Open Surgical Insertion of Tenkchoff Straight Catheter Without Guide Wire
Shi-feng Yang, Wu-jun Xue,Ai-ping Yin, Li-yi Xie, and Wan-hong Lu
Department of Nephrology, First Affiliated Hospital of Medical School, Xi'an Jiaotong University, Xi'an 710061, China
PERITONEAL dialysis (PD) is an effective renal replacement therapy widely used in patients with end stage renal diseases (ESRD). Availability of peritoneal access is the prerequisite for a successful PD program, so PD catheter (PDC) insertion is required before PD. The Tenckhoff catheter is the most widely used type of PDC worldwide in the past 40 years.1There are several ways to insert Tenckhoff catheters.2Although peritoneoscopy or laparoscopy3is becoming the most preferred option in the United States and some other developed countries, open surgical PDC insertion is still commonly practiced in China.4
Successful PD depends on keeping the PDC unobstructed for dialysate injection and drainage. Post-operative compli- cations, such as catheter obstruction, catheter displacement, and dialysate leakage, may tamper with the function of catheter and lead to catheter removal. Several studies suggested that the function and longevity of PDC are greatly determined by the methods of PDC insertion.5,6
In traditional open surgical PDC insertion, guide wire is commonly used to aid placing catheter into the peritoneal cavity. However, guide wire has not been used in PDC insertion in the Department of Nephrology in our hospital. Although guide wire has been proved useful in PDC insertion,7it would be more convenient if PDC insertion without guide wire is equally effective so that PDC insertion can be conducted when guide wire is not available, especially in emergency cases.
To our knowledge, there have been no studies so far exploring the difference between PDC insertion with guide wire and PDC insertion without guide wire. Therefore, this retrospective study compared the clinical outcomes of these 2 different techniques of PDC insertion.
PATIENTS AND METHODS
Patients and follow-up
From January 2005 to January 2011, 238 patients received double-cuff Tenkchoff straight catheter insertion in our department, including 117 cases with the guidance of guide wire (group A) and 121 cases without guide wire (group B). Post-operative complications were recorded, such as catheter obstruction, catheter diaplacement, dialysate leakage, and bloody dialysate. Complications were identified based on the international guidline for PD.8Early complications were defined as occuring within 30 days of PDC insertion, and late complications after 30 days.9Overweight patients was difined as having body mass index (BMI) over 28 kg/m2.10The endpoint event of catheter survival was catheter loss, defined as catheter removal as a result of irreversible mechanical malfunction, infection, or peritoneal membrane failure.
Operation methods
The guide wire used was a copper, flexible, and curved wire with a blunt end. High frequency electrotome was not used in operation.
In group A, the patients were placed in the supine position, and local anaesthesia was induced before surgery. A vertical incision about 5-7 cm was made at the left paramidline and 10 cm above the upper border of pubic symphysis. The subcutaneous tissue was dissected, and the sheath of the rectus abdominis was reached. The anterior rectus sheath was dissected and the muscle fibres separated bluntly along the direction of its fibers. The posterior rectus sheath was dissected and the peritoneum exposed. The peritoneal cavity was entered via a 0.5-cm opening in the peritoneum layer, and a purse-string suture was placed around this opening. The guide wire was put through catheter, with the end left 1 cm away from the inner end of the catheter. The guide wire was inserted into the peritoneal cavity toward pubic symphysis. The intraperitoneal segment of the catheter was slid off the guide wire, and the inner cuff remained in the preperitoneal space. The peritoneum and posterior rectus sheath were closed with purse-string suture. The catheter was tested to ensure adequate inflow and outflow. The inner cuff was laid parallel to the rectus abdominis. The anterior rectus sheath was sutured, leaving the inner cuff within the rectus muscle. A tunnel was created to the exit site, which located lateral and caudal to the entrance site using a needle. Ensure that the exit site was facing downward. The outer cuff was placed subcutaneously, 2 cm from the exit site. The subcutaneous layer and skin was then sutured.
In group B, the procedure of PDC insertion was the same with that in group A, except that the catheter was inserted without guide wire. During insertion, the catheter was pressed close to the abdominal wall, the inner end of which remained toward pubic symphysis, then slowly advanced into the peritoneal cavity. The position of the end of the catheter within lesser pelvis was confirmed with X-ray after operation.
All the patients started PD soon after operation. Small volumes of dialysis solutions were given in the first week and continuous ambulatory PD started after 1 week.
