薄皮干酪菌发酵液的化学成分研究
陆云德,郭怀宇,刘吉开,王 刚,吴培云*
1安徽中医药大学药学院,现代中药安徽省重点实验室,合肥230031;2中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,昆明650201
薄皮干酪菌(Tyromyces chioneus)属于多孔菌科(Polyporaceae)干酪属(Tyromyces)高等真菌,菌盖直径1~9 cm,纯白色,后变成污白至淡黄色,生于阔叶树桩上,在我国主要分布于河北、山西、黑龙江、陕西等地[1]。刘东泽从该种真菌发酵液中分离鉴定了7个化合物,包括一个新的cadinane型倍半萜有比较显著的抗HIV-1活性,其EC50=3.02μg/mL(SI=25.4)[2]。为了进一步从薄皮干酪菌中寻找具有生物活性的次生代谢产物,笔者对薄皮干酪菌发酵液进行了系统的化学成分研究,从其乙酸乙酯提取物中分离得到13个已知化合物,经波谱解析分别鉴定为:角鲨烯(1)、亚油酸甲酯(2)、棕榈酸(3)、过氧麦角甾醇(4)、麦角甾-4,6,8(14),22-四烯-3-酮(5)、麦角甾-5,7,22-三烯-3β-醇(6)、麦角甾-7,22-二烯-3β,5α,6β-三醇 (7)、5α,6α-环氧麦角甾-8,22-二烯-3β,7α-二醇(8)、对羟基苯乙醇(9)、1-苯基丙烷-1,2-二醇(10)、5-苯基戊烷-2,3-二醇(11)、5-羟甲基糠醛(12)、(+)-3β-hydroxy-αmuurolene(13)。
1 仪器与材料
熔点由四川大学科学仪器厂生产的 XRC-1型显微熔点仪测定,温度计未校正;质谱由 VG Auto-Spec-3000质谱仪测定;核磁共振由 Bruker AV-400和DRX-500测定,内标为 TMS;分析型和制备型HPLC为Agilent1100 HPLC,色谱柱为 Agilent Zorbax SB-C18和YMC柱;柱层析硅胶(80~100目和200~300目)以及GF254薄层硅胶板均为青岛海洋化工厂生产;反相材料 Lichroprep Rp-18(40~63μm)为Merck公司产品;Sephadex LH-20为瑞典 Amersham Biosciences公司产品;显色方法为荧光灯下波长254 nm和365 nm处观察荧光,10% 硫酸乙醇溶液和硫酸香草醛处理后加热显色及碘蒸气显色。
薄皮干酪菌(Tyromyces chioneus)于2003年7月采自云南哀牢山,由昆明植物研究所纪大干研究员鉴定,标本存于昆明植物研究所标本馆。
2 培养与发酵
由昆明植物研究所李正辉工程师采用斜面转摇瓶液体培养的方法对该菌种进行发酵培养。培养基:葡萄糖5%,猪肉蛋白胨0.15%,酵母粉0.5%,KH2PO4和 MgSO4各0.05%,160 ~165 rpm,25 ℃摇床发酵25 d,装液量为350 mL,发酵液总量为20 L。
3 提取与分离
薄皮干酪菌发酵液(20 L)用乙酸乙酯萃取3次,合并减压浓缩得12 g粗提物,经硅胶柱色谱,以三氯甲烷-甲醇(100∶0→0∶100)梯度洗脱得8个组分(A、B、C、D、E、F、G、H)。组分 A(三氯甲烷∶甲醇-100∶0)经石油醚-乙酸乙酯(100∶0→0∶100)梯度洗脱得到9个亚组分,其中A3经Sephadex LH-20柱色谱,以三氯甲烷-甲醇(1∶1)洗脱得化合物1(3.6 mg),A6经硅胶柱色谱以石油醚-乙酸乙酯(50∶1)洗脱再经反相 RP-18用甲醇-水(85∶15→0∶100)梯度洗脱得到化合物2(17 mg)。组分D(三氯甲烷∶甲醇-100∶2)经反相 RP-18 以甲醇-水(30∶70→0∶100)梯度洗脱得到13个亚组分,其中 D3经Sephadex LH-20柱色谱,以甲醇洗脱得化合物12(11.9 mg),D5经硅胶柱色谱以三氯甲烷-丙酮(10∶1)洗脱,再经反相-高效液相色谱法(RP-HPLC)制备柱色谱得化合物10(3 mg),D10经硅胶柱色谱以石油醚-丙酮(15∶1→1∶1)梯度洗脱得化合物 13(1.9 mg),D11经Sephadex LH-20柱色谱,以三氯甲烷-甲醇(1∶1)洗脱再重结晶化合物3(4.1 mg),D12经重结晶得到化合物4(6.3 mg),D13经硅胶柱色谱以石油醚-丙酮(15∶1→1∶1)梯度洗脱再经 Sephadex LH-20柱色谱以三氯甲烷-甲醇(1∶1)洗脱得到化合物5(4.9 mg)和化合物6(5.4 mg)。