Combined transplantation of GDAsBMP and hr-decorin in spinal cord contusion repair****○
Liang Wu , Jianjun Li Liang Chen Hong Zhang Li Yuan Stephen JA Davies
1 School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China
2 Department of Neural Functional Reconstruction of Spine and Spinal Cord, China Rehabilitation Research Center, Beijing 100068, China
3 Rehabilitation Center, Beijing Xiaotangshan Rehabilitation Hospital, Beijing 102211, China
4 Department of Neurosurgery, University of Colorado Denver, 1250 14th Street Denver, Colorado 80217, USA
INTRODUCTION
After central nervous system damage, injured axons are unable to produce functional connections because axons cannot effectively regenerate and an inhibitory microenvironment is formed[1].A variety of cells have been examined for transplantation after spinal cord injury, such as human umbilical cord blood stem cells[2-4], marrow stromal cells[5-6],Schwann cells[7-8], astrocytes[9-10], olfactory ensheathing glia[11-14], and oligodendrocyte progenitor cells[15-18].Because of structural damage and scar formation in the white matter of the spinal cord[19-25], as well as axonal growth inhibitors present in the glial scar tissue[26-28]and myelin of the central nervous system[29-31], insufficient endogenous regenerated axons traversed the lesion site after various cell transplantation into or around the injured spinal cord[32-34].
After spinal cord injury, active astrocytes can induce migration of nerve cells.Mature astrocytes mainly maintain nerve morphology, but they also play an important role in providing nerve growth factor and communication between cells[35].However, mature astrocytes also affect nerve regeneration and hinder axonal extension through the secretion of harmful factors that form chemical glial barriers[36].Therefore, it is very important to favorably regulate astrocyte expression after spinal cord injury.Glial-restricted precursor cells from the embryonic spinal cord, which are the precursors of oligodendrocytes and astrocytes[37-39],can improve the microenvironment, regulate astrocyte expression, inhibit glial scar formation and delay the expression of proteoglycans.Glial-restricted precursor-derived astrocytes induced by bone morphogenetic protein (BMP)-4 (GDAsBMP) not only have common characteristics of glial-restricted precursor cells, but can also fill the injured interface, rearrange tissues, protect injured axons, and promote axonal regeneration and functional recovery after acute spinal cord injury[40].Decorin decreases fibrosis, as the core protein of decorin, can inhibit collagen fiber proliferation and neutralize transforming growth factor-β[40-42].Decorin within the nervous system not only regulates the extracellular collagen matrix,it also promotes axon fibronectin adhesion,Schwann cell proliferation and neural cell survival[43].Human recombinant decorin(hr-decorin) has been shown to inhibit inflammation, glial scar formation and chondroitin sulfate proteoglycan expression, and may promote axonal growth across the injured interface after acute spinal cord injury[44-46].
After spinal cord injury, astrocytes become activated and begin to proliferate.It is very difficult to effectively control the proliferation of astrocytes and inhibit the formation of the glial scar through a single method.To provide a favorable microenvironment for spinal cord regeneration, the stationary,activated and proliferative states of the astrocytes should be considered comprehensively after spinal cord injury.In this experiment, rats with T8spinal cord contusion were subjected to GDAsBMPand hr-decorin transplantation.The response of a transection model of spinal cord injury could not objectively mimic clinical pathological changes of spinal cord injury as spinal cord contusion accounts for the vast majority of clinical cases.Hr-decorin inhibited astrocyte proliferation and glial scar formation, and even degraded preformed glial scar.GDAsBMPtransplantation to contusive spinal cord promoted astrocyte to form a linear arrangement that provided axonal growth channels, which promoted axonal connectivity and recovery of sensory and motor functions below the level of spinal cord contusion.
To confirm that combined GDAsBMPand hr-decorin transplantation could effectively promote the recovery of sensory and motor functions below the level of spinal cord contusion, this study observed and analyzed the size of the cavity, the degree of spinal cord atrophy, the expression of astrocytes,and the extent of motor and sensory axonal regeneration in rats with T8spinal cord contusion after treatment with GDAsBMPand hr-decorin transplantation.
RESULTS
Quantitative analysis of experimental animals
Before T8spinal cord contusion, 72 female Sprague-Dawley rats were randomly divided into hr-decorin injection group, GDAsBMPtransplantation group, combined GDAsBMPand hr-decorin transplantation group,and spinal cord injury group (each groupn=18).The spinal cord injury group served as a control and did not receive any treatment.According to the time after spinal cord contusion, each group was randomly divided into 3-,7- and 28-day subgroups (each subgroupn=6).Five rats died of anesthetic accident and seven rats died of excessive bleeding during surgery.New rats were used to supplement the dead rats.Seventy-two rats were included in the final analysis.
Effects of combined GDAsBMP and hr-decorin transplantation on the angles between astrocytelined axons in rats with spinal cord contusion
The angles between adjacent glial fibrillary acidic proteinpositive astrocyte-lined axons were measured with Image Pro Plus 6.0 software at 28 days after spinal cord contusion, and the extent of linear arrangement was estimated by comparing the angles (0–90°; the smaller the degree, the more linear the arrangement).The angles in the hr-decorin treatment group and spinal cord injury group were far bigger than the GDAsBMPtreatment group and combined GDAsBMPand hr-decorin transplantation group (P<0.05; Figure 1), indicating that GDAsBMPtransplantation alone or combined transplantation could effectively promote linear arrangement of astrocyte-lined axons but spinal injection of hr-decorin alone had no such effect.
Effect of combined GDAsBMP and hr-decorin transplantation on the cavity size and crosssectional area in the contusive spinal cord of rats
The cavities and cross-sectional area of spinal cord in glial fibrillary acidic protein-positive astrocyte fluorescence images (Figure 2) were measured and analyzed with Image Pro Plus 6.0 software.At 3 days after spinal cord contusion, hr-decorin injection or GDAsBMPtransplantation alone inhibited astrocyte proliferation, resulting in large cavities in the spinal cord center.GDAsBMPalone or combined transplantation partially filled the injured spinal cord, so the cross-sectional areas of spinal cord were relatively larger than the other two groups.Seven days after treatment, hr-decorin significantly inhibited astrocyte proliferation (P<0.05), resulting in the enlarged cavities in the hr-decorin injection group and thin spinal cord in the combined transplantation group.Twenty-eight days later, the cavities were obviously reduced in the GDAsBMPalone or combined transplantation groups, but were further enlarged in the spinal cord injury group (Figures 3–5).
