Spectroscopic Analysis of Structural Transformation in Biodiesel Oxidation
Wu Jiang; Chen Boshui; Fang Jianhua; Wang Jiu
(Department of Petrochemistry, Logistical Engineering University, Chongqing 401311)
Spectroscopic Analysis of Structural Transformation in Biodiesel Oxidation
Wu Jiang; Chen Boshui; Fang Jianhua; Wang Jiu
(Department of Petrochemistry, Logistical Engineering University, Chongqing 401311)
The oxidation behavior of three biodiesels of different origins, viz. rapeseed oil derived biodiesel, soybean oil derived biodiesel and waste oil based biodiesel, were tested on an oxidation tester. The chemical compositions of the biodiesels were characterized by gas chromatography. Thereafter, the structural transformation of fatty acid methyl ester (FAME) of the biodiesels was analyzed by an infrared spectrometer and an ultraviolet absorption spectrometer. The results demonstrated that the oxidation behavior of biodiesels of different origins was closely related to the composition and distribution of FAMEs. Higher concentration of unsaturated FAME with multi-double bonds exhibited poorer oxidation resistance. Furthermore, cis-trans isomerization transformation occurred in the unsaturated FAME molecules and conjugated double-bond produced during the oxidation process of biodiesel. Greater cis-trans variations corresponded to deeper oxidation degree. The higher the content of unsaturated FAME with multi-double bonds in a biodiesel, the more the conjugated double bonds was formed.
biodiesel; oxidation; structural transformation; spectroscopic analysis
1 Introduction
Biodiesel, derived from renewable vegetable oils or animal fats with a process of transesterification and commonly referred to as fatty acid methyl esters (FAME), has proven itself as an prominent substitute for petroldiesel[1-5]. Virtually, biodiesel is similar to conventional petrodiesel in the fuel properties and is competitive with petrodiesel and offers a number of advantages over petrodiesel such as enhanced biodegradability, reduced toxicity, lower emissions and increased lubricity[6]. However, there exist some significant drawbacks that have limited its application. One of the major problems associated with the use of biodiesel as the diet of diesel engines is its poor oxidation stability. As we know, most vegetable oils and animal fats are triacylglycerols with long-chain fatty acid groups attached by ester linkages to a glycerol backbone. Biodiesel derived from such feedstocks contain a relatively high concentration (80%—90%) of unsaturated long-chain fatty acid alkyl esters. Unsaturated organic compounds are significantly more reactive to oxidation than saturated compounds. Therefore biodiesel can be easily oxidized during storage, negatively affecting fuel quality and thus engine performance[7]. Maintaining fuel quality of biodiesel for widespread use will depend on the development of technologies to increase its resistance to oxidation during long-term storage. As a result, oxidation prevention of biodiesel has in recent years attracted considerable attention, mainly focusing on the oxidation evaluation methods[8-12]and the oxidation inhibiting technologies[13-16]. Investigations on oxidation mechanism of biodiesel, especially the structural transformation during biodiesel oxidation, were scarcely reported. It is indeed important to fully understand the behavior of structural transformation of biodiesel in order to better improve the fuel quality and thus promote practical applications of biodiesel. In this paper, the structural transformation during biodiesel oxidation was analyzed by IR and UV spectroscopic methods.
2 Experimental
2.1 Preparation of biodiesel
Rapeseed oil methyl ester (RME), soybean oil methyl ester (SME) and waste oil methyl ester (WME) were prepared by transesterification of the corresponding originating oil, viz. rapeseed oil, soybean oil and waste cooking oil, respectively, with methanol (the molar ratio of methanol to oil=6:1) under alkaline condition (1% of sodium methoxide). The reaction was carried out for 60 minutes under reflux at 60—65℃ with agitation. After reaction, the reaction mixture was allowed to stand overnight and the methyl ester layer was separated from the glycerol layer using a separatory funnel. The residual amount of glycerol in the crude methyl ester was removed by centrifugation. The methyl ester was purified by distilling off the unreacted methanol under atmospheric pressure, followed by washing several times with water, centrifugation, and drying with anhydrous Na2SO4.