组分E(三氯甲烷∶甲醇-100∶2)经反相 RP-18 以甲醇-水(50∶50→0∶100)梯度洗脱得到9个亚组分,其中E1经Sephadex LH-20柱色谱以甲醇洗脱,再经硅胶柱色谱以三氯甲烷-甲醇(15∶1)洗脱得化合物 9(5.2 mg),E2经Sephadex LH-20柱色谱以三氯甲烷-甲醇(1∶1)洗脱再经硅胶柱色谱以石油醚-丙酮(8∶1)洗脱得化合物11(4.1 mg)。组分 F(三氯甲烷∶甲醇-100∶4)经反相 RP-18 以甲醇-水(30∶70→80∶20)梯度洗脱得到12个亚组分,其中F9经Sephadex LH-20柱色谱以三氯甲烷-甲醇(1∶1)洗脱再重结晶得化合物7(3.8 mg),F10经硅胶柱色谱以三氯甲烷-丙酮(20∶1→5∶1)梯度洗脱再经Sephadex LH-20柱色谱以三氯甲烷-甲醇(1∶1)洗脱经硅胶柱色谱以石油醚-丙酮(6∶1)洗脱得化合物 8(3.6 mg)。
4 结构鉴定
化合物1 角鲨烯,C30H50,无色透明油状液体;FAB-MS m/z:411.3[M+H]+;1H NMR(CDCl3,400 MHz)δ:5.08 ~5.15(6H,m,H-3,H-7,H-11,H-14,H-18 and H-22),1.96 ~2.11(20H,m,H-4,H-5,H-8,H-9,H-12,H-13,H-16,H-17,H-20 and H-21),1.68(6H,s,H-1 and H-24),1.60(18H,s,2-CH3,6-CH3,10-CH3,15-CH3,19-CH3and 23-CH3);13CNMR(CDCl3,100 MHz)δ:135.0(s,C-6 and C-19),134.8(s,C-10 and C-15),131.1(s,C-2 and C-23),124.4(d,C-3 and C-22),124.3(d,C-7,C-11,C-14 and C-18),39.7(t,C-5,C-9,C-16 and C-20),28.3(t,C-12 and C-13),26.8(t,C-4,C-8,C-17 and C-21),25.6(q,C-1 and C-24),17.6(q,2-CH3and 23-CH3),16.0(q,6-CH3,10-CH3,15-CH3and 19-CH3)。以上波谱数据与文献[3]数据报道一致,确定该化合物为角鲨烯。
化合物2 亚油酸甲酯,C19H3402,淡黄色油状物;EI-MS m/z(%):294[M]+(45),263(34),220(2),149(18),109(40),95(77),81(100),74(31),67(88);1H NMR(CDCl3,400 MHz)δ:5.35(4H,m,H-9,H-10,H-12,H-13),3.66(3H,s,-OCH3),2.77(2H,t,J=6.8 Hz,H-11),2.30(2H,t,J=7.2 Hz,H-2),2.04(4H,m,H-8 and H-14),1.60(2H,m,H-3),1.25~1.37(m,H-4~H-7 and H-15~ H-17),0.88(3H,t,J=6.8 Hz,H-18);13C NMR(CDCl3,100 MHz)δ:174.3(s,C-1),130.1(d,C-9),130.0(d,C-10),128.0(d,C-12),127.8(d,C-13),51.4(q,-OCH3),34.0(t,C-2),31.9(t,C-8),31.5(t,C-14),29.7 ~22.5(t,C-3 ~ C-7 and C-15 ~C-17),14.0(q,C-18)。以上数据与文献[4]数据报道一致,确定该化合物为亚油酸甲酯。
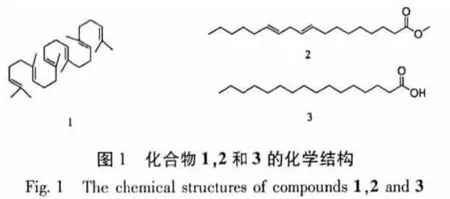