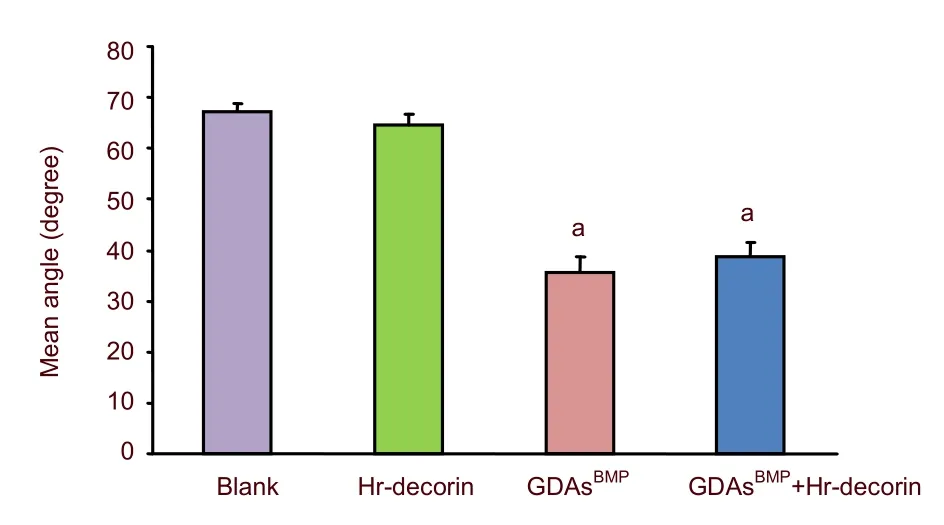
Figure 1 Effect of combined GDAsBMP and hr-decorin transplantation on the angles between the astrocyte-lined axons in the contusive spinal cord of rats at 28 days after injury.
Glial scar formation in the damaged region of rats undergoing combined GDAsBMP and hr-decorintransplantation at 28 days after spinal cordcontusion
The integrated absorbance values (area × average absorbance) of glial fibrillary acidic protein-positive astrocytes in the spinal cord injury group were significantly higher than the three treatment groups (P<0.05).Hr-decorin inhibition of astrocyte proliferation and glial scar formation was slightly stronger than in the GDAsBMPtransplantation group, and combined transplantation enhanced this effect but it was not significant (P> 0.05) between the three treatment groups (Figure 6).
Effect of combined GDAsBMP and hr-decorin transplantation on sensory and motor axons within contusive spinal cord of rats at 28 days after spinal cord contusion
A range of injured axons spread to 1 mm away from the injury center of the spinal cord.The injured axon terminals in the spinal cord injury group showed thinning,neuroma formation, bifurcation, germination, swelling,winding or disorganized growth within the injured interface at 28 days after spinal cord contusion (Figure 2).The growth ability of sensory and motor axons was reduced, and passing rates of all axons in each point were significantly lower in the spinal cord injury group than the three treatment groups (P<0.05).Hr-decorin injection,GDAsBMPtransplantation alone, or combined transplantation could effectively promote regeneration and growth of sensory and motor axons; nearly half of the sensory axons and some of the motor axons passed through the injury center; a small part of the sensory axons and motor axons reached a range of 5 mm from the injury center(Figures 7–9).
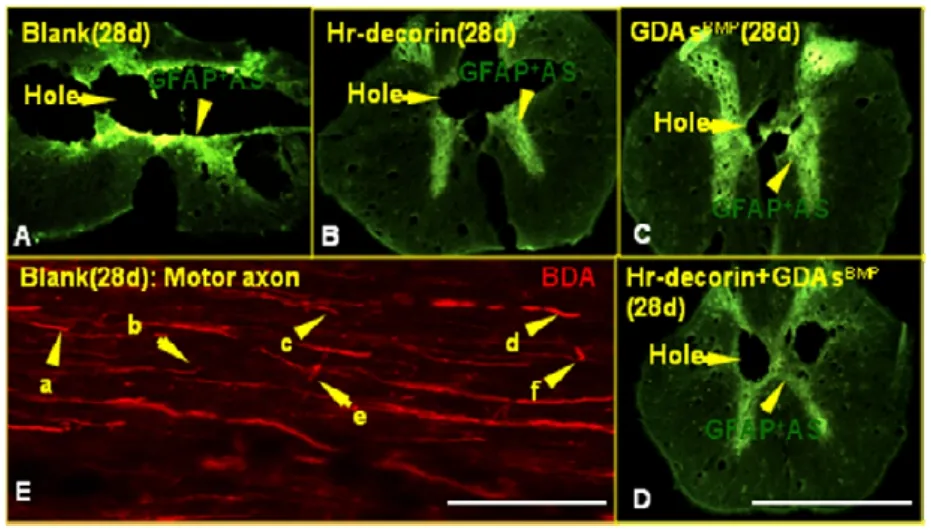
Figure 2 Expression of astrocytes, cavities and change of axonal terminal morphology in the center of the injured spinal cord at 28 days after injury.
Effects of combined GDAsBMP and hr-decorintransplantation on hindlimb motor function in rats with spinal cord contusion
At 1 day after spinal cord contusion, the Basso-Beattie-Bresnahan scores of rats in each group were 0.Three days later, Basso-Beattie-Bresnahan scores of all rats were no more than 2 points, indicating that there was no significant recovery of hindlimb motor function.However,7 days later, Basso-Beattie-Bresnahan scores in the treatment groups were significantly higher than the spinal cord injury group (P<0.05).Twenty-eight days later, the recovery level of hindlimb motor function in the treatment groups increased significantly, especially in the combined GDAsBMPand hr-decorin transplantation group(approaching 17 points;P<0.05), while Basso-Beattie-Bresnahan scores in the spinal cord injury group were around 10 points (Figure 10).
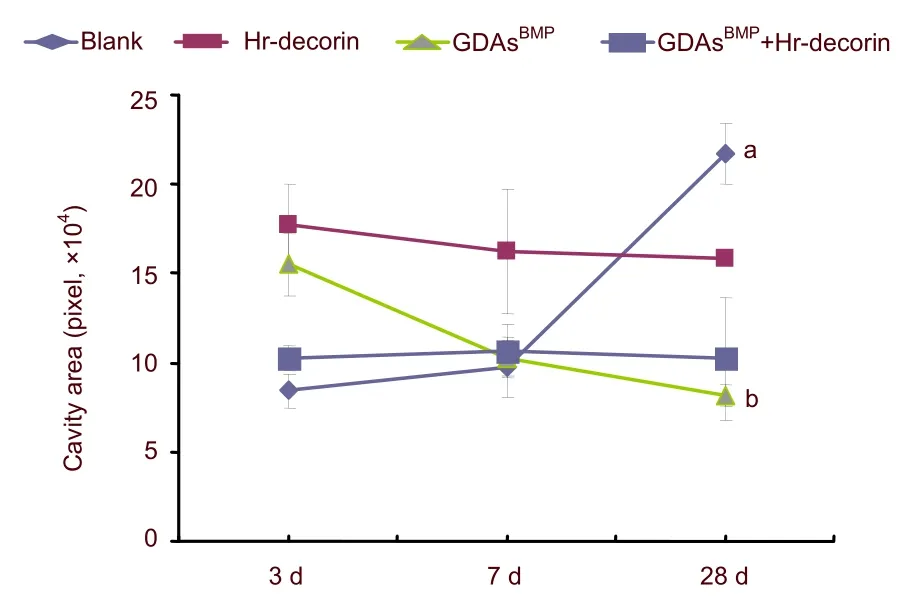
Figure 3 Effect of combined GDAsBMP and hr-decorin transplantation on the cavity area in the contusive spinal cord of rats.
