Which form of collagen is suitable for nerve cell culture?*
Mohsen Fathi Najafi, Saber Zahri, Fatemeh Vahedi, Leila Esmaililian Toosi, Nazila Ariaee,
1 Razi Vaccine and Serum Research Institute, Mashhad, Iran
2 Departamnt of Biology, University of Mohaghegh Ardebili, Ardebil, Iran
3 Inflammation and Inflammatory Diseases Research Center, Mashhad University of Medical Science, Mashhad, Iran
INTRODUCTION
The mammalian central nervous system has little capacity for self-repair[1].There is a critical need to design new strategies to repair damaged nerve tissues[2].Transplantation of exogenous nerve cells and tissues is one of desired approaches for functional recovery following brain and spinal cord injuries and neurodegenerative diseases[3-4].Since adult normal cells have a very limited capability to proliferate[5], the use of embryonic cells has become the focus for replacement therapy of injured central nervous system[6].Neural stem cells are anchorage-dependent and require a solid matrix to attach to and grow.In the central nervous system, these cells adhere to the fibrillar protein meshwork known as extracellular matrix[7-8].Collagen, specially type I collagen,is the major class of insoluble fibrous proteins in the extracellular matrix that makes up one third of all proteins of the body and is responsible for framework modeling of connective tissues[9-11].
Collagen can stimulate cell attachment and intracellular communication between some kinds of cells[12-13].It can initiate a number of cellular processes, including activation of some receptors and induction of various gene transcriptions in nerve cells[14].A previous study has shown that neural stem and progenitor cells derived from embryonic tissue, cultured on collagen matrices, are able to expand actively and generate neurons and neurites with developed neuronal polarity[15].
The differentiation was accompanied by a shift in expression of functional receptors[16-17].Some experiments have suggested the strong chemotropism of growing neurites and axons in the presence of collagen[18].It has been proven that some cells, like macrophages, adhere to natural collagen matrix poorly.In contrast, these cells adhere greatly to monomeric, heat-inactivated or modified type I collagen[19].These data support the theory that cells may have different responses to variable forms of collagen.However, most of these studies on nerve cells focused on three-dimensional collagen matrix[16-17].In this study, the effects of different treatments with type I collagen, specially hydrolyzed collagen as a model of damaged extracellular matrix, on nerve cell culture were evaluated.
RESULTS
Effects of different concentrations of collagen on primary cultured nerve cells
The optimal growth of primary cultured nerve cells was observed in the presence of 15 µg/mL and 5 µg/mL hydrolyzed or non-hydrolyzed collagen.High concentration of hydrolyzed collagen (70 µg/mL) significantly inhibited the growth of primary neural cells (Figure 1).
Effects of hydrolyzed collagen and two-dimensional non-hydrolyzed collagen matrix on viability of nerve cells
In the presence of 15 µg/mL hydrolyzed collagen, the viability of nerve cells increased at 7 days and decreased thereafter.In contrast,hydrolyzed collagen in the presence of two-dimensional matrix supported growth over 14 days, reaching its peak on day 14.The viability of primary cultured nerve cells increased by 54% and 45% on days 7 and 14,respectively in the presence of two-dimensional collagen matrix or hydrolyzed collagen (Figure 2).

Figure 1 The viabiliy of primary cultured nerve cells in the presence of different concentrations of hydrolyzed and nonhydrolyzed collagen after 7 days as determined by 3-[4,5-dimethyl thiazol-2-yl]-2,5- diphenyltetrazolium bromide (MTT)colorimetric assay.
