Et3NHCl-AlCl3Ionic Liquids as Catalyst for Alkylation of Toluene with 2-Chloro-2-methylpropane
Chen Han; Luo Guohua; Xu Xin; Wang Yanli; Xia Jiajia
(1. Department of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 1026172; 2. College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029)
Et3NHCl-AlCl3Ionic Liquids as Catalyst for Alkylation of Toluene with 2-Chloro-2-methylpropane
Chen Han1; Luo Guohua1; Xu Xin1; Wang Yanli2; Xia Jiajia2
(1. Department of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 1026172; 2. College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029)
Alkylation of toluene with 2-chloro-2-methylpropane (t-Bu-Cl) to synthesize para-tert-butyltoluene (PTBT) was carried out in the presence of triethylamine hydrochloride-aluminum chloride ionic liquids used as the catalyst. The ionic liquids were prepared with different molar ratios of Et3NHCl to AlCl3, and the effect of the molar ratio between AlCl3and Et3NHCl, the reaction time, the reaction temperature, the ionic liquid dosage, as well as the molar ratio of toluene to chloro-2-methylpropane on the alkylation reaction of toluene with chloro-2-methyl-propane was investigated. The test results showed that the acidic ionic liquids prepared with Et3NHCl and AlCl3had good activity and selectivity for the alkylation reaction of toluene with alkyl chloride to produce PTBT. The optimal reaction conditions were specified at an AlCl3to Et3NHCl ratio of 1.6, a reaction temperature of 20 ℃, a mass fraction of toluene to ionic liquid of 10%, and a chloro-2-methylpropane to toluene molar ratio of 0.5. Under the suitable reaction conditions, a 98% conversion of chloro-2-methylpropane and an 82.5% selectivity of PTBT were obtained. Ionic liquids could be reused 5 times with its catalytic activity unchanged, and the regenerated ionic liquids can be recycled.
alkylation; ionic liquid; toluene; 2-chloro-2-methylpropane; Et3NHCl-AlCl3
1 Introduction
Para-tert-butyltoluene (PTBT) is a valuable raw material in spice industry and an important intermediate for manufacture of pharmaceutics and insecticides[1-2]. PTBT has been synthesized via the Friedel-Crafts alkylation of toluene and isobutylene in the presence of catalysts consisting of homogeneous acid such as AlCl3, H2SO4, HF and other Lewis acid catalysts used in the spice industry[3]. However, almost all of the processes have encountered some common problems, such as heavy environmental pollution, and tough problems associated with product recovery and purification[4]. Therefore, the investigation and development of environmentally friendly catalytic technology have become the focus of attention in chemical industry.
Recently, the ionic liquids that are known as environmentally benign catalysts and solvents have been extensively used in the alkylation reaction[5-9]. Ionic liquids uniquely integrate many valuable properties including high polarity, low viscosity, high liquid temperature range, high thermal stability, wide tunability, immiscibility with certain organic solvents, and absence of effective vapor pressure. The main advantages of ionic liquid catalysis are its ability to greatly enhance reaction rates, along with higher activity at low temperature and higher selectivity[10-15]. Lowmelting ionic liquids composed of an organic chloride and aluminum chloride are used as solvents and catalysts for the Friedel–Crafts reactions as referred to in literature reports[16-17]. The alkylation of toluene with 2-chloro-2-methylpropane (t-Bu-Cl) to synthesize PTBT was carried out in the presence of triethylamine hydrochloridealuminum chloride ionic liquids used as the catalyst under optimal reaction conditions. The effect of process parameters like the reaction time, the reaction temperature, and the reactants ratio was investigated. Furthermore, the recyclability and regeneration of ionic liquids were also studied. As a result, some useful basic data for industrial production can be provided.
2 Experimental
2.1 Synthesis of ionic liquid
Ionic liquid prepared from triethylamine hydrochloride and anhydrous AlCl3was selected as the catalyst from a series of ionic liquids for the alkylation of toluene witht-Bu-Cl. Et3NHCl-AlCl3(Al-IL) was prepared by slow addition of a desired amount of anhydrous AlCl3to the reagent triethylamine hydrochloride[18-19]. The reaction was carried out under stirring for 3 hours at room temperature, in order to yield a perfect compound of triethylamine hydrochloride with AlCl3(Al-IL)[20-24]. The whole process was kept under blanketing with a dry nitrogen atmosphere to avoid the hydrolysis of AlCl3. The Al-IL, once prepared, could be stored for a long time in a dry inert atmosphere.
