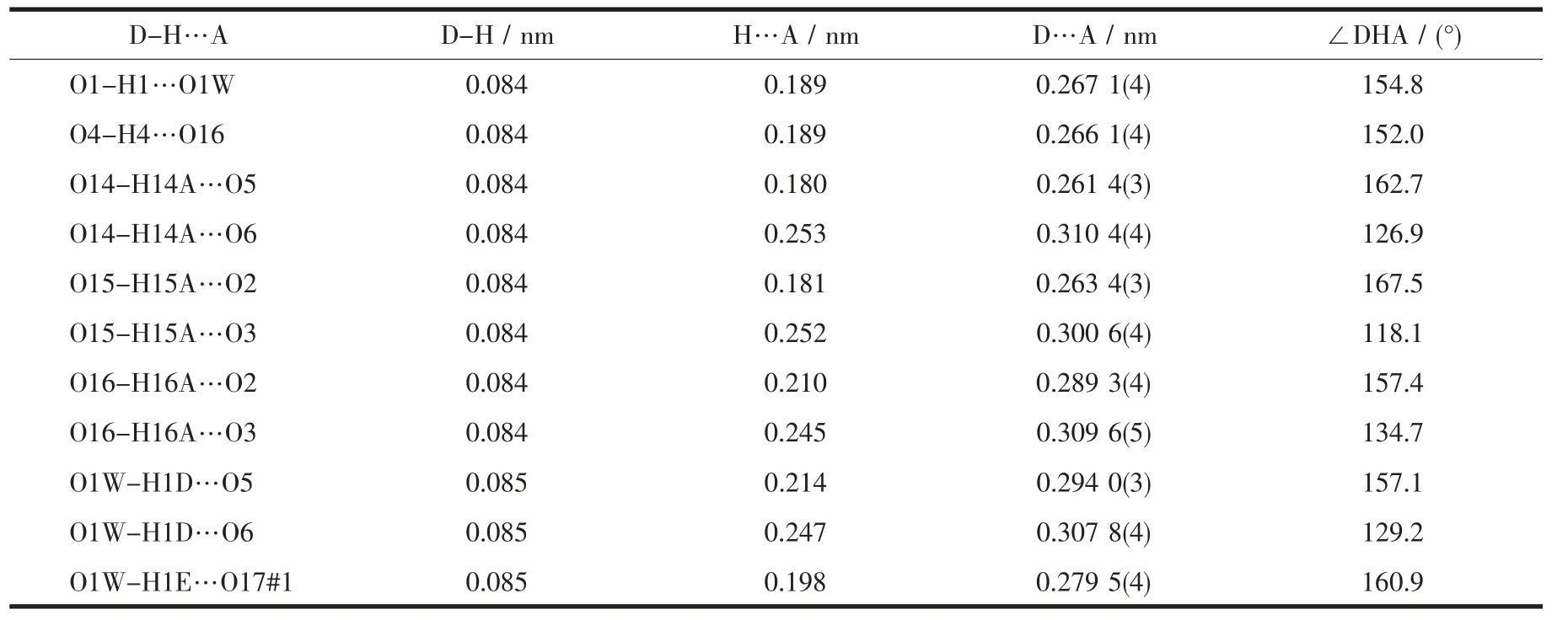
Table 3 Intramolecular hydrogen-bond parameters in 1
2.3 Thermogravimetric analyses
The TGA curve of complex 1 (Fig.3) shows complex 1 is not stable from room temperature. The initial weight loss of 11.69% from room temperature to 150 ℃, may attribute to the removal of four and a half methanol molecules (two coordinated ones and two and a half lattice ones, Calcd. 13.24%). And the following weight loss of 11.91% from 150 ℃to 260 ℃may correspond to the removal of the deprotonated ovanillin molecule (Calcd. 13.75%), indicative of the decomposition of complex 1. Then complex 1 continues to decompose upon further heating.