DISCUSSION
GDAsBMP transplantation after spinal cord contusion effectively filled the cavity in contusive spinal cord
Glial-restricted precursor cells can be directly isolated from embryonic spinal cord[47], and neural stem cells and embryonic stem cells[48-49]can also be separately isolated from the central nervous system of mice and humans[40,50-54].

Figure 4 Effect of combined GDAsBMP and hr-decorin transplantation on the cross-sectional area in the contused spinal cord of rats.
After severe spinal cord contusion, a large cavity gradually forms in the injury center that seriously affects regeneration and growth.Small cavities are initially formed because of an early serious inflammatory response, intramedullary hemorrhage, spinal cord edema, and glial cell activation and proliferation after severe spinal cord contusion.However, in the later stages, huge cavities are gradually formed in the spinal cord injury center due to gray and white matter necrosis; a large glial scar forms surrounding the cavity; most axonal paths are disconnected; and a very small number of motor axons cross the injury center.Many methods have been investigated for filling these cavities, but little success has been achieved.After treatment with GDAsBMPfrom glial-restricted precursor cells induced with bone morphogenetic protein-4[40,55-57], although cavities formed in the first 7 days after spinal cord contusion, GDAsBMPinhibited astrocyte proliferation and the inflammatory response, and drastically reduced cavity size after day 7,through the proliferation of GDAsBMPand astrocytes, and regeneration of nerve tissue.At 3 days after spinal cord contusion, the ability of hr-decorin to inhibit astrocyte proliferation and the inflammatory response reduced,resulting in obvious spinal cord edema.Subsequently,the spinal cord became tapered as acute inflammation subsided.Following this, the cross-sectional area of the spinal cord gradually recovered because most of the cavities were filled.The cavities decreased and the recovery of cross-sectional area of spinal cord provided a carrier for axonal regeneration and growth.
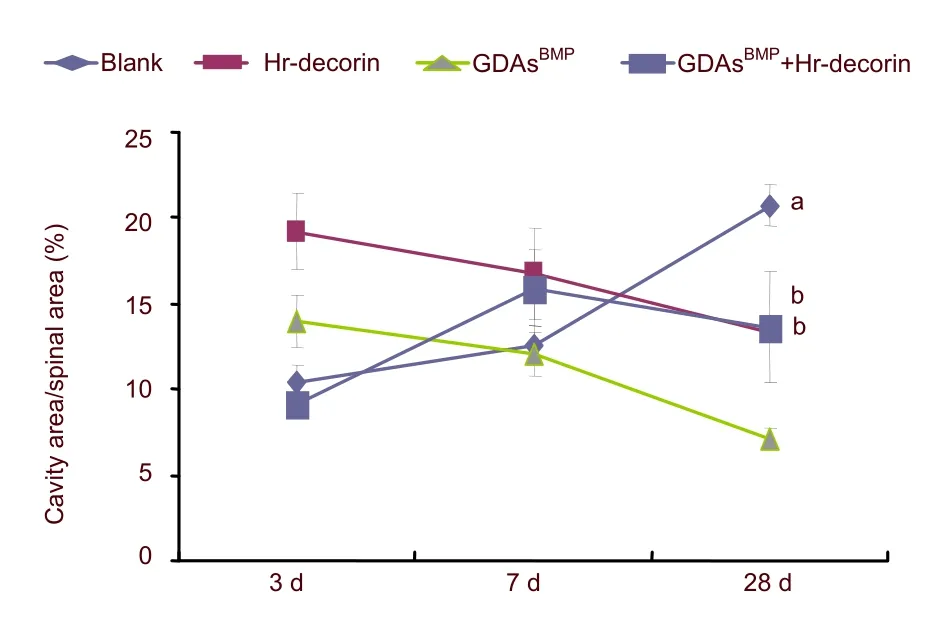
Figure 5 Effect of combined GDAsBMP and hr-decorin transplantation on the ratio between the cavity and cross-sectional areas in the contusive spinal cord of rats.
Formation of astrocyte linear arrangement and reduction in glial scar
The formation of a linear arrangement of astrocytes in the contusive spinal cord created the shortest path for axonal growth, which enhanced the efficiency of axons passing through the injury interface[40].After severe spinal cord contusion, a serious glial scar, characterized by an increased number and density of astrocytes, is formed within the injury interface.This blocks axonal growth, resulting in axon terminal atrophy, neuroma formation, and buckling or winding growth.Although many methods have been examined previously, removal of the glial scar remains a difficult issue.
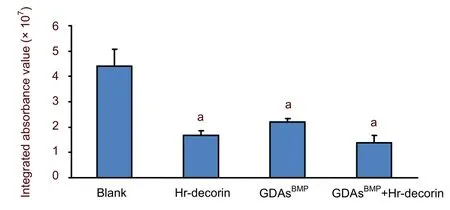
Figure 6 Effect of combined GDAsBMP and hr-decorin transplantation on glial fibrillary acidic protein-positive astrocytes within the contusive spinal cord of rats at 28 days after injury.
Thus, numerous studies have changed strategy to investigate mechanisms of preventing the formation of the glial scar in the early stages of spinal cord injury.After GDAsBMPtransplantation, the angle of astrocyte-lined axons significantly reduced, which denoted that the glial scar was significantly reduced and astrocyte-lined axons were evenly distributed.The results showed that GDAsBMPtransplanted into contusive spinal cord could effectively promote the linear arrangement of astrocyte-lined axons, while treatment with PBS or hr-decorin injection had no such effect.
Promotion of axonal regeneration and growth
GDAsBMPderived from glial-restricted precursor cells are a particular type of astrocyte possessing numerous functions, such as support, nutrition, and signal contact.After GDAsBMPtransplantation into the spinal cord, the vast majority of the cavities were filled by astrocytes and GDAsBMPpromoted the linear arrangement of astrocyte-lined axons, which provided channels for axonal regeneration and growth in the host.