The ionic liquid used in this work had an AlCl3to triethylamine hydrochloride (Et3NHCl) molar ratio of 2.0.
The ratio (XAlCl3) is defined as:
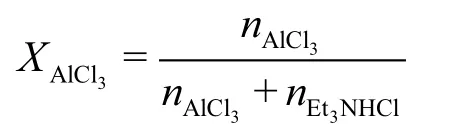
The reaction for synthesis of ionic liquid was carried out under an inert gas atmosphere, owing to the hydrophilicity of the catalyst towards moisture. The solvents were dried according to the procedure referred to in the literature[25-26].
2.2 Alkylation reaction
The liquid-phase alkylation reaction of toluene andt-Bu-Cl in the presence of ionic liquids used as the catalyst was performed in a glass reactor, fitted with a reflux condenser and magnetic stirrer. In a typical run, toluene after being dehydrated with anhydrous calcium chloride together with an appropriate amount of newly-made catalyst, were added in the reactor prior to slow addition of a specified amount oft-Bu-Cl in the reactor under constant stirring. The reactor was then heated in a water bath by an electric heater to the desired temperature, at which the reaction was initiated in the specified temperature range. After several minutes, the reaction mixture was cooled down to room temperature with the catalyst being separated from the reaction mixture.
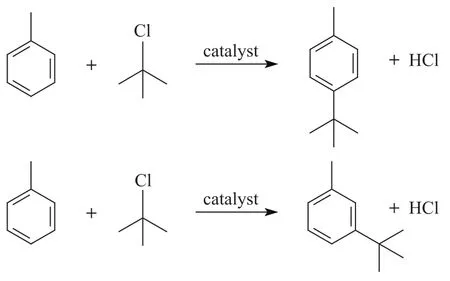
The liquid samples were analyzed by GC equipped with a flame ionization detector (FID) provided with a 5% phenyl methylsilicone capillary column (measuring 30 m×0.25 mm) using nitrogen as the carrier gas. Reference substances and GC–MS (GC 6890–MS 5973N) were used for identification of the products. Ortho-tert-butyltoluene did not appear as product due to the orientation effect and space steric effect of methyl group which could be verified by chromatographic analysis.
3 Results and Discussion
3.1 Influence of the acidity of ionic liquids on reaction
Et3NHCl-nAlCl3was synthesized at the Et3NHCl/AlCl3molar ratios varying in the range of 1:n. The molar fraction of AlCl3had a significant influence on the acidity of ionic liquids, which could decide the catalytic activity. When the molar ratio between Et3NHCl and AlCl3was more than one, the molten salt remained in a state of viscous glassy material at room temperature and atmospheric pressure, which would reduce the catalytic activity of the ionic liquid itself. Furthermore, the reaction cannot be observed as long as the Et3NHCl/AlCl3molar ratio is 1:1 according to the data collected from experiments. It is reported that the acidity of ionic liquids should be present as long as the molar ratio of Et3NHCl and AlCl3is under 1:1. The acidity of the ionic liquids showed an upward trend with the increase ofXAlCl3. As it can be seen from the data collected from experimental results, when the molar ratio of Et3NHCl and AlCl3reached 1:1.1, the alkylation reaction could be catalyzed effectively since the formation of carbocation could greatly contribute to alkylation reaction of toluene, denoting that the increasing Lewis acidity of catalyst was responsible for its activity. Furthermore, the molar fraction of AlCl3had a decisive influenceon the product selectivity. The influence of the acidity of ionic liquids on the alkylation reaction is shown in Figure 1. It can be seen from the data that the conversion oft-Bu-Cl and selectivity of PTBT reached 98% and 83.3%, respectively, when the molar ratio of Et3NHCl to AlCl3was 1:1.6, indicating that the acidity, which is determined by anions in the ionic liquid, should be treated as the essential factor that can affect the selectivity of catalyst. In conclusion, the selectivity of the catalyst could be regulated through adjusting the molar ratio of AlCl3.