2.2 Determination of FAME contents
The FAME contents of biodiesels of different origins were determined by a gas chromatograph (GC2010, Shimadzu, Japan) equipped with a hydrogen flame ionization detector and a Rtxwax capillary column (30 m×0.25 mm×0.25 μm). GC analysis was conducted at a nitrogen carrier gas flow rate of 50 mL/min, a hydrogen flow rate of 30 mL/min, and an air flow rate of 350 mL/min. The injector, detector and column were maintained at 250 ℃, 250 ℃, and 230 ℃, respectively. 1.0 µL of the sample was injected for each GC analysis.
2.3 Oxidation test
The oxidation behavior of RME, SME and WME was tested respectively on an oxidation tester assembled previously by the authors, the schematic diagram of which is shown in Figure 1.

Figure 1 The schematic diagram of oxidation tester
For each test run, 100 g of the individual biodiesel sample were used. The oxidation test was performed at an oxygen flow rate of 300 mL/min under the catalytic action of copper, aluminum and iron strips at 95 ℃ for 10 hours. After oxidation, the acid value, peroxide value and kinematic viscosity at 40 ℃ of the oxidized fuel was determined, respectively, and the tested fuel samples were ready for further structural analysis.
2.4 Structural analysis of biodiesel
The structures of RME, SME and WME before and after oxidation were characterized by a Fourier transform infrared spectrometer (PE-1725X) and an UV absorption spectrometer (UV-2501PC), respectively. After IR and UV characterizations, the structural transformation of RME, SME and WME before and after oxidation was compared.
3 Results and Discussion
3.1 FAME contents of biodiesels
The saturated and unsaturated FAME contents of RME, SME and WME obtained from GC analysis are presented in Table 1.

Table 1 FAME contents of biodiesel samplesw, %
It can be seen from Table 1 that biodiesel was composed predominantly of unsaturated FAME coupled with a small percentage of saturated FAME. The total content of unsaturated FAMEs in RME was 93.7%, which was higher than those in SME (85.3%) and WME (76.4%). Furthermore, the contents of unsaturated FAME with multidouble bonds are also different. For example, the content of linoleic acid methyl ester (with two double bonds in the molecule) and linolenic acid methyl ester (with three double bonds in the molecule) in SME, WME and RMEwas 62.2%, 35.7% and 30.5%, respectively. The oxidation behavior of biodiesels of different origins may be closely related with the composition and distribution of FAMEs. The oxidation test results in the present study has shown that higher concentration of unsaturated FAME with multi-double bonds provided poorer oxidation resistance as evidenced by higher acid value, peroxide value and viscosity increase, as depicted in Table 2.

Table 2 Oxidation test results of biodiesel
3.2 Structural transformations of biodiesel
3.2.1 Infrared spectroscopic analysis
Figures 2, 3 and 4 show the IR spectra of RME, SME and WME before and after oxidation.