GDAsBMPproduce brain-derived neurotrophic factor,which can promote survival of neurons in the contusive spinal cord and prevent axonal atrophy[40,58].GDAsBMPcan also delay the expression of chondroitin sulfate proteoglycans, thereby indirectly promoting axonal regeneration[40].At 28 days after GDAsBMPtransplantation, a number of motor axons crossed the injury center and reached the distal end of the spinal cord by growing along nerve channels established by astrocytes.The sensory axons in the spinal cord surface were increased,and moved around the cavity or through the injury center to reach the distal end of the spinal cord.In addition,GDAsBMPmay also have promoted the growth of other motor-related axons, such as the reticular tract and lateral corticospinal tract.
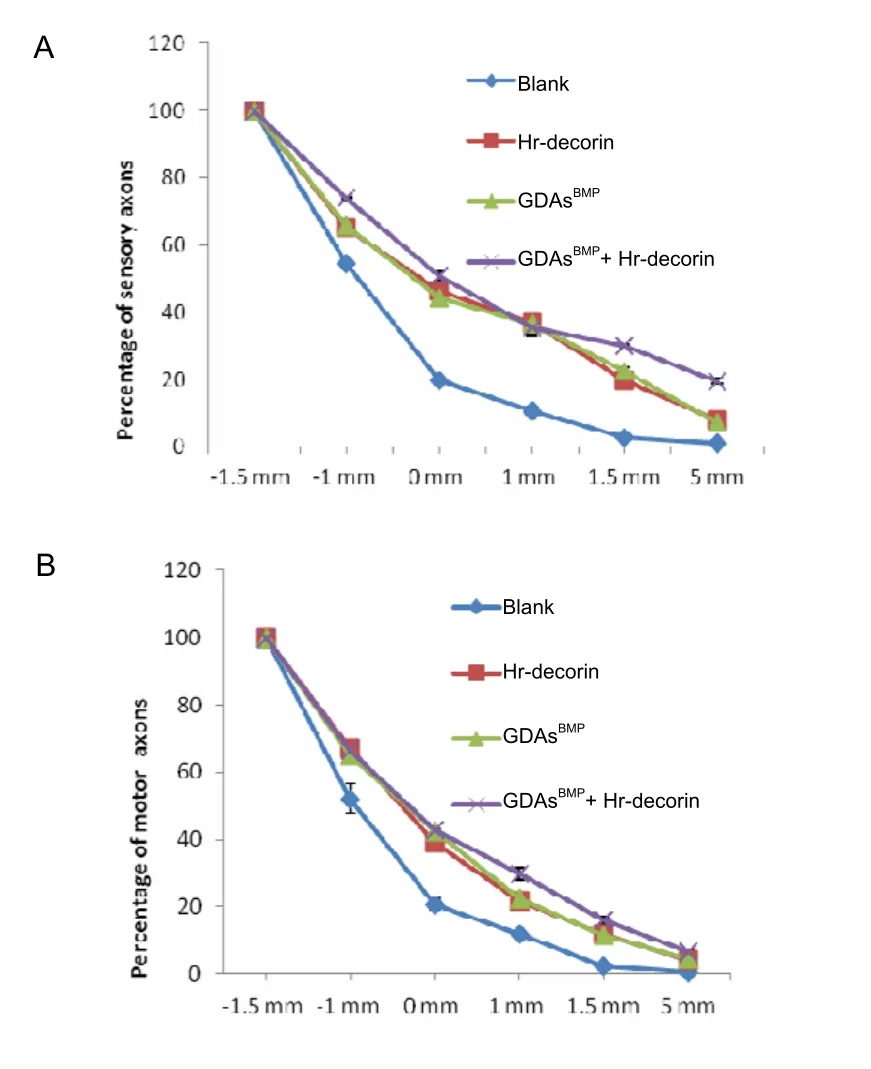
Figure 7 Passing rates of sensory (A) and motor (B)axons at different points (1.5 mm and 1 mm in front of the injury center [–1.5 mm, –1 mm], the injury center and 1 mm, 1.5 mm and 5 mm posterior to the injury center[1.5 mm, 5 mm]) at 28 days after spinal cord contusion in each group.
Hr-decorin injection after spinal cord contusion effectively inhibited glial scar formation and promoted glial scar degradation
Decorin is a small leucine-rich chondroitin sul-fate/dermatan sulfate proteoglycan, which is very rich in the vascular border zone, the brain surface, the ependyma and mesh tissue, as well as being expressed in the central nervous system after injury.Hr-decorin can promote endothelial cell formation of blood vesselsin vitroand the generation of capillariesin vivo, especially during the inflammatory stage.The degree of spinal cord blood stasis was significantly reduced at 7 days and disappeared at 28 days after hr-decorin injection by respectively comparing gross specimens of contusive spinal cord in rats, indicating that hr-decorin promoted the absorption of stagnant blood in the injured spinal cord.Hr-decorin has also been shown to inhibit inflammatory factors (transforming growth factor-β) and reduce the expression of I, III procollagen mRNA in cells, so it can effectively inhibit scar fibroblast proliferation.

Figure 8 Effect of combined GDAsBMP and hr-decorin transplantation on sensory axonal regeneration in the contusive dorsal spinal cord of rats at 28 days after injury.

Figure 9 Effect of combined GDAsBMP and hr-decorin transplantation on motor axonal regeneration within the contusive spinal cord of rats at 28 days after injury.
After hr-decorin treatment for 3 days, the inflammatory response and the proliferation of astrocytes were effectively suppressed and the product of the inflammatory response was degraded, which resulted in larger cavities than the spinal cord injury group.Following this,hr-decorin promoted plasminogen/plasmin synthesis,which not only inhibited synthesis of chondroitin sulfate proteoglycans, it also promoted degradation of chondroitin sulfate proteoglycans.Hr-decorin injection again at 14 days helped to inhibit scar formation and also degrade the glial scar that had formed in the earlier stages.
Protection of axons and promotion of axonal regeneration
Hr-decorin within the nervous system regulates collagen extracellular matrix, and promotes the fibronectin adhesion of axons, Schwann cell proliferation and neural cell survival[43].Hr-decorin effectively inhibited the early inflammatory response and promoted the formation of tiny blood vessels, prevented further secondary damage to injured axons, and provided a better microenvironment for axonal regeneration and growth.Hr-decorin inhibited astrocyte proliferation (early) and degraded the glial scar(late), resulting in a reduction in the inhibition of axonal regeneration and growth.Hr-decorin could not effectively induce a linear arrangement of astrocytes within the spinal cord, but could reduce the density of astrocytes,allowing axons to extend into the cavity.Hr-decorin promoted the synthesis of plasmin, which activated neurotrophic factors, degraded a variety of axonal growth inhibitors, and promoted reconstruction of the central nervous system[44].The experimental results demonstrate that hr-decorin not only effectively protected the injured spinal cord, it also promoted axonal regeneration to a certain extent.