化合物3 棕榈酸,C16H3202,白色无定型粉末;mp160~162℃;Negative FAB-MS m/z(%):255[MH]-;EI-MS(%):256[M]+(89),239[M-OH]+(5),227[M-C2H5]+(19),213(54),199(20),185(30),171(26),157(29),143(15),129(68),115(23),97(27),85(38),73(100),57(75);1H NMR(400 MHz,CDCl3)δ:2.35(2 H,t,J=7.2 Hz,H-2),1.63(2 H,m,H-3),1.24(br.s,多个-CH2-),0.88(3 H,t,J=6.8 Hz,-CH3)。以上数据与文献[5]数据报道一致,确定该化合物为棕榈酸。
化合物4 过氧麦角甾醇,C28H4403,无色针晶;mp 177~178℃;EI-MS m/z(%):428[M]+(10),410(4),396(100),363(35),271(7),255(37),251(14),152(30),107(22),69(63);1H NMR(CDCl3,400 MHz)δ:6.50(1H,d,J=8.4 Hz,H-7),6.24(1H,d,J=8.4 Hz,H-6),5.22(1H,dd,J=15.2,7.4 Hz,H-22),5.13(1H,dd,J=15.2,7.4 Hz,H-23),3.97(1H,m,H-3),0.99(3H,d,J=6.8 Hz,H-21),0.90(3H,d,J=6.8 Hz,H-28),0.88(3H,s,H-19),0.83(3H,d,J=6.8 Hz,H-27),0.82(3H,s,H-18),0.80(3H,d,J=6.8 Hz,H-26);13C NMR(CDCl3,100 MHz)δ:135.4(d,C-6),135.2(d,C-22),132.3(d,C-23),130.7(d,C-7),82.1(s,C-5),79.4(s,C-8),66.4(d,C-3),56.2(d,C-17),51.6(d,C-14),51.0(d,C-9),44.5(s,C-13),42.7(d,C-24),39.7(d,C-20),39.3(t,C-12),36.9(t,C-4),36.9(s,C-10),34.7(t,C-1),33.0(d,C-25),30.1(t,C-2),28.6(t,C-15),23.4(t,C-11),20.8(q,C-21),20.6(t,C-16),19.9(q,C-27),19.6(q,C-26),18.1(q,C-19),17.5(q,C-28),12.8(q,C-18)。以上波谱数据与文献[6]数据报道一致,确定该化合物为过氧麦角甾醇。
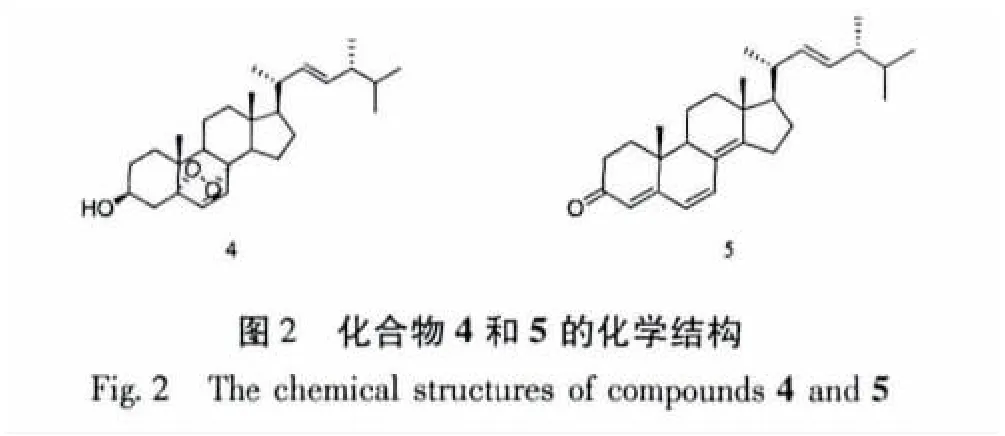
化合物 5 麦角甾-4,6,8(14),22-四烯-3-酮,C28H40O,浅黄色晶体;mp 112~114℃ ;EI-MS m/z(%):392[M]+(15),377(3),349(4),268(100),253(30),214(26),173(23),69(47);1H NMR(CDCl3,400 MHz)δ:6.58(1H,d,J=9.4 Hz,H-7),6.00(1H,d,J=9.4 Hz,H-6),5.70(1H,s,H-4),5.24(1H,dd,J=15.2,7.2 Hz,H-23),5.18(1H,dd,J=15.2,7.2 Hz,H-22),1.21 ~2.53(18H,m,甾体母核),1.03(3H,d,J=6.8 Hz,H-21),0.97(3H,s,H-19),0.93(3H,s,H-18),0.90(3H,d,J=6.8 Hz,H-28),0.82(3H,d,J=6.8 Hz,H-27),0.78(3H,d,J=6.8 Hz,H-26);13C NMR(CDCl3,100 