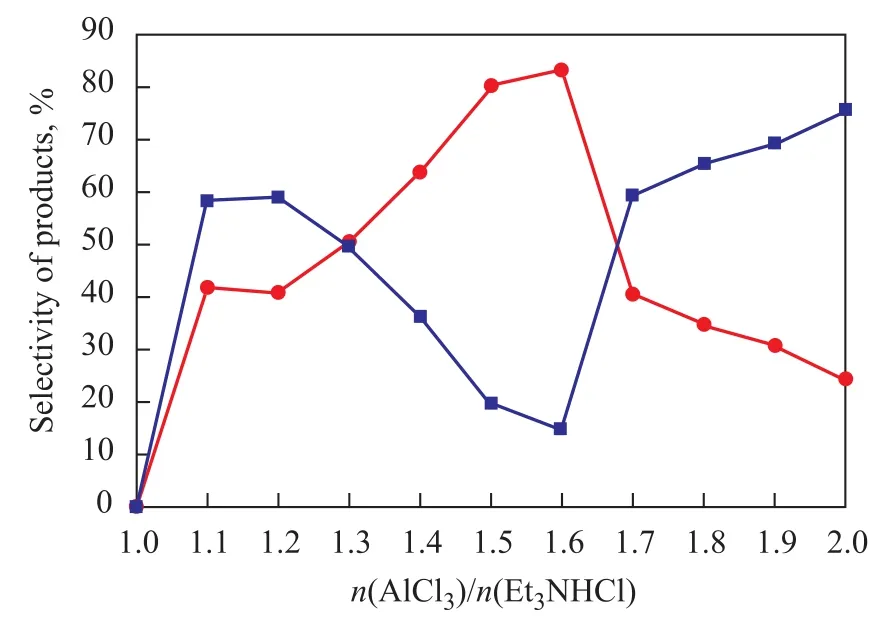
Figure 1 Influence of molar ratio of AlCl3to Et3NHCl on alkylation reaction
3.2 Influence oft-Bu-Cl to toluene molar ratio on the alkylation reaction
The influence of the molar ratio on the alkylation reaction is shown in Figure 2. It was observed that with an increasing amount oft-Bu-Cl, the conversion of toluene increased and the selectivity of PTBT also increased provided that thet-Bu-Cl /toluene molar ratio was less than 0.5. The selectivity was determined by the molar ratio between chloro-2-methylpropane and toluene when other conditions were fixed and this effect was made up of two factors. On the one hand, not only did the probability of reaction between toluene and chloro-2-methylpropane rise, but also the opportunity for formation of deep alkylate of chloro-2-methylpropane with MTBT and PTBT declined due to the dilution effect caused by toluene. On the other hand, the increasing concentration of toluene would be beneficial to the disproportionation reaction between toluene and poly-butylated toluene to transform the byproducts into PTBT and MTBT(m-tert-butyltoluene), so that the selectivity of target product would increase. However, an excessive molar ratio between chloro-2-methylpropane and toluene would reduce the efficiency of reaction unit and increase the load of separation unit. According to the experimental results, the selectivity of PTBT accounted for 82.5% when the molar ratio between chloro-2-methylpropane and toluene was 0.5:1, and the selectivity would be affected if this molar ratio increased excessively. In conclusion, the appropriate molar ratio between chloro-2-methylpropane and toluene should be 0.5:1.
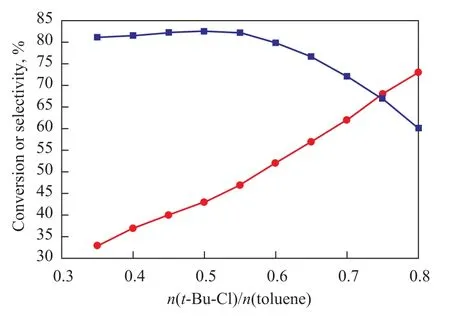
Figure 2 Influence of t-Bu-Cl to toluene molar ratio on target product selectivity of alkylation reaction
3.3 Influence of catalyst amount on alkylation reaction
The influence of the amount of ionic liquids on the alkylation reaction is shown in Figure 3, when alkylation oft-Bu-Cl with toluene was carried out with various percentage of aluminum chloride radicals in ionic liquid ranging from 2% to 20% (based on the total weight of toluene). When the amount of ionic liquid increased under the same reaction conditions, the conversion oft-Bu-Cl increased. When the catalyst dosage was 10%, the conversion oft-Bu-Cl reached 98.2%. The reason why the catalytic activity did not perform perfectly during the alkylation reaction might be attributed to the insufficient amount of the catalyst to provide a necessary amount ofacid sites. The trend of increase in conversion of chloro-2-methylpropane could be explained by the fact that the quantity of acid sites increased with an increasing dosage of catalyst.