Figure 2 Infrared spectrograms of RME efore and after oxidation
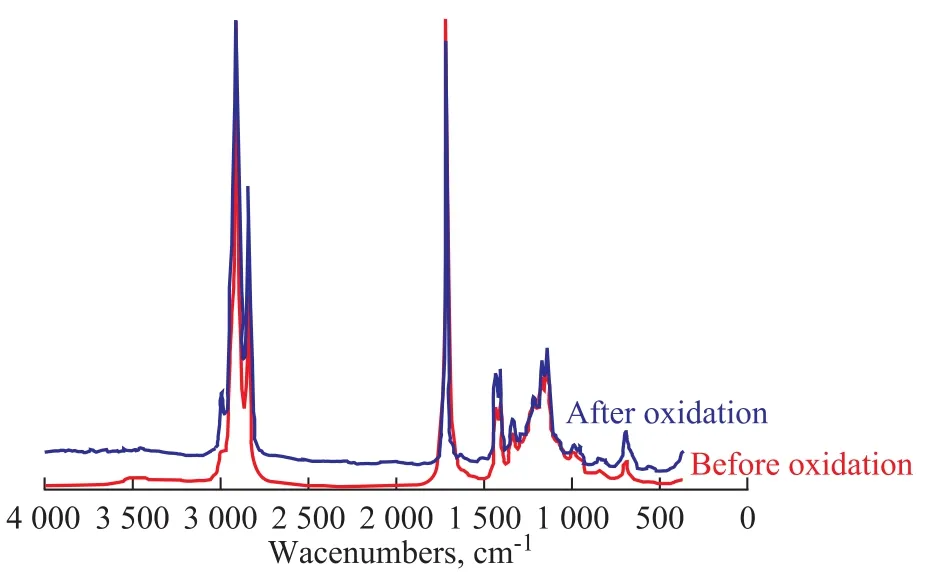
Figure 3 Infrared spectrograms of SME
It can be seen clearly from Figures 2—4 that the infrared absorbance of RME, SME and WME are different before and after oxidation. For the oxidated RME, SME and WME, there were absorbance peaks of —OOH functional groups in FAME at wave numbers of 3 446 cm-1, 3 443 cm-1and 3 446 cm-1, respectively. The absorbance peaks of C=C cis-double bonds at the wave numbers from 3 000 cm-1to 3 040 cm-1decreased with the increase of—OOH radicals. At 980—960 cm-1, the absorbance peak of C=C trans-double bond structure could also be observed. A certain increase of the absorbance peak area was found at 1 700—1 750 cm-1, which might be resulted from the oxidation decomposition products of —OOH radicals, such as ketone and aldehyde. The results indicated that cis-trans isomerization transformation might occur in unsaturated FAME molecules during oxidation, which could be verified through variations of cis-trans absorbance values of biodiesel before and after oxidation as shown in Table 3.
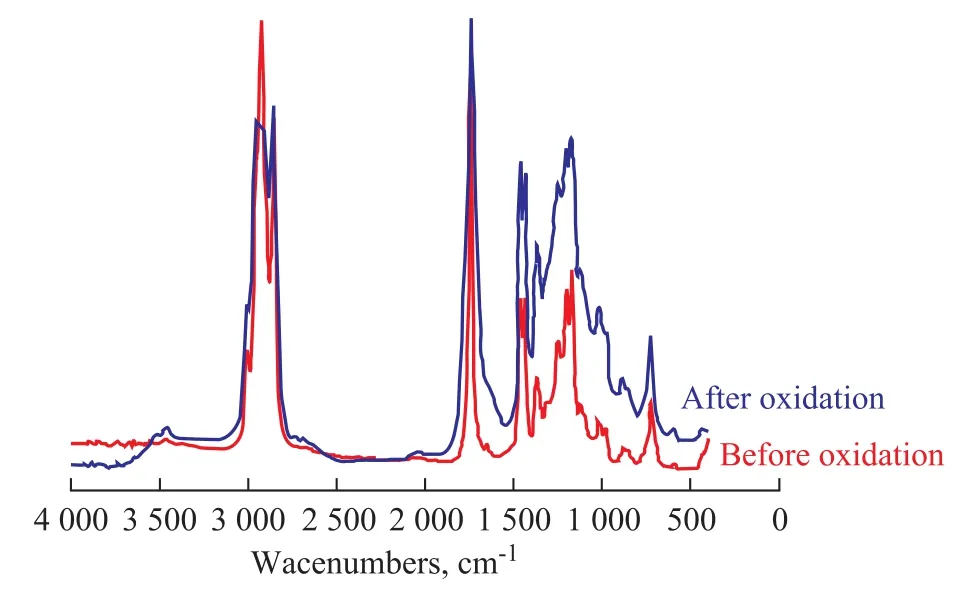
Figure 4 Infrared spectrograms of WME before and after oxidation
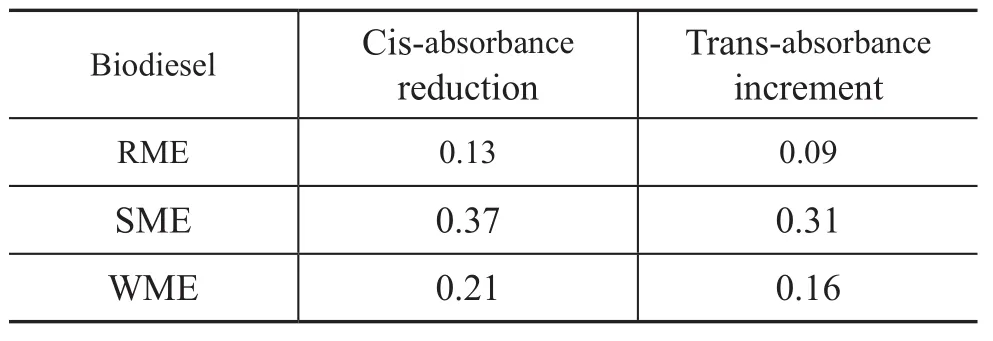
Table 3 Variations of cis-trans absorbance values for biodiesel samples before and after oxidation
The oxidation degree of biodiesel could also be judged indirectly from the cis-trans transformation variations. Larger cis-trans variations corresponds to deeper oxidation degree, which is in good agreement with the biodiesel oxidation results shown in Table 2.
3.2.2 UV spectroscopic analysis
The UV spectrograms of RME, SME and WME before andafter oxidation are shown in Figures 5, 6 and 7, respectively.

Figure 5 UV spectrograms of RME before and after oxidation

Figure 6 UV spectrograms of SME before and after oxidation

Figure 7 UV spectrograms of WME before and after oxidation