MHz)δ:199.3(s,C-3),164.2(s,C-5),156.0(s,C-14),135.0(d,C-22),133.9(d,C-7),132.6(d,C-23),124.5(d,C-6),124.3(s,C-8),123.0(d,C-4),55.8(d,C-17),44.0(d,C-9),44.0(s,C-13),42.9(d,C-24),39.2(d,C-20),36.8(s,C-10),35.7(t,C-15),34.2(t,C-12),34.1(t,C-1),33.1(d,C-25),27.7(t,C-16),25.4(t,C-11),21.2(q,C-21),20.0(q,C-27),19.7(q,C-26),19.0(t,C-2),18.9(q,C-19),17.6(q,C-28),16.7(q,C-18)。以上波谱数据与文献[7]数据报道一致,确定该化合物为麦角甾-4,6,8(14),22-四烯-3-酮。
化合物 6 麦角甾-5,7,22-三烯-3β-醇,C28H44O,白色晶体;mp 152~154℃ ;EI-MS m/z(%):396[M]+(55),363(61),337(40),271(17),253(48),211(37),197(28),185(23),171(30),157(55),143(57),131(27),119(30),91(28),81(27),69(100),55(56);1H NMR(CDCl3,400 MHz)δ:5.58(1H,m,H-6),5.38(1H,m,H-7),5.14~5.26(2H,m,H-22 and H-23),3.60(1H,m,H-3),1.03(3H,d,J=6.8 Hz,H-21),0.95(3H,s,H-19),0.92(3H,d,J=6.8 Hz,H-28),0.84(3H,d,J=6.8 Hz,H-27),0.82(3H,d,J=6.8 Hz,H-26),0.61(3H,s,H-18);13C NMR(CDCl3,100 MHz)δ:141.3(s,C-8),139.8(s,C-5),135.6(d,C-22),132.1(d,C-23),119.6(d,C-6),116.4(d,C-7),70.5(d,C-3),55.9(d,C-17),54.6(d,C-14),46.4(d,C-9),42.9(s,C-13),40.8(d,C-24),40.9(t,C-4),40.4(d,C-20),39.2(t,C-12),38.4(t,C-1),37.1(s,C-10),33.1(d,C-25),32.1(t,C-2),28.3(t,C-16),23.0(t,C-15),21.2(t,C-11),21.2(q,C-21),19.9(q,C-26),19.7(q,C-27),17.6(q,C-28),16.3(q,C-19),12.1(q,C-18)。以上波谱数据与文献[8]数据报道一致,确定该化合物为麦角甾-5,7,22-三烯-3β-醇。

化合物 7 麦角甾-7,22-二烯-3β,5α,6β-三醇,C28H46O3,白色粉末;mp 224~226℃ ;EI-MS m/z(%):430[M]+(35),412(35),394(37),379(65),376(15),269(33),251(62),69(100);1H NMR(C5D5N,400 MHz)δ:5.74(1H,br.s,H-7),5.24(1H,dd,J=15.2,7.4 Hz,H-23),5.16(1H,dd,J=15.2,7.4 Hz,H-22),4.84(1H,m,H-3),4.32(1H,br.d,J=4.8 Hz,H-6),1.53(3H,s,H-19),1.07(3H,d,J=6.8 Hz,H-21),0.94(3H,d,J=6.8 Hz,H-28),0.85(3H,d,J=6.8 Hz,H-27),0.84(3H,d,J=6.8 Hz,H-26),0.67(3H,s,H-18);13C NMR(C5D5N,100 MHz)δ:141.6(s,C-8),136.2(d,C-22),132.5(d,C-23),120.4(d,C-7),76.5(s,C-5),74.3(d,C-6),67.6(d,C-3),56.5(d,C-17),55.2(d,C-14),43.9(s,C-13),43.8(d,C-9),43.0(d,C-24),42.0(t,C-4),40.7(d,C-20),40.1(t,C-12),38.1(s,C-10),33.8(t,C-1),33.1(d,C-25),32.6(t,C-2),28.2(t,C-16),23.5(t,C-15),22.4(t,C-11),21.3(q,C-21),20.1(q,C-27),19.9(q,C-26),18.8(q,C-19),17.6(q,C-28),12.3(q,C-18)。