However, the conversion oft-Bu-Cl still remained stable at 98% even though the amount of catalyst increased to 20% (Figure 3), which indicated that the ionic liquid could be treated as a solvent so that once the reactant was diluted by a redundant amount of solvent, the catalytic activity did not perform perfectly in the reaction. This trend also illustrated that 10% of catalyst in the catalytic system would have enough acid strength and concentration to effectively catalyze the alkylation reaction, leading to a 98.2% conversion oft-Bu-Cl and also an 83% selectivity to PTBT.
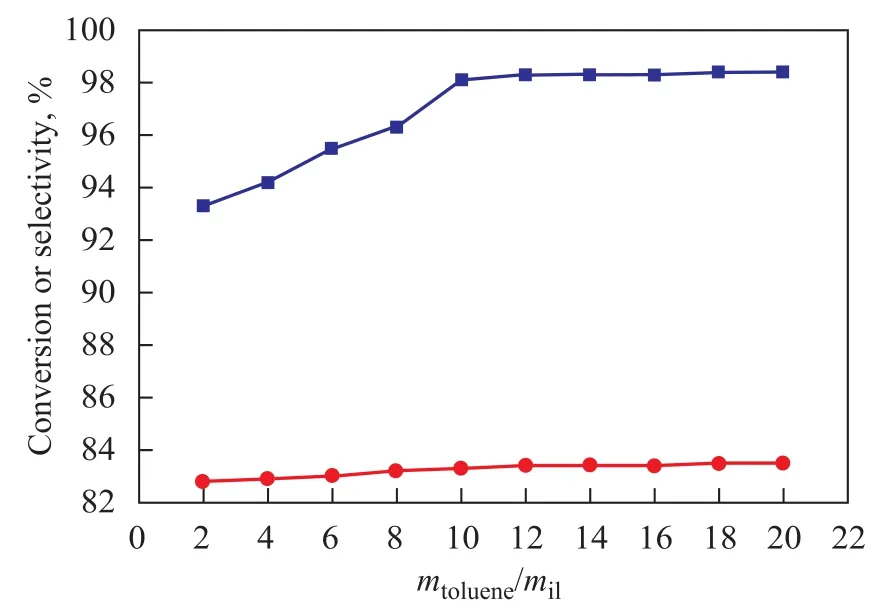
Figure 3 Influence of amount of ionic liquid on alkylation reaction
3.4 Influence of temperature on the alkylation reaction
Reaction temperature is regarded as one of the essential factors to the activity of catalyst, because the reaction temperature not only can influence both the activity of catalyst and the reaction rate during alkylation, but also can affect the diffusion rate of both reactants and products. Alkylation reaction between toluene and chloro-2-methylpropane is an exothermic reaction with remarkable reaction heat release, which implies that a lower temperature is more suitable to the reaction. The influence of the reaction temperature on toluene alkylation reaction was also investigated and the results are shown in Figure 4. The maximum selectivity of PTBT was obtained at a temperature of about 20 ℃ (293K). Meanwhile, the selectivity of MTBT saw an upward trend with the increase of reaction temperature, while the selectivity of PTBT witnessed a downward trend simultaneously. The reason why this phenomenon might occur was that the isomerization reaction played a significant role at high temperature, because there was a trend on converting PTBT to MTBT, which could be treated as an important factor causing a sliding selectivity of PTBT. However, the reaction kinetics must be taken into account in order to guarantee an unrestricted flowability of ionic liquid and also an appropriate reaction velocity, even though the selectivity of PTBT was higher at lower temperature. Therefore, a temperature of 20 ℃ (293K) would be an adequate compromise between thermodynamic and kinetic factors.