It can be seen from Figures 5—7 that at a wave length of 230 nm, UV absorbance values of RME, SME and WME after oxidation increased to a certain degree compared with those before oxidation. This indicated that doublebond transfer might occur and cis-trans conjugated double bonds might thus be generated during the oxidation of unsaturated FAME with multi-double bonds. Ultraviolet absorbance values of the biodiesel samples before and after oxidation are shown in Table 4.
It can be seen from Table 4 that the absorbance value increment for RME, SME and WME decreased in the following order: SME>WME>RME, which well coincided with the contents of unsaturated FAME with multi-double bonds such as linoleic acid methyl ester and linolenic acid methyl ester in RME, SME and WME verified by GC analyses. The results indicated that the content of unsaturated FAME with multi-double bonds is a governing factor in the conjugated double bonds formation. More content of unsaturated FAME with multi-double bonds will produce more conjugated double bonds during the oxidation process, thus resulting in more significant increase of absorbance values.

Table 4 Ultraviolet absorbance values of biodiesel before and after oxidation
4 Conclusions
Based on the results of GC, IR and UV analyses, it can be concluded that the oxidation behavior of biodiesels of different origins are closely related with the composition and distribution of FAMEs. Higher concentration of unsaturated FAME with multi-double bonds exhibited poorer oxidation resistance. Furthermore, cis-trans isomerization transformation occurred in the unsaturated FAME molecules and conjugated double-bonds produced due to transfer of double-bond in the oxidation process of biodiesel. Greater cis-trans variations corresponded to deeper oxidation degree. The content of unsaturated FAME with multi-double bonds governed the conjugated double bonds formation in the oxidation of biodiesel. The higher the content of unsaturated FAME with multi-double bonds in a biodiesel, the more the conjugated double bonds was formed.
Acknowledgement:The authors gratefully acknowledge the financial support from the National Natual Science Foundation of China (No.51375491), the Natural Science Foundation of Chongqing (Project No. 2011JJA90020) and the Science Foundation for Young Teachers of Logistical Engineering University.