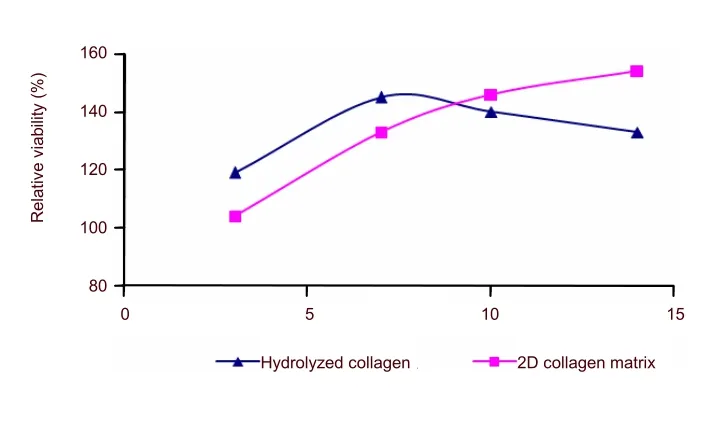
Figure 2 The viability of nerve cells in the presence of 15 µg/mL hydrolyzed and two-dimensional collagen matrix at 2 weeks as determined by (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)colorimetric assay.
Effects of collagen on attachment of nerve cells
To evaluate the effect of hydrolyzed collagen or two-dimensional non-hydrolyzed collagen matrix, after 7 days of treatment,the percentage of attached and suspended cells was assessed by MTT colorimetric assay.In the presence of hydrolyzed collagen (15 µg/mL), viable suspended cells outnumbered attached cells, but in the presence of two-dimensional non-hydrolyzed collagen matrix, attached cells were increased over floating cells (Figure 3).The increase rate of the overall viability of nerve cells after each treatment was increased by 30% compared to control conditions (Figure 3).

Figure 3 Effects of 15 µg/mL hydrolyzed collagen suspension and two-dimensional collagen matrix on nerve cell attachment after 7 days as determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay.
Effect of collagen on morphology of primary cultured nerve cells
During culture of nerve cells, an increased number of viable cells were detected upon collagen treatment.Nerve cell colonies were observed in the presence of 15 µg/mL hydrolyzed collagen or three-dimensional collagen matrix.Formation of nerve cell colonies indicates dendrogenesis and neurite outgrowth after treatment with 15 µg/mL hydrolyzed collagen and three-dimensional collagen matrix.In addition, these changes were not observed in two-dimensional collagen matrix (Figure 4).
DISCUSSION
Nerve injury and neurodegenerative diseases are common and sometimes lead to loss of nerve function, resulting in serious functional impairment[4,20].Repair strategies using transplantation of exogenous functional nerve cells have attracted a lot of attention.The key to successful tissue engineering is the use of biodegradable material that mimics the capabilities of original tissue so that the host tissue can eventually replace the substitute inert materials[21].Since endogenous and exogenous delivered nerve cells have a limited capacity for differentiation and growth, some adjuvant treatments are needed to stimulate cell proliferation, support cell survival and trigger neurite outgrowth.

Figure 4 Nerve cell colony formation and neurite outgrowth of primary cultured nerve cells in the presence of different collagen forms (inverted microscope, ×100).
In this study, we tested the effects of type I collagen onin vitrogrowth, attachment and differentiation of primary cultured nerve cells.Type I collagen has been shown to enhance the growth and differentiation of many cells to a larger extent than other substrates such as plastic or glass[22-23].In this study, four kinds of collagen treatments were evaluated to find an optimal treatment to induce neurite outgrowth and morphogenesis.Primary nerve cell attachment was determined by inverted microscopy and confirmed by MTT colorimetric assay.The number of viable cells and cell attachment increased after treatments with different kinds of non-hydrolyzed collagen including two-dimensional collagen matrix and collagen suspension.