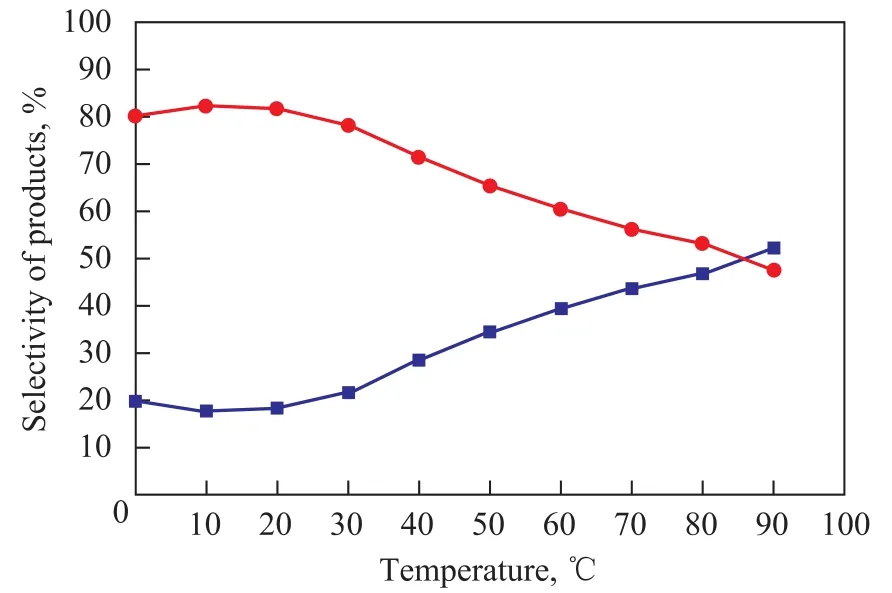
Figure 4 Influence of temperature on alkylation reaction
3.5 Influence of reaction time on alkylation reaction
The influence of reaction time on the conversion oft-Bu-Cl and the PTBT selectivity of ionic liquids is given in Figure 5. It can be readily seen from Figure 5 that the reaction time had only a slight influence on the outcome of alkylation reaction. Within 5 min, the catalytic activity and product selectivity reached an equilibrium (which is clearly shown in Figure 5); an 83% selectivity of desired product (PTBT) was achieved and no decomposition of PTBT and secondary alkylation reaction were detected when the alkylation reaction terminated. So, this is a quick reaction to achieve kinetic and thermodynamic equilibrium.
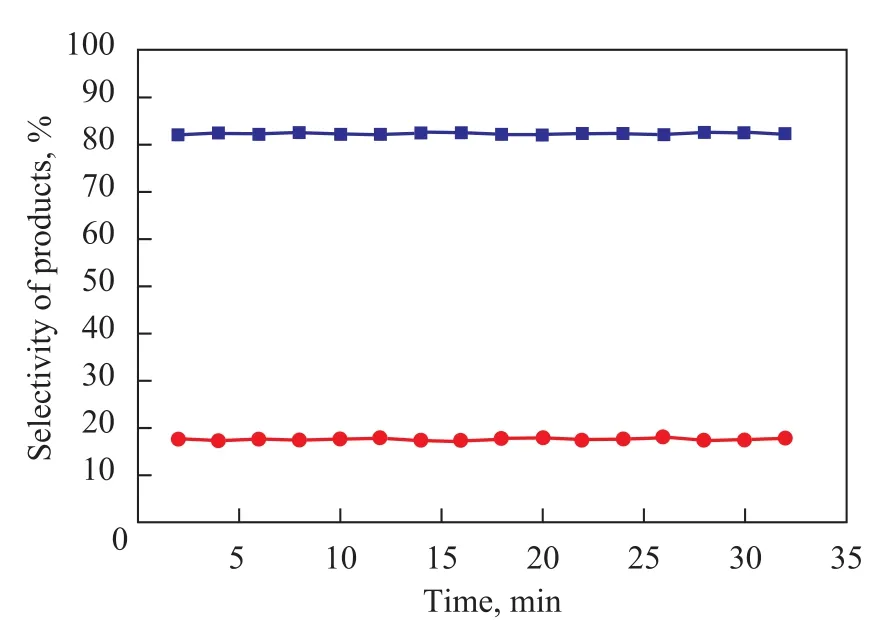
Figure 5 Selectivity of products obtained at different reaction time
3.6 Characterization of catalysts
FT-IR spectral analysis was performed because the acidity of ionic liquid could be regarded as a prominent factor that could have influence on the selectivity of alkylate. In this experiment, pyridine was used as the probe molecule and the related spectrograms are shown in Figure 6. It can be seen from Figure 6 that there were two characteristic peaks (near 1 450 cm-1and 1 540 cm-1simultaneously), which implied that both Lewis acid sites and Brønsted acid sites were contained in the ionic liquid Et3NHCl-AlCl3. With an increasing molar fraction of AlCl3, the wave number shifted to 1 448.5 cm-1after the occurrence of reaction between pyridine and ionic liquid (with different Lewis acidity). It was a kind of hypsochromic shift (moving from 1 446.4 cm-1to 1 448.5 cm-1), indicating that the Lewis acidity saw an upward trend once the amount of AlCl3increased.