Figure 10 Effect of combined GDAsBMP and hr-decorin transplantation on the Basso-Beattie-Bresnahan (BBB)scores in rats with contusive spinal cord.
Combined GDAsBMP and hr-decorin transplantation after spinal cord contusion more effectively inhibited the inflammatory response and reduced secondary axonal injury
Three days after spinal cord contusion, hr-decorin and GDAsBMPtransplantation inhibited the inflammatory response and may have decreased astrocyte proliferation,with no obvious thinning of the spinal cord; the remaining spinal cord tissue significantly increased as transplanted GDAsBMPfilled the cavity and both hr-decorin and GDAsBMPeffectively protected the spinal cord.Seven days later, because the inflammatory response and spinal cord edema decreased along with the inhibition of astrocyte proliferation by hr-decorin and GDAsBMPtreatment, the spinal cord had obviously thinned.At 28 days after spinal cord contusion, the inflammatory response had disappeared; GDAsBMPand hr-decorin had constantly inhibited astrocyte proliferation and glial scar formation, while hr-decorin alone also possibly inhibited GDAsBMPproliferation.Nevertheless, because of GDAsBMPproliferation, there were no significant changes in the areas of residual spinal cords and cavities, and the spinal cord tended to be stable.
Decreased glial scar and promotion of astrocyte linear arrangement
Combined GDAsBMPand hr-decorin transplantation for contusive spinal cord constantly inhibited the early inflammatory response and astrocyte proliferation, which substantially reduced glial scar formation.At 28 days after spinal cord contusion, treatment with either hr-decorin injection, GDAsBMPtransplantation or combined GDAsBMPand hr-decorin transplantation effectively suppressed astrocyte proliferation and scar formation.Although there was no statistically significant difference between the treatment groups, the decreasing trend of glial scar appeared in the combined transplantation group.The angles between astrocyte-lined axons after combined transplantation and GDAsBMPtransplantation were smaller, suggesting that both GDAsBMPtransplantation alone or combined transplantation effectively promoted the linear arrangement of astrocyte-lined axons in the host.
Significantly improved abilities of axonal regeneration and growth
The results suggest that combined transplantation promoted regeneration and growth of sensory and motor axons more effectively than hr-decorin injection or GDAsBMPtransplantation.First, combined transplantation further strengthened inhibition of the inflammatory response, promoted the formation of spinal cord capillaries and reduced chondroitin sulfate proteoglycan expression.Second, GDAsBMPpromoted the linear arrangement of astrocytes to provide channels for axonal growth.Third,GDAsBMPsecreted brain-derived neurotrophic factor to protect axons and neurons from atrophy in the early stages, and promoted axonal growth in the later stages.Finally, hr-decorin and GDAsBMPprovided a favorable microenvironment for axonal regeneration and growth at an early stage, which extended the time window for axonal regeneration in the acute stage.
Enhanced hindlimb motor function
Previous studies demonstrated that GDAsBMPtransplantation at 3 days after spinal cord promoted axonal regeneration and plasticity of axonal connections, and resulted in recovery of motor function[40].At 28 days after spinal cord contusion, GDAsBMPtransplantation or hr-decorin injection significantly promoted recovery of hindlimb motor function in rats.However, the mechanisms of functional recovery for both treatments were different.The inhibitory effects of hr-decorin on the early inflammatory response and glial scar formation were stronger than GDAsBMP, whereas GDAsBMPtransplantation provided brain-derived neurotrophic factor, which in turn prevented axonal and neuronal atrophy.GDAsBMPtransplantation induced astrocytes to form a linear arrangement within the host that provided growth channels for axons passing through the injury center and interfaces, whereas hr-decorin alone could not change astrocyte arrangement.Because hr-decorin injection and GDAsBMPtransplantation had different mechanisms for the treatment of spinal cord contusion, a combined transplantation was adopted.Hr-decorin inhibited the inflammatory response in the early stages, and protected axons and neurons together with GDAsBMP, thus creating a better microenvironment for enhancing axonal regeneration and growth and significantly promoting the recovery of hindlimb function.Furthermore, the recovery of hindlimb motor function was more obvious with time in the combined treatment group, indicating that the speed and extent of motor axonal regeneration were stronger in the later stages.Together, these results indicate that combined transplantation established axonal regeneration channels, reduced the glial scar and provided a favorable microenvironment for axonal regeneration and growth, which obviously enhanced the speed and degree of motor axon regeneration.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Laboratory of Academy of Military Medical Sciences, Beijing Institute of Neuroscience, Capital Medical University, China from 2009 to 2011.
Materials
A total of 72 female Sprague-Dawley rats weighing 200 ±10 g and one embryonic day 13.5 rat were obtained from the Academy of Military Medical Sciences, Beijing, China(license No.SCXK (Military) 2007-004).