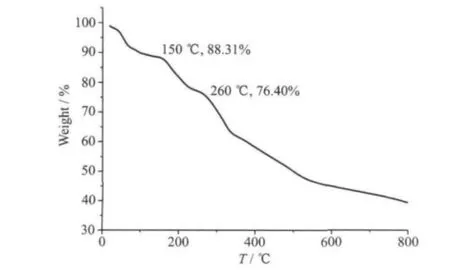
Fig.3 TGA curve of 1
2.4 CD spectra
Considering that complex 1 is chiral, the solidstate circular dichroism (CD) spectra were recorded to probe the whole chiral nature of the bulk samples. As shown in Fig.4, the results of solid-state circular dichroism (CD) measurements show the chiral nature of complex 1 in crystalline state, revealing that the bulk crystals of complex 1 show a negative Cotton effect at 360, 525 and 702 nm, respectively, indicative of the homochiral nature of complex 1.
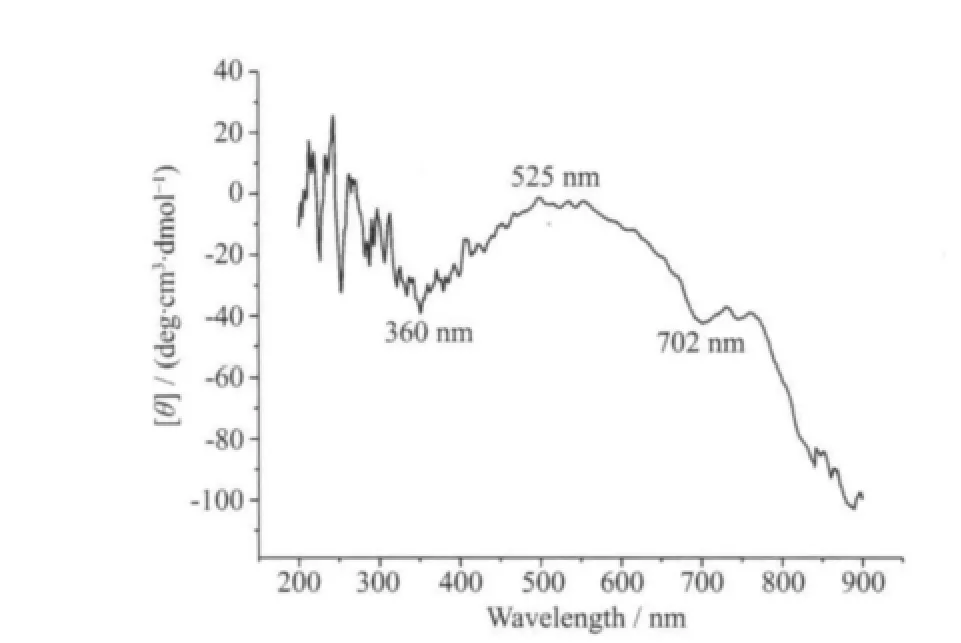
Fig.4 Solid-state CD spectra of 1
[1] Sui Y, Li D P, Li C H, et al. Inorg. Chem., 2010,49:1286-1288
[2] Wang C F, Gu Z G, Lu X M, et al. Inorg. Chem., 2008,47:7957-7959
[3] Zhang J F, Meng S C, Song Y L, et al. Chem. Eur. J., 2010,16:13946-13950
[4] Cao G J,Fang W H,Zheng S T,et al. Inorg.Chem.Commun.,2010,47:1047-1049
[5] Hernandez-Molina R,Gonzalez-Platas J,Kovalenko K A,et al.Eur. J. Inorg. Chem., 2011:683-693
[6] Thielemann D T, Fernández I, Roesky P W. Dalton Trans.,2010,39:6661-6666
[7] Algarra A G, Basallote M G, Fernández-Trujillo M J, et al.Inorg. Chem., 2010,49:5935-5942
[8] WU Ren-Tao(吴仁涛), ZHANG Hai-Xia(张海霞), SONG Juan(宋 娟), et al. Journal of Taishan University(Taishan Xueyuan Xuebao), 2009,31(3):39-43
[9] ZHANG Yan-Yan(张燕燕).Thesis for the Master of Yangzhou University(扬州大学硕士论文). 2008.
[10]Fan L L, Guo F S, Yun L, et al. Dalton Trans., 2010,39:1771-1780
[11]XIANG Hua(向华), LI Huan-Yong(李焕勇), ZHENG Xiao-Dan(郑 晓 丹), et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2012,28(8):1763-1768
[12]CrysAlis RED, Version 1.171.31.7, Oxford Diffraction Ltd.,2006.
[13]Sheldrick G M. Acta Crystallogr.,Sect.A:Found.Crystallogr.,2008,64:112-122