以上波谱数据与文献[9]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,6β-三醇。
化合物 8 5α,6α-环氧麦角甾-8,22-二烯-3β,7α-二醇,C28H44O3,无色晶体;EI-MS m/z(%):428[M]+(13),410(60),395(29),392(23),377(86),285(63),284(43),267(100),231(90),213(95);1H NMR(CDCl3,400 MHz)δ:5.16 ~ 5.24(2H,m,H-22 and H-23),4.21(1H,br.d,J=2.5 Hz,H-7β),3.92(1H,m,H-3),3.30(1H,d,J=2.5 Hz,H-6β),1.13(3H,s,H-19),1.01(3H,d,J=6.6 Hz,H-21),0.90(3H,d,J=6.8 Hz,H-28),0.83(3H,d,J=6.5 Hz,H-27),0.81(3H,d,J=6.5 Hz,H-26),0.57(3H,s,H-18);13C NMR(CDCl3,100 MHz)δ:135.5(d,C-22),134.4(s,C-8),132.0(d,C-23),126.9(s,C-9),68.5(d,C-3),67.0(d,C-7),65.7(s,C-5),62.6(d,C-6),53.6(d,C-17),49.5(d,C-14),42.8(d,C-24),42.0(s,C-13),40.4(d,C-20),39.1(t,C-4),38.0(s,C-10),35.6(t,C-12),33.0(d,C-25),30.8(t,C-1),30.2(t,C-2),29.0(t,C-16),23.8(t,C-15),23.4(t,C-11),22.8(q,C-19),20.9(q,C-21),19.9(q,C-27),19.6(q,C-26),17.6(q,C-28),11.2(q,C-18)。以上波谱数据与文献[10]数据报道一致,确定该化合物为 5α,6α-环氧麦角甾-8,22-二烯-3β,7α-二醇。
化合物9 对羟基苯乙醇,C8H10O2,无色结晶;EI-MS m/z(%):138[M]+(28),107(100),77(14);1H NMR(CDCl3,500 MHz)δ:6.94(2H,d,J=8.5 Hz,H-2 and H-6),6.66(2H,d,J=8.5 Hz,H-3 and H-5),3.61(2H,t,J=6.8 Hz,H-8),2.63(2H,t,J=6.8 Hz,H-7);13C NMR(CDCl3,125 MHz)δ:154.8(s,C-4),129.6(d,C-2 and C-6),129.4(s,C-1),115.0(d,C-3 and C-5),64.2(t,C-8), 37.8(t,C-7)。以上波谱数据与文献[11]报道一致,确定该化合物为对羟基苯乙醇。

化合物 10 1-苯基丙烷-1,2-二醇,C9H12O2,无色油状物;GC-MS(70 eV):m/z(%)=135[MOH]+(15),108[C7H7O]+(95),45[C2H5O]+(20);1H NMR(CDCl3,600 MHz)δ:7.28 ~ 7.35(5H,m,Ph-H),4.34(1H,br.d,J=7.3 Hz,H-1),3.84(1H,m,H-2),2.90(1H,br.s,OH),2.69(1H,br.s,OH),1.04(3H,d,J=6.3 Hz,H-3);13C NMR(CDCl3,150 MHz)δ:141.1(s,C-1'),128.5(d × 2,C-3'and C-5'),128.2(d ×2,C-2'and C-6'),126.9(d,C-4'),79.5(d,C-1),72.2(d,C-2),18.8(q,C-3)。以上波谱数据与文献[12]报道一致,确定该化合物为1-苯基丙烷-1,2-二醇。