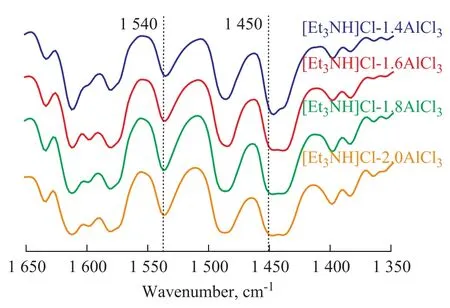
Figure 6 FT-IR spectra of ionic liquids with different mole ratios of AlCl3/Et3NHCl
3.7 Reusability of the catalyst
The reusability of the ionic liquids was investigated and the related results are presented in Table 1. Both the amount of reactants and catalyst was increased in order to investigate the reusability of the catalyst and to examine the sensitivity of the chloroaluminate ionic liquid catalyst to moisture and its loss in the separation process. The catalyst was separated out through a separatory funnel after the alkylation reaction and then reused for the next experiment under the same experimental conditions.
The data (as shown in Table 1) which were collected from the cycle No.1 to the cycle No.5 suggested that the Et3NHCl-AlCl3ionic liquids could be reused thanks to its favourable stability, and a high conversion oft-Bu-Cl and a good selectivity of PTBT could still be observed even though the ionic liquid was reused for 5 times. However, the data which were acquired from the cycle No. 6 to the cycle No. 7 of operation on reused catalyst implied that the conversion of chloro-2-methylpropane had dropped to 81.1% steeply, which demonstrated that the activity of ionic liquids tumbled drastically since AlCl3had dissolved in the system. It was the AlCl3solution that had broken down the ionization equilibrium:+AlCl3, which could impair the Lewis acidity, resulting in a weakened activity of catalyst.
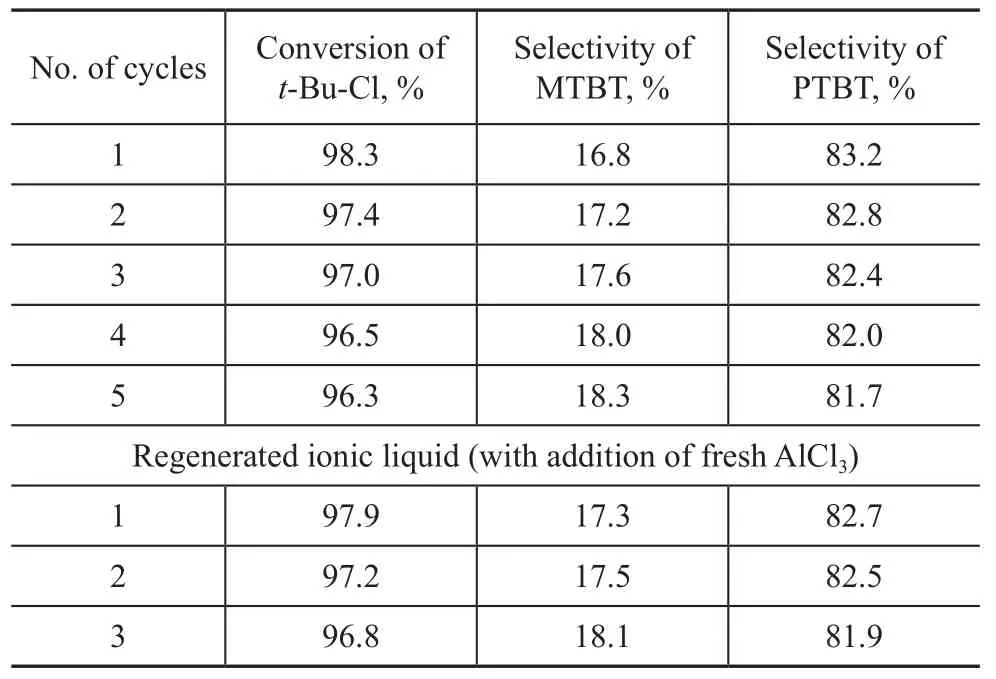
Table 1 Results on recycle of Et3NHCl-1.6AlCl3ionic liquid used in alkylation reaction
Furthermore, the regeneration of inactivated ionic liquid must be taken into account in order to abide by the environmental protection regulations, since the active component of this catalyst system is consumed in the course of alkylation reaction, leading to a decline of both acidity and activity of the catalyst. The recovery and reuse of the catalyst can be realized when a certain amount of fresh AlCl3was added to the inactive ionic liquids, which is a topic that needs to be further investigated. This finding implies that the ionic liquid catalyst can be recovered and recycled efficiently.