Results from this study demonstrated that 5 µg/mL non-hydrolyzed collagen suspension was not very effective to increase viability and attachment of primary cultured nerve cellsviaformation of a collagen network in comparison with other collagen treatments.Higher viability and neurite outgrowth of cells were found in the presence of hydrolyzed collagen.There are several reasons to be considered.Firstly, these differences between non-hydrolyzed collagen and hydrolyzed collagen may be due to collagen uptake during cell proliferation[24],suggesting that collagen polymers of small size would be more useful than non-hydrolyzed collagen for cell growth.In addition, it has been shown that some kinds of cells respond differently to natural and deformed collagens.Deformed collagen resulting from damaged extracellular matrix is able to induce cell proliferation and morphological changes effectively[19].It might be a common mechanism of the action underlying hydrolyzed or damaged collagen in some normal cells like nerve cells.It is proposed that cell culture media treated with hydrolyzed collagen could be a model of damaged extracellular matrixin vitro[19].On the other hand, hydrolyzed collagen suspension did not improve cell attachment, which might be due to the disability of small pieces of collagen to form a network and act as a scaffold for cell anchorage.Hydrolyzed collagen may be therapeutically used in strategies and research programs because it can mimic damaged extracellular matrix.
Although non-hydrolyzed collagen could improve cell attachment, highest level of attachment of primary cultured nerve cells was observed on two-dimensional and three-dimensional matrices, which confirms the capacity of collagen to support cell anchorage.Furthermore, collagen could be an important factor of cell-cell interaction,which results in higher rate of cell growth and viability.Type I collagen is a natural extracellular matrix highly suitable for nerve repair[25]and it benefits cell culture media treatments as in tissue engineering materials like matrigel.
In conclusion, results from this study show that type I collagen had different effects on nerve cell culture and these differences depend on the form of collagen, such as matrices (two-dimensional or three-dimensional) and suspension (non-hydrolyzed or hydrolyzed).
MATERIALS AND METHODS
Materials
Reagents and equipment
Fetal calf serum (New York, NY, USA), 0.5% trypsin,0.2% ethylenediaminetetraacetic acid disodium salt(EDTA) (Sigma, St.Louis, MO, USA), dulbecco's modified eagle’s medium (DMEM)/F12 medium (Gibco,Carlsbad, CA, USA), insulin (Sigma), (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)(Sigma), inverted microscope (Nicon, Japan) were used in this study.Type I collagen and protease enzyme were obtained from Razi Vaccine and Serum Research Institute of Mashhad, Iran.Collagen solution was prepared by dissolving collagen lyophilized powder in 0.02 mol/L acetic acid, and diluted with 0.15 mol/L PBS (pH 7.2) to achieve a final concentration of 1 mg/mL and sterilized by 0.22 μm millipore syringe filter.
Methods
Cell preparation
Nerve cells were obtained from the forebrain cortex of 18-day BALB/c mouse embryos[2].Briefly, the forebrain cortex tissues were dissociated by mincing into about 1-mm3pieces with a sterile scalpel, washed with PBS,incubated by trypsin and EDTA at 37 °C for 20 minutes,and then gently dispersed by pipetting.After fetal calf serum was added to inhibit trypsin activity, the suspension was centrifuged at 300 ×gfor several times for 4 minutes each to completely remove blood cells[26].Finally,cell pellets were re-suspended in DMEM/F12 supplemented with 10% fetal calf serum and 1 000 unit insulin(1 × 104cells/mL) and then cultured for 3 days at 37 °C in 95% humidified air containing 5% CO2.
Preparation of two-dimensional matrix
After loading collagen solution (50 μg/cm2) on the surface of tissue culture dishes, the tissue culture dishes were incubated at room temperature for 2 hours under sterile conditions.Once two-dimensional matrix was prepared, cells and media were added.
Preparation of three-dimensional matrix
To prepare gels, collagen solution was diluted with growth media to achieve a final collagen concentration of 1 mg/mL.After adjusting the pH of collagen solution to 7.4 by addition of 1 mol/L NaOH, the solution was chilled on ice to prevent gel formation.Cells were added at the desired density, along with more cell media, if necessary,to get a final collagen concentration of 0.4 mg/mL and then 0.2 mL of collagen suspension was poured into 96-well tissue culture plates.Subsequently, the plates were placed in an incubator (37°C, 5% CO2and 95%humidity) for 1–2 hours to allow gel formation.Once the gel had set, the media was added to the top of the gels.