Methods
Glial-restricted precursor cell isolation and GDAsBMP generation
After intramuscular injection with 1% pentobarbital sodium solution (45 mg/kg), spinal cords of embryonic day 13.5 rat embryos were isolated under an anatomical microscope[40,48].After the embryonic spinal cords were cut into 1 mm × 1 mm pieces and digested with 0.25%trypsin/ethylene diamine tetraacetic acid (Invitrogen,Carlsbad, CA, USA) solution, residual trypsin/ethylene diamine tetraacetic acid solution was neutralized by Dulbecco’s modified Eagle’s medium (DMEM; Hyclone,Logan, UT, USA) with a standard 10% fetal bovine serum(Hyclone Co.).The digested spinal cord tissues were gently pipetted with a Pasteur pipette, and the cells were then collected and diluted to 1 × 106cells/mL.After adding anti-A2B5 (Sigma-Aldrich Co., St.Louis, MO, USA)(0.1 mg/mL) and phycoerythrin anti-mouse IgM (eBioscience Co., San Diego, CA, USA; 1:100) in sequence,A2B5- positive cells accounted for 33.18% by flow cytometry analysis, then the primary A2B-positive glialrestricted precursor cells (90.65%) were ultimately sorted by fluorescence-activated cell sorting.The primary glialrestricted precursor cells were maintained on a fibronectin/laminin substrate (Sigma-Aldrich) in DMEM/F12 (Hyclone Co.) Sato-medium with 20 ng/mL rat basic fibroblast growth factor (Sigma-Aldrich)in vitro.Culture media containing DMEM/F12 and rat basic fibroblast growth factor were changed every 2 days.After serially passaging for no more than three generations, more glial-restricted precursor cells were obtained.To differentiate glial-restricted precursor cells into GDAsBMP(A2B5-negative/glial fibrillary acidic protein-positive), glial-restricted precursor cells were seeded on a fibronectin/laminin substrate in DMEM/F12 Sato-medium with 20 ng/mL basic fibroblast growth factor and 10 ng/mL of human recombinant bone morphogenetic protein-4 (R&D Systems Co., Ltd., Minneapolis, MN, USA) for 7 days.GDAsBMPwere collected by centrifugation and diluted by adding an appropriate volume of PBS.Subsequently, the anti-A2B5 and rabbit anti-rat glial fibrillary acidic protein polyclonal antibody (Sigma-Aldrich) was added.After incubation for 1 hour in a 37°C CO2incubator, the sec-ondary antibody including phycoerythrin anti-mouse IgM and goat anti-rabbit IgG-fluoresceine isothiocyanate(FITC) fluorescent antibody (Sigma-Aldrich) were added for 1 hour.A2-positive cells were removed by fluorescence-activated cell sorting, and purified A2B-negative glial fibrillary acidic protein-positive GDAsBMPwere obtained.The purified GDAsBMPwere collected by centrifugation, and diluted into cell suspensions (30 000 cells/μL)with Dulbecco’s modified Eagle’s medium/F12 for transplantation.
Preparation of T8 spinal cord contusion model
After intraperitoneal injection with 1% pentobarbital sodium(Merck Co., Darmstadt, Hessen, Germany; 35 mg/kg),female rats (body weight 200±10 g) were fixed on the operating table in the prone position.The T8–9spinous process and lamina were exposed along the posterior midline of the spine (T7–10), T8and the upper part of T9spinous process and laminar were carefully removed to fully expose the dura.Subsequently, the T8spinal cord was bruised with an NYU device (New York University, New York, NY, USA; 10 g × 50 mm).Contusive spinal cord suffered from swelling and congestion, and motor and sensory function in rats was lost completely[59].The Basso-Beattie-Bresnahan scores of all rats were 0 the next day and no more than 2 points at 3 days after spinal cord contusion, indicating that the combat strength fully met the requirements of the experiment.Later, rats with spinal cord contusion were injected with penicillin (50 000 U/kg per day) for 3 days, and urinated manually at least twice a day until autonomic micturition reflex appeared.
Drug injection or GDAsBMP transplantation
The strategies of drug injection or GDAsBMPtransplantation to rat spinal cord were divided into three cross-sections (Figure 11A), two injection points on each cross-section (Figure 11A, B) and two levels on each point(Figure 11A).The rats with T8spinal cord contusion were fixed in the prone position under rat brain stereotaxic apparatus with a micropipe installed with a 100 μm tiny glass needle.According to the design, the needle with drug or GDAsBMPwas slowly injected to 1.5 mm depth from the spinal cord dorsal surface directly for 1 minute, then withdrawn to 0.5 mm depth for 10 minutes.
Treatment of T8 spinal cord contusion in rats
Rats in the hr-decorin injection group, GDAsBMPtransplantation group, combined hr-decorin and GDAsBMPtransplantation group and spinal cord injury group were respectively injected with hr-decorin (R&D Systems Co.,Ltd.;1 μg/μL), GDAsBMPsuspension (30 000 cells/μL), hrdecorin and GDAsBMPsuspension (the same concentration and density as above), and PBS solution (0.1 mol/L)into the 12 injection sites within the damaged area (each site 0.5 μL; a total of 6 μL).Two weeks later, rats in the 28-day subgroup with hr-decorin injection received intraperitoneal injection of 0.5 mL hr-decorin solution(12 μg/mL).The rats undergoing GDAsBMPtransplantation received intramuscular injection of cyclosporine A(Novartis AG, Basel, Switzerland;10 mg/kg) every day.
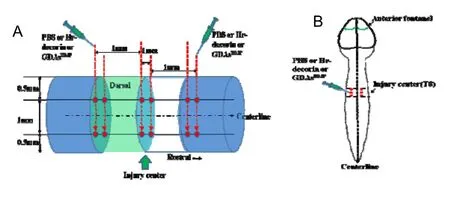
Figure 11 Schematic illustration of PBS injection or human recombinant decorin (Hr-decorin) injection or glial-restricted precursor-derived astrocytes induced by bone morphogenetic protein-4 (GDAsBMP) transplantation mode.
Motor function of rats scored by Basso-Beattie-Bresnahan scoring
At 3, 7, 14, 21 and 28 days after spinal cord contusion,behavioral changes and hindlimb motor function of rats were observed to obtain Basso-Beattie-Bresnahan function scores[60].These included the number and scope of hindlimb joint activities, load level of hindlimbs, coordination of forelimbs and hindlimbs, claws and tail activities.Basso-Beattie-Bresnahan function scores of rats were divided into 22 grades (0 to 21 grades): zero indicated complete paralysis and 21 indicated normal function.
Tracking of biotinylated dextran amine anterograde motor or sensory axons
The ability of axonal regeneration or growth after spinal cord contusion was observed with biotinylated dextran amine (Molecular Probes Co., Ltd., Junction, OR, USA)anterograde motor or sensory axonal tracking.At 2 weeks before rats were executed, 10% biotinylated dex-tran amine 10 000 was injected into both sides of the cerebral motor cortex in rats for anterograde corticospinal tract tracking[61].At 8 days before rats were executed,ascending endogenous axons were tracked by injecting 10% biotinylated dextran amine 10 000 into cuneate and gracile white matter at the T10spinal level in both sides of the spinal cord[40].
For biotinylated dextran amine anterograde motor axonal tracking, the rats were fixed under a stereotaxic apparatus (Stoelting, Chicago, IL, USA) in the prone position.A total of 16 holes (eight holes in each side; diameter of 0.5 mm) located in the 16 points were respectively drilled.Biotinylated dextran amine was slowly (0.1 μL/min) injected into the 16 points (Figure 12A, 1.0 mm depth to the cerebral motor cortex surface; each point 0.5 μL) for 5 minutes.For biotinylated dextran amine anterograde sensory axonal tracking, T10dura mater was fully exposed, and then the rats were fixed under a stereotaxic apparatus in prone position.Biotinylated dextran amine was slowly (0.1 μL/min) injected into two points (Figure 12B); 0.5 mm depth to the spinal cord surface; each point 0.5 μL) for 10 minutes.