化合物 11 5-苯基戊烷-2,3-二醇,C11H16O2,无色油状物;ESI-MS m/z(%):203[M+Na]+(72),181[M+H]+(4),91(11);1H NMR(Acetone,400MHz)δ:7.12 ~7.27(5H,m,Ph-H),3.59(1H,m,H-2),3.41(1H,m,H-3),2.85(1H,m,H-5β),2.64(1H,m,H-5α),1.84(1H,m,H-4β),1.64(1H,m,H-4α),1.11(3H,d,J=6.2 Hz,H-1);13C NMR(Acetone,100 MHz)δ:143.8(s,C-1'),129.3(d ×2,C-3'and C-5'),129.1(d × 2,C-2'and C-6'),126.4(d,C-4'),75.30(d,C-3),71.1(d,C-2),35.6(t,C-4),32.9(t,C-5),18.8(q,C-1)。以上波谱数据与文献[13]报道一致,确定该化合物为5-苯基戊烷-2,3-二醇。
化合物12 5-羟甲基糠醛,C6H6O3,黄色油状物;EI-MS m/z(%):126[M]+(13),125(13),109(100),97(27),81(14),69(6),53(5);1H NMR(CDCl3,500 MHz)δ:9.56(1H,s,2-CHO),7.22(1H,d,J=3.5 Hz,H-3),6.51(1H,d,J=3.5 Hz,H-4),4.71(1H,s,5-CH2OH);13C NMR(CDCl3,125 MHz)δ:177.7(d,CHO),160.7(s,C-5),152.3(s,C-2),122.9(d,C-3),109.9(d,C-4),57.5(t,CH2OH)。以上波谱数据与文献[14]报道一致,确定该化合物为5-羟甲基糠醛。
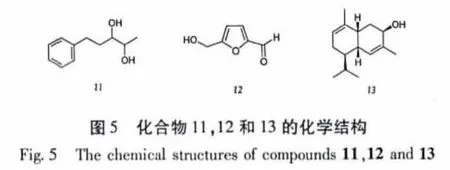
化合物 13 (+)-3β-hydroxy-α-muurolene ,C15H24O,无色固体;[α]24D+131(c 1.67 CHCl3);IR(ATR)νmax3408,2956,2927,1714,1671,1367,1222 cm-1;ESI-MS m/z 221[M+H]+;HREI-MS m/z 220.1825;1H NMR(MeOD,300 MHz)δ:5.68(1H,dd,J=1.1 and 4.6 Hz,H-5),5.45(1H,br.s,H-9),3.93(1H,t,J=3.4 Hz,H-3),2.34(1H,m,H-1),2.10(1H,m,H-6),1.98(1H,m,H-11),1.93(1H,m,H-2β),1.88(2H,m,H-8),1.84(3H,br.s,H-14),1.73(3H,br.s,H-15),1.66(1H,m,H-2α),1.47(1H,m,H-7),0.94(3H,d,J=7 Hz,H-12),0.90(3H,d,J=6.6 Hz,H-13);13C NMR(MeOD,75 MHz)δ:136.9(s,C-10),136.4(s,C-4),129.1(d,C-5),122.6(d,C-9),68.5(d,C-3),41.0(d,C-7),38.2(d,C-6),35.2(d,C-1),35.1(t,C-2),28.1(d,C-11),25.5(t,C-8),21.7(q,C-12),21.6(q,C-15),21.4(q,C-14),16.3(q,C-13)。以上波谱数据与文献[15]报道一致,确定该化合物为(+)-3β-hydroxy-α-muurolene。