Preparation of hydrolyzed collagen
The collagen was digested with immobilized protease.The immobilization of protease was performed by entrapment method using poly acrylamide gel[24].A mixture of 7% (w/v) polyacrylamide gel containing 23 units (70 µg) of the protease was made and mixed gently for 20 minutes.Then, tetramethylethylenediamine and ammonium persulfate were added.The resulting gel was cut into pieces and then incubated with 50 μg/mL of collagen in the presence of 20 mmol/L Tris-Cl (pH 8.0) for 2 hours.Then,immobilized protease gels were removed and the hydrolyzed collagen solution was sterilized using 0.22 μm millipore syringe filter.The hydrolyzed collagen was tested by paper chromatography and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)[24].
Collagen treatment
In the first step, different concentrations of hydrolyzed and non-hydrolyzed collagen (5, 15, 30, 50 and 70 µg/mL)were added to find the optimum concentrations.Cells were treated with selected concentrations for 7 days(37°C, 5% CO2and 95% humidity), and then cell viability was assessed by MTT colorimetric assay.In the second step, the viability of nerve cells in two-dimensional collagen matrix and hydrolyzed collagen suspension were observed at 3, 7, 10 and 14 days and compared with the blank group in which cells and growth media were treated without collagen.Nerve cell adhesion and cell attachment were also detected in the presence of hydrolyzed collagen and two-dimensional non-hydrolyzed collagen matrix at 7 days.In this study, MTT colorimetric assay was separately performed for cells that attached to matrix and suspended cells which could not attach.Any changes in cell appearance, final attachment, colony formation, dendritogenesis and neurite outgrowth, were considered in cell evaluation after each treatment of primary nerve cells.Cells were observed under inverted microscope during 21 days.
MTT assay
Cell viability was evaluated by MTT colorimetric assay[27].Briefly, 100 μL MTT (Sigma) solution (5 mg/mL in PBS)was added to each well.The plates were incubated for 3 hours at 37°C, and then the supernatants were removed.For solubilization of the MTT crystals, 1 mL of dimethyl sulfoxide and 125 μL of 100 mmol/L glycine (pH 10.5)were added to the wells.The plates were placed on a shaker for 15 minutes for complete solubilization of crystals and then the absorbance of each well was determined at 492 nm[24].
Statistical analysis
SPSS software (SPSS, Chicago, IL, USA) was used to analyze the data.Graphs were made in Excel (Microsoft Office 2010).
[1]Ma W, Fitzgerald W, Liu QY, et al.CNS stem and progenitor cell differentiation into functional neuronal circuits in threedimensional collagen gels.Exp Neurol.2004;190(2):276-288.
[2]O'Connor SM, Stenger DA, Shaffer KM, et al.Primary neural precursor cell expansion, differentiation and cytosolic Ca(2+) response in three-dimensional collagen gel.J Neurosci Methods.2000;102(2):187-195.
[3]Liu N, Tang ZP, Yu ZY, et al.Morphological properties and proliferation analysis of olfactory ensheathing cells seeded onto three-dimensional collagen-heparan sulfate biological scaffolds.Neural Regen Res.2012;7(16):1213-1219.
[4]Wang Q, Yang XL, Ren M, et al.Effect of chitosan/type I collagen/gelatin composites in biocompatibility and nerve repair.Neural Regen Res.2012;7(15):1179-1184.
[5]Buzańska L, Habich A, Jurga M, et al.Human cord blood-derived neural stem cell line--possible implementation in studying neurotoxicity.Toxicol In Vitro.2005;19(7):991-999.
[6]Nakajima M, Ishimuro T, Kato K, et al.Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation.Biomaterials.2007;28(6):1048-1060.