Figure 12 Biotinylated dextran amine (BDA)anterograde motor or sensory axonal tracking in rats.
Double immunofluorescence histochemistry
A 3, 7 and 28 days after spinal cord contusion, six rats from each group were executed for double immunofluorescence histochemistry to observe the ability of motor and dorsal ascending sensory axonal regeneration(biotinylated dextran amine marked), and astrocyte expression within the contusive spinal cord (glial fibrillary acidic protein expression).After cardiac perfusion, the spine was completely removed and post-fixed in 4%paraformaldehyde solution for 2 hours.The osseous structure of the spine was stripped off; the spinal cord was completely removed and protected in 30% sucrose/PBS solution (4°C) overnight.The spinal cord was embedded in optimal cutting temperature medium (SAKURA, Tokyo, Japan) for serial frozen sectioning.Serial longitudinal sections (50 μm) were obtained from the dorsal to spinal medullary canal.Cross-sections (50 μm)were respectively obtained at –1.5 mm, –1 mm, 0 mm,+1 mm , +1.5 mm and +5 mm points (“0”, “–” and “+”indicating the spinal cord injury center, approaching and withdrawing from the biotinylated dextran amine injection point).After antigen repairing, sections were immersed in sodium citrate buffer with 0.1% Triton X-100 for 5 minutes, treated with 5% standard goat serum and closed for 30 minutes at room temperature.Subsequently, rabbit glial fibrillary acidic protein polyclonal antibody(Sigma-Aldrich; 1:100) was added at 4°C overnight.The next day, sections were incubated with Avidin-Cy3(Sigma-Aldrich;1:50; marked biotinylated dextran amine)and goat anti-rabbit IgG-FITC (Zymed Laboratories Co.,San Diego, CA, USA) (1:50) fluorescent antibody for 2 to 3 hours at room temperature, and then immersed in PBS with 0.1% Tween-20.Finally, biotinylated dextran amine(red) and glial fibrillary acidic protein (green) expression of the spinal cord sections were respectively observed with DMLA 4000B fluorescence microscope (Leica,Wetzlar, Germany) and TCS SP5 confocal microscope(Leica), and measured with the Image Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Data were expressed as mean±SEM and analyzed using a two-samplet-test and one-way analysis of variance with SPSS 11.5 software (SPSS, Chicago, IL, USA).APvalue<0.05 was considered statistically significant.
[1]Fry EJ.Central nervous system regeneration: mission impossible? Clin Exp Pharmacol Physiol.2001;28(4):253-258.
[2]Park DH, Lee JH, Borlongan CV, et al.Transplantation of umbilical cord blood stem cells for treating spinal cord injury.Stem Cell Rev.2011;7(1):181-194.
[3]Liu J, Götherström C, Forsberg M, et al.Human neural stem/progenitor cells derived from embryonic stem cells and fetal nervous system present differences in immunogenicity and immunomodulatory potentials in vitro.Stem Cell Res.2013;10(3):325-337.
[4]Kim JY, Kim DH, Kim JH, et al.Umbilical cord blood mesenchymal stem cells protect amyloid-β42 neurotoxicity via paracrine.World J Stem Cells.2012;4(11):110-116.
[5]Han X, Yang N, Cui Y, et al.Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat.Neurosci Lett.2012;521(2):136-141.
[6]Ozdemir M, Attar A, Kuzu I, et al.Stem cell therapy in spinal cord injury: in vivo and postmortem tracking of bone marrow mononuclear or mesenchymal stem cells.Stem Cell Rev.2012;8(3):953-962.
[7]Wiliams RR, Bunge MB.Schwann cell transplantation: a repair strategy for spinal cord injury? Prog Brain Res.2012;201:295-312.
[8]Hill CE, Brodak DM, Bartlett Bunge M.Dissociated predegenerated peripheral nerve transplants for spinal cord injury repair: a comprehensive assessment of their effects on regeneration and functional recovery compared to schwann cell transplants.J Neurotrauma.2012;29(12):2226-2243.
[9]Joosten EA, Veldhuis WB, Hamers FP.Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury.J Neurosci Res.2004;7(1):127-142.
[10]Fan C, Zheng Y, Cheng X, et al.Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury.Int J Biol Sci.2013;9(1):78-93.
[11]Radtke C, Kocsis JD.Peripheral nerve injuries and transplantation of olfactory ensheathing cells for axonal regeneration and remyelination: factor fiction? Int J Mol Sci.2012;13(10):12911-12924.
[12]Yazdani SO, Pedram M, Hafizi M, et al.A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat.Tissue Cell.2012;44(4):205-213.
[13]Roet KC, Eggers R, Verhaagen J.Noninvasive bioluminescence imaging of olfactory ensheathing glia and schwann cells following transplantation into the lesioned rat spinal cord.Cell Transplant.2012;21(9):1853-1865.
[14]Li BC, Xu C, Zhang JY, et al.Differing Schwann cells and olfactory ensheathing cells behaviors, from interacting with astrocyte, produce similar improvements in contused rat spinal cord's motor function.J Mol Neurosci.2012;48(1):35-44.
[15]Sharp J, Keirstead HS.Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells.Curr Opin Biotechnol.2007;18(5):434-440.
[16]Sharp J, Frame J, Siegenthaler M, et al.Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury.Stem Cells.2010;28(1):152-163.
[17]Bazley FA, Pourmorteza A, Gupta S, et al.DTI for assessing axonal integrity after contusive spinal cord injury and transplantation of oligodendrocyte progenitor cells.Conf Proc IEEE Eng Med Biol Soc.2012;2012:82-85.
[18]Piao JH, Wang Y, Duncan ID.CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord.Glia.2013;61(3):361-367.
[19]Rajasekaran S, Kanna RM, Shetty AP, et al.Efficacy of diffusion tensor anisotropy indices and tractography in assessing the extent of severity of spinal cord injury: an in vitro analytical study in calf spinal cords.Spine J.2012;12(12):1147-1153.
[20]Valsasina P, Rocca MA, Absinta M, et al.Cervical cord FMRI abnormalities differ between the progressive forms of multiple sclerosis.Hum Brain Mapp.2012;33(9):2072-2080.
[21]Bramlett HM, Dietrich WD.Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies.Prog Brain Res.2007;161:125-141.
[22]Davies SJ, Fitch MT, Memberg SP, et al.Regeneration of adult axons in white matter tracts of the central nervous system.Nature.1997;390(6661):680-683.
[23]Shechter R, Raposo C, London A, et al.The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair.PLoS One.2011;6(12):e27969.
[24]Parry PV, Engh JA.Promotion of neuronal recovery following experimental SCI via direct inhibition of glial scar formation.Neurosurgery.2012;70(6):N10-11.