1 Mao XL(卯晓岚).The Macrofungi in China(中国大型真菌).Zhengzhou:Henan Science and Technology Press,2000.435.
2 Liu DZ,Wang F,Yang LM,et al.A New Cadinane Sesquiterpene with Significant Anti-HIV-1 Activity from the Cultures of the Basidiomycete Tyromyces chioneus.J Antibiot,2007,60:332-334.
3 Du ZW(杜子伟),Liu JS(刘劲松),Wu PY(吴培云),et al.Chemical constituents of Hexagonia speciosa.Chin Tradit PatMed(中成药),2012,34:1305.
4 Chen SC,Hong LL,Chang CY.Antiprolferative constituents from Gynura divaricata subsp.Formasana.Chin Pharm J,2003,55:109.
5 Xu CL(徐从立),Chen HS(陈海生),Tan XQ(谭兴起),et al.Studies on the active constituents of asparagi radix.Nat Prod Res Dev(天然产物研究与开发),2005,17:128-130.
6 Xiang C(项晨),Liu JK(刘吉开),Du ZW(杜子伟),et al.Chemical constitutuents of Lyophyllum connatum.Anhui Med Pharm J(安徽医药),2011,15:1482-1484.
7 Gao JM,Dong ZJ,Liu JK.A new ceramide from the basidiomycete Russula cyanoxantha.Lipids,2001,36:175.
8 Du ZW(杜子伟),Liu JK(刘吉开),Xiang C(项晨),et al.Chemical Constituents of Boletinus pictus.Nat Prod Res Dev(天然产物研究与开发),2012,24:618-621.
9 Gao JM,Hu L,Liu JK.A novel sterol from Chinese truffle s Tuber indicum.Steroids,2001,66:771.
10 Yue JM,Chen SN,Lin ZW,etal.Sterols from the fungus Lactarium volemus.Phytochemistry,2001,56:801-806.
11 Yin W(尹伟),Wu PY(吴培云),Liang YM(梁益敏),et al.Chemical constituents of sarcocarp of Crescentia cujete.Chin Tradit PatMed(中成药),2012,34:1523-1528.
12 Justyna K,Robert CS,Christopher AR,et al.Stereoselective synthesis of bulky 1,2-diols with alcohol dehydrogenases.Catalysis Sci Technol,2012,2:1580-1589.
13 Liu R(刘荣).Investigation on chemical constituens of eight higher fungi and onemedicinal plant.Kunming:Kunming Institute of Botany,Chinese Academy of Sciences(中国科学院昆明植物研究所),PhD.2010.71.
14 Yin HQ(尹宏权),Qi XL(齐秀兰),Hua HM(华会明),et al.The chemical constituents from Saussurea lappa C.B Clarke.Chin JMed Chem(中国药物化学杂志),2005,15:217-220.
15 Sven A,Stefan K,Heike W,et al.Dietary Derived Sesquiterpenes from Phyllodesmium lizardensis.JNat Prod,2009,72:298-300.