4 Conclusions
Alkylation of toluene witht-Bu-Cl was performed in an attempt to optimize the process regime and study the influence of process parameters, including the reaction time, the reaction temperature, the reactants mole ratio, and the catalyst (ionic liquid) to toluene mass ratio, on the conversion oft-Bu-Cl and selectivity of PTBT. A series of comparative experiments were used in order to acquire the optimum data. The optimum parameters for conducting the batch alkylation of toluene witht-Bu-Cl using Et3NHCl-AlCl3ionic liquid as the catalyst include: a reaction temperature of 293 K, at-Bu-Cl to toluene ratio of 0.5:1, a catalyst (IL) to toluene mass ratio of 1:10 and a reaction time of 10 min. This set of optimum parameters can result in a maximum of 98.2% conversion oft-Bu-Cl and a maximum of 83.5% selectivity of PTBT. The initial catalytic activity can be maintained even though the Et3NHCl-AlCl3ionic liquid has been utilized for 5 times. The Et3NHCl-AlCl3ionic liquid has demonstrated a stably reusable performance and it can be regenerated for repeated use when it becomes inactivated.
Acknowledgments:The authors are grateful for the financial support from the Beijing University of Chemical Technology. We also thank the Key Laboratory of Advanced Chemical Engineering and Technology, Beijing Institute of Petrochemical Technology, for the analysis of samples.
[1] Anand B, Halgeri, Jagannath Das. Recent advances in selectivation of zeolites forpara-disubstituted aromatics[J]. Catalysis Today, 2002, 73(1/2): 65-73
[2] Perego C, Amarilli S, Carati A, et al. Mesoporous silicaaluminas as catalysts for the alkylation of aromatic hydrocarbons with olefins[J]. Microporous Mater, 1999, 27(2/3): 345-354
[3] Tang A S, Shen D L, Zhou C F, et al. Studies onp-tertbutyltoluene synthesis[J]. Zhejiang Chem, 1993, 4(1): 6-8 (in Chinese)
[4] Xu L X, Yin J H, Chen X X. Progress in environmentally friendly chemical process of heterogeneous catalysts[J]. Petrochemical Technology & Application, 2003, 2l(6): 444-447 (in Chinese)
[5] Shen H Y, Judeh Z M A, Ching C B, et al. Comparative studies on alkylation of phenol withtert-butyl alcohol in the presence of liquid or solid acid catalysts in ionic liquids[J]. Journal of Molecular Catalysis A: Chemical, 2004, 212(1/2): 301-308
[6] Gök Y, Özdemir İ, Çetinkaya E. Ionic liquids as solvents/ catalysts for selective alkylation of amines with alkyl halides[J]. Chin J Catal, 2007, 28(6): 489-491
[7] Zhao Z K, Qiao W H, Wang G R, et al. Alkylation of α-methylnaphthalene with long-chain alkenes catalyzed by butylpyridinium bromochloroaluminate ionic liquids[J]. Journal of Molecular Catalysis A: Chemical, 2005, 231(1/2): 137-143
[8] Kondamudi K, Elavarasana P, Dyson P J, et al. Alkylation ofp-cresol with tert-butyl alcohol using benign Bronsted acidic ionic liquid catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2010, 321(1/2): 34-41
[9] Qiao C Z, Zhang Y F, Zhang J C, et al. Activity and stability investigation of [BMIM][AlCl4] ionic liquid as catalyst for alkylation of benzene with 1-dodecene[J]. Apply Catalysis A: General, 2004, 276(1/2): 61-66
[10] Han P, Zhang H M, Qiu X P, et al. Palladium within ionic liquid functionalized mesoporous silica SBA-15 and its catalytic application in room-temperature Suzuki coupling reaction[J]. Journal of Molecular Catalysis A: Chemical, 2008, 295(1/2): 57-67