[1] Ali Y, Hanna M A. Alternative diesel fuels from vegetable oils[J]. Bioresource Technology, 1994, 50(2): 153-163
[2] Nye M J, Southwell P H. Esters from rapeseed oil as diesel fuel[C]. Proceedings of Vegetable Oil as Diesel Fuel Seminar Ш. Peoria: Northern Agricultural Energy Center, 1983: 78
[3] Agarwal A K, Das L M. Biodiesel development and characterization for use as a fuel in compression ignition engines[J]. Journal of Engineering for Gas Turbines and Power- Transactions of the ASME, 2001, 123(2): 440-447
[4] Demirbas A. Progress and recent trends in biofuels[J]. Progress in Energy and Combustion Science, 2007, 33(1): 1-18
[5] Demirbas A. Diesel fuel from vegetable oil via transesterification and soap pyrolysis[J]. Energy Sources, 2002, 24(9): 835-841
[6] Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters[J]. Fuel Processing Technology, 2005, 86(10): 1059-1070
[7] Haseeb A S M A, Fazal M A. Compatibility of automotive materials in biodiesel: A review[J]. Fuel, 2011, 90(3): 922-931
[8] Kivevele T T, Mbarawa M M, Bereczky A, et al. Evaluation of the oxidation stability of biodiesel produced from Moringa oleifera oil[J]. Energy & Fuels, 2011, 25(11): 5416-5421
[9] Siddharth J, Sharma M P. Oxidation stability of blends of Jatropha biodiesel with diesel[J]. Fuel, 2011, 90(10): 3014-3020
[10] Meira M, Quintella C M, Tanajura A D S, et al. Determination of the oxidation stability of biodiesel and oils by spectrofluorimetry and multivariate calibration[J]. Talanta, 2011, 85(1): 430-434
[11] Karavalakis G, Stournas S, Karonis D, et al. Evaluation of the oxidation stability of diesel/biodiesel blends[J]. Fuel, 2010, 89(9): 2483-2489
[12] Bannister C D, Ali H M, Hawley J G. Investigation and analysis into the impact of rapeseed methyl ester biodiesel on the diesel oxidation catalyst performance[J]. Proceedings of the Institution of Mechanical Engineers Part D - Journal of Automobile Engineering. 2012, 226 (D11): 1525-1535
[13] Caramit R P, de Freitas Andrade A G, de Souza J B G, et al. A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi-walled carbon nanotube screenprinted electrodes [J]. Fuel, 2013, 105: 306-313
[14] Kivevele T T, Mbarawa M M. Impact of antioxidant additives on the oxidation stability of biodiesel produced from Croton Megalocarpus oil [J]. Fuel Processing Technology, 2011, 92(6): 1244-1248
[15] Moser B R. Efficacy of gossypol as an antioxidant additive in biodiesel[J]. Renewable Energy, 2012, 40(1): 65-70
[16] de Araujo T A, Barbosa A M J, Viana L H, et al. Electroanalytical determination of TBHQ, a synthetic antioxidant, in soybean biodiesel samples[J]. Fuel, 2011, 90(2): 707-712
Recieved date: 2013-02-26; Accepted date: 2013-03-12.
Prof. Chen Boshui, Telephone: +86-23-86730832; E-mail: boshuichen@163.com.
- 中国炼油与石油化工的其它文章
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Synthesis of MgO-Al2O3/ZSM-5 by Solid State Reaction for Propane Dehydrogenation
- Synthesis of TS-1 Films on Porous Supports for Epoxidation of Allyl Chloride by Hydrogen Peroxide
- Effects of Moisture on Rheological Properties of PAV Aged Asphalt Binders
- Microwave-Assisted Decarboxylation of Sodium Oleate and Renewable Hydrocarbon Fuel Production
- Research and Commercial Application of CPP Technology for Producing Light Olefins from Heavy Oil