[25]Wu J, Pajoohesh-Ganji A, Stoica BA, et al.Delayed expression of cell cycle proteins contributes to astroglial scar formation and chronic inflammation after rat spinal cord contusion.J Neuroinflammation.2012;9:169.
[26]Kuerten S, Gruppe TL, Laurentius LM, et al.Differential patterns of spinal cord pathology induced by MP4, MOG peptide 35-55, and PLP peptide 178-191 in C57BL/6 mice.APMIS.2011;119(6):336-346.
[27]De Winter F, Oudega M, Lankhorst AJ, et al.Injury-induced class 3 semaphorin expression in the rat spinal cord.Exp Neurol.2002;175(1):61-75.
[28]Tang X, Davies JE, Davies SJ.Changes in distribution,cell associations, and protein expression levels of NG2,neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue.J Neurosci Res.2003;71(3):427-444.
[29]Chen MS, Huber AB, van der Haar ME, et al.Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1.Nature.2000;403(6768):434-439.
[30]Wang KC, Koprivica V, Kim JA, et al.Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth.Nature.2002;417(6892):941-944.
[31]Zendedel A, Nobakht M, Bakhtiyari M, et al.Stromal cell-derived factor-1 alpha (SDF-1α) improves neural recovery after spinal cord contusion in rats.Brain Res.2012;1473:214-226.
[32]Han SS, Liu Y, Tyler-Polsz C, et al.Transplantation of glial-restricted precursor cells into the adult spinal cord:survival, glial-specific differentiation, and preferential migration in white matter.Glia.2004;45(1):1-16.
[33]Hill CE, Proschel C, Noble M, et al.Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration.Exp Neurol.2004;190(2):289-310.
[34]Hofstetter CP, Holmstrom NA, Lilja JA, et al.Allodynia limits the usefulness of intraspinal neural stem cell grafts;directed differentiation improves outcome.Nat Neurosci.2005;8(3):346-353.
[35]Giménez y Ribotta M, Menet V, Privat A.The role of astrocytes in axonal regeneration in the mammalian CNS.Prog Brain Res.2001;132:587-610.
[36]Hobohm C, Günther A, Grosche J, et al.Decompsition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats.J Neurosci Res.2005;80(4):539-548.
[37]Gregori N, Proschel C, Noble M, et al.The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocytetype-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function.J Neurosci.2002;22(1):248-256.
[38]Liu Y, Wu Y, Lee JC, et al.Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development.Glia.2002;40(1):25-43.
[39]Phillips AW, Falahati S, De Silva R, et al.Derivation of glial restricted precursors from E13 mice.J Vis Exp.2012;(64):3462.
[40]Davies JE, Huang C, Proschel C, et al.Astrocytes derived from glial-restricted precursors promote spinal cord repair.J Biol.2006;5(3):7.
[41]Schachtrup C, Ryu JK, Helmrick MJ, et al.Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage.J Histochem Cytochem.J Neurosci.2010;30(17):5843-5854.
[42]Kritis A, Kapoukranidou D, Michailidou B, et al.Sciatic nerve crush evokes a biphasic TGF-beta and decorin modulation in the rat spinal cord.Hippokratia.2010;14(1): 37-41.
[43]Hanemann CO, Kuhn G, Lie A, et al.Expression of decorin mRNA in the nervous system of rat.J Histochem Cytochem.1993;41(9):1383-1391.
[44]Davies JE, Tang X, Bournat JC, et al.Decorin promotes plasminogen/plasmin expression within acute spinal cord injuries and by adult microglia in vitro.J Neurotrauma.2006;23(3-4):397-408.
[45]Minor KH, Bournat JC, Toscano N, et al.Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue.Brain.2011;134(Pt 4):1140-1155.
[46]Minor K, Tang X, Kahrilas G, et al.Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons.Neurobiol Dis.2008;32(1):88-95.
[47]Rao MS, Noble M, Mayer-Pröschel M.A tripotential glial precursor cell is present in the developing spinal cord.Proc.Proc Natl Acad Sci U S A.1998;95(7):3996-4001.
[48]Rao MS, Mayer-Proschel M.Glial-restricted precursors are derived from multipotent neuroepithelial stem cells.Dev Biol.1997;188(1):48-63.
[49]Mayer-Proschel M, Kalyani AJ, Mujtaba T, et al.Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells.Neuron.1997;19(4):773-785.
[50]Ben-Hur T, Rogister B, Murray K, et al.Growth and fate of PSA-NCAM+ precursors of the postnatal brain.J Neurosci.1998;18(15):5777-5788.
[51]Mujtaba T, Piper DR, Kalyani A, et al.Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells.Dev Biol.1999;214(1):113-127.
[52]Dietrich J, Noble M, Mayer-Proschel M.Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells.Glia.2002;40(1):65-77.
[53]Lepore AC, O'Donnell J, Kim AS, et al.Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS.PLoS One.2011;6(10):e25968.
[54]Sandrock RW, Wheatley W, Levinthal C, et al.Isolation,characterization and preclinical development of human glial-restricted progenitor cells for treatment of neurological disorders.Regen Med.2010;5(3):381-394.
[55]Baptiste DC, Tighe A, Fehlings MG.Spinal cord injury and neural repair: focus on neuroregenerative approaches for spinal cord injury.Expert Opin Investig Drugs.2009;18(5):663-673.
[56]Noble M, Davies JE, Mayer-Pröschel M, et al.Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury.Neurotherapeutics.2011;8(4):677-693.
[57]Haas C, Neuhuber B, Yamagami T, et al.Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration.Exp Neurol.2012;233(2):717-732.
[58]Kobayashi NR, Fan DP, Giehl KM, et al.BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration.J Neurosci.1997;17 (24):9583-9595.
[59]Fukui N, Fukuda A, Kojima K, et al.Suppression of fibrous adhesion by proteoglycan decorin.J Orthop Res.2001;19(3):456-462.
[60]Davies JE, Tang X, Denning JW, et al.Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries.Eur J Neurosci.2004;19(5):1226-1242.
[61]Anderson AJ, Robert S, Huang W, et al.Activation of complement pathways after contusion-induced spinal cord injury.J Neurotrauma.2004;21(12):1831-1846.
[62]Basso DM, Beattie MS, Bresnahan JC.A sensitive and reliable locomotor rating scale for open field testing in rats.J Neurotrauma.1995;12(1):1-21.
[63]Zhou HL, Yang HJ, Li YM, et al.Changes in glial cell line-derived neurotrophic factor expression in the rostral and caudal stumps of the transected adult rat spinal cord.Neurochem Res.2008;33(5):927-937.