[11] Yue C B, Fang D, Liu L, et al. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions[J]. Journal of Molecular Liquids, 2011, 163(3): 99-121
[12] Olivier-Bourbigou H, Magna L, Morvan D. Ionic liquids and catalysis: Recent progress from knowledge to applications[J]. Applied Catalysis A: General, 2010,373(1/2): 1-56
[13] Blanco C G, Banciella D C, Azpíroz M D G. Alkylation of naphthalene using three different ionic liquids[J]. Journal of Molecular Catalysis A: Chemical, 2006, 253(1/2): 203-206
[14] Ranu B C, Banerjee S, Das A. Catalysis by ionic liquids: Cyclopropyl carbinyl rearrangements catalyzed by [pmim] Br under organic solvent free conditions [J]. Tetrahedron Letters, 2006, 47(6): 881-884
[15] Zhao D B, Wu M, Yuan K, et al. Ionic liquids: Applications in catalysis [J]. Catalysis Today, 2002, 74(1/2): 157-189
[16] Sun X W, Zhao S Q, Wang R A. Mechanism of alkylation of benzene with ethylene catalyzed by [bmim]Cl/FeCl3ionic liquid[J]. Chinese Journal of Catalysis, 2004, 25(3): 247-251 (in Chinese)
[17] Valkenberg M H, deCastro C, Holderich W F, et al. Friedel-Crafts acylation of aromatics catalysed by supported ionic liquids[J]. Applied Catalysis A: General, 2001, 215(1/2): 185-190
[18] Zhang Jie, Huang Chongpin, Chen Biaohua. Isobutane/2-butene alkylation catalyzed by chloroaluminate ionic liquids in the presence of aromatic additives[J]. Journal of Catalysis, 2007, 249(2): 261-268
[19] Cai X J, Cui S H, Qu L P, et al. Alkylation of benzene and dichloromethane to diphenylmethane with acidic ionic liquids [J]. Catalysis Communications, 2008, 9(6): 1173-1177
[20] Liu Huibin, Meng Xianghai, Zhang Rui, et al. Reaction performance and disappearance kinetics ofn-pentane isomerization catalyzed by chloroaluminate ionic liquid [J]. Catalysis Communications, 2010, 12(3): 180-183
[21] Cai Q H, Li J, Bao F X. et al. Tunable dimerization of α-methylstyrene catalyzed by acidic ionic liquids [J]. Applied Catalysis A: General, 2005, 279(1-2): 139-143
[22] Philipp Keil, Axel König. Enthalpies of solution of 1-ethyl-3-methyl-imidazolium chloride and aluminum chloride in molten chloroaluminate ionic liquids [J]. Thermochimica Acta, 2011, 524(1/2): 202-204
[23] Li Z Y, Liu Y, Liu H T, et al. Synthesis and ionic conductivity of polymeric ion gel containing room temperature ionic liquid and phosphotungstic acid [J]. Solid State Ionics, 2006, 177(15/16): 1281-1286
[24] Cui P, Zhao G Y, Ren H L, et al. Ionic liquid enhanced alkylation ofiso-butane and 1-butene [J]. Catalysis Today, 2013, 200(1): 30-35
[25] Xing Xueqi, Zhao Guoying, Cui Jianzhong, et al. Isobutane alkylation using acidic ionic liquid catalysts [J]. Catalysis Communications, 2012, 26(5): 68-71
[26] Sun X W, Zhao S Q. [bmim]Cl/[FeCl3] ionic liquid as catalyst for alkylation of benzene with 1-octadecene1 [J]. Chinese Journal of Chemical Engineering, 2006, 14(3): 289-293 (in Chinese)
Recieved date: 2012-10-17; Accepted date: 2012-12-01.
Professor Luo Guohua, E-mail: luoguohua@bipt.edu.cn.
- 中国炼油与石油化工的其它文章
- Alumina Supported Vanadium Oxide Catalysts for Residue Hydrotreating
- Commercial Applications of Paraxylene Adsorbents RAX-2000A and RAX-3000
- Hydrocarbon Composition of Different VGO Feedstocks and Its Correlation with FCC Product Distribution
- Purification of Crude Glycerol from Waste Cooking Oil Based Biodiesel Production by Orthogonal Test Method
- Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
- Highlights on Planned Grassroots Styrene Units and Expansion of Existing Styrene Units in China