[7]Raspanti M, Congiu T, Alessandrini A, et al.Different patterns of collagen-proteoglycan interaction: a scanning electron microscopy and atomic force microscopy study.Eur J Histochem.2000;44(4):335-343.
[8]Ruoslahti E.Stretching is good for a cell.Science.1997;276(5317):1345-1346.
[9]Gutiérrez Morales JC, Gutiérrez Morales SE, Ruiz Moya BE, et al.Neural surfaces coverage with “collagen films and cigarettes”: A revisited and modified method of protection and retraction during microsurgical approaches to craniospinal lesions.Clin Neurol Neurosurg.2010;112(2):144-148.
[10]Suri S, Schmidt CE.Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering.Tissue Eng Part A.2010;16(5):1703-1716.
[11]Yue Z, Liu X, Molino PJ, et al.Bio-functionalisation of polydimethylsiloxane with hyaluronic acid and hyaluronic acid--collagen conjugate for neural interfacing.Biomaterials.2011;32(21):4714-4724.
[12]Rafiuddin Ahmed M, Jayakumar R.Peripheral nerve regeneration in RGD peptide incorporated collagen tubes.Brain Res.2003;993(1-2):208-216.
[13]Wen F, Chang S, Toh YC, et al.Development of poly(lactic-co-glycolic acid)-collagen scaffolds for tissue engineering.Mater Sci Eng.2007;27(2):285-292.
[14]Jones JM, Cohen RL, Chambers DA.Collagen modulates gene activation of plasminogen activator system molecules.Exp Cell Res.2002;280(2):244-254.
[15]Edelman DB, Keefer EW.A cultural renaissance: in vitro cell biology embraces three-dimensional context.Exp Neurol.2005;192(1):1-6.
[16]O’Connor SM, Stenger DA, Shaffer KM, et al.Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices.Neurosci Lett.2001;304(3):189-193.
[17]O’Connor SM, Andreadis JD, Shaffer KM, et al.Immobilization of neural cells in three-dimensional matrices for biosensor applications.Biosens Bioelectron.2000;14(10-11):871-881.
[18]Guthrie S, Lumsden A.Neuroprotocoles: A companion method in neurosciences.San Diego: Academic press.1994.
[19]Gowen BB, Borg TK, Ghaffar A, et al.Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors.Matrix Biol.2000;19(1):61-71.
[20]Wang Y, Yao M, Zhou J, et al.The promotion of neural progenitor cells proliferation by aligned and randomly oriented collagen nanofibers through β1 integrin/MAPK signaling pathway.Biomaterials.2011;32(28):6737-6744.
[21]Guan J, Tong W, Ding W, et al.Neuronal regeneration and protection by collagen-binding BDNF in the rat middle cerebral artery occlusion model.Biomaterials.2012;33(5):1386-1395.
[22]Chen L, Cheng WL, Ke YN, et al.Aortic artery elastic lamina degradation, collagen remodeling, oxides stress and inflammation in the apolipoprotein E deficient mice with or without aortic banding.Zhonghua Xin Xue Guan Bing Za Zhi.2012;40(6):505-510.
[23]Chen SY, Shiau AL, Li YT, et al.Suppression of collagen-induced arthritis by intra-articular lentiviral vector-mediated delivery of Toll-like receptor 7 short hairpin RNA gene.Gene Ther.2012;19(7):752-760.
[24]Najafi MF, Vahedi F, Ahmadi S, et al.Effect of collagen Type I (Rat Tail) on cell proliferation and adhesion of BHK-21.Biomed.2008: p.806-809.
[25]Deyle DR, Khan IF, Ren G, et al.Normal collagen and bone production by gene-targeted human osteogenesis imperfecta iPSCs.Mol Ther.2012;20(1):204-213.
[26]Griffiths A, Doyle JB, Newell DG, et al.Cell and Tissue Culture: Laboratory Procedures.New York: John Wily &Sons.1998.
[27]Mosmann T.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays.J Immunol Methods.1983;65(1-2):55-63.