Statistical analysis
The statistical analysis was performed using SPSS software version 14.0 (Chicago, IL, USA). Continuous variables were expressed as means±SD and analyzed using T-test. Categorical data were presented as absolute values and percentages, analyzed using chi-square test or Fischer's exact test when the theoretical frequency did not add up to the standard. Compliation-assciated factors were evaluated with logistic regression analysis. Catheter survival and patient survival were evaluated with log-rank test. The significance level was defined as α= 0.05.
RESULTS
Comparability of baseline
The baseline data of the 2 groups were showed in Table 1. There were no differences of gender, age, BMI, and other parameters between the 2 groups. The main causes of ESRD were glomerulonephritis (94/238, 39.5%), diabetic nephropathy (69/238, 29.0%), and hypertension (49/238, 20.6%). There were no intergroup differiences regarding the causes of ESRD (all P>0.05). The everage follow-up time were 27.5±20.3 months and 26.0±21.4 months in group A and group B, respectively (P=0.6). In a period of 6 years, a totall of 104 patients (43.7%) died, 85 patients (35.7%) survived on PD, 42 (17.6%) survived on hemodialysis, and 7 (2.9%) resorted to renal transplantion at the end of the study. The mortality rates of two groups were not significantly different (P=0.7). The main causes of death were cardiova- scular events (68/104, 65.4%), infection(23/104, 22.1%), malnutrition (8/104, 7.7%), and others (5/104, 4.8%).
Post-operative complications
The rates of catheter obstruction, catheter displacement, and dialysate leakage were not different between the 2 groups (all P>0.05), but the rate of early bloody dialysate was lower in group B than in group A (7.4% vs. 16.2%, P=0.04, Table 2). Multiple regression analysis showed that guide wire was a significant contributing factor of bloody dialysate. The patients with early bloody dialysate were treated with delaying initation of PD, and no catheters were replaced due to bloody dialysate.
A total of 11 cases were found with catheter obstruction, of which 6 cases were due to protein clot and treated with urokinase flushes, 5 were due to omental wrapping and the catheters were repalced. Catheter displacement was found in 31 patients, in which 23 were treated by manipulation, and the other 8 who failed with manipulation received catheter replacement. Dialysate leakage were found in 20 patients. The 13 cases developing early leakage were managed by reducing filling volumes or delaying initiation of PD, and the 7 cases developing late leakage that could not be resolved were treated by removing their catheters.
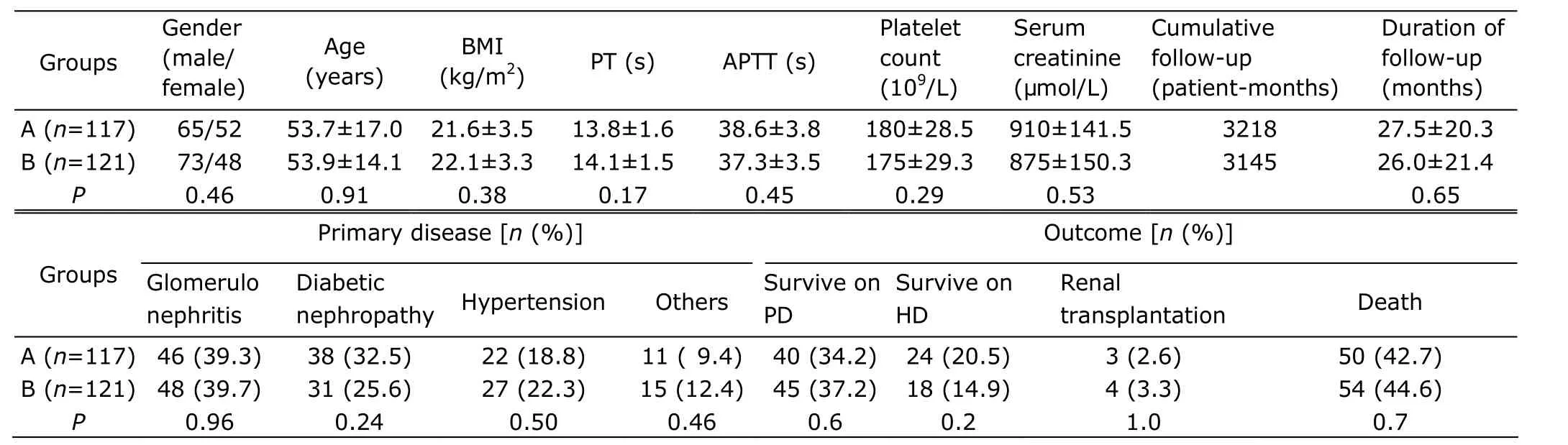
Table 1. Baseline characteristics of the 2 groups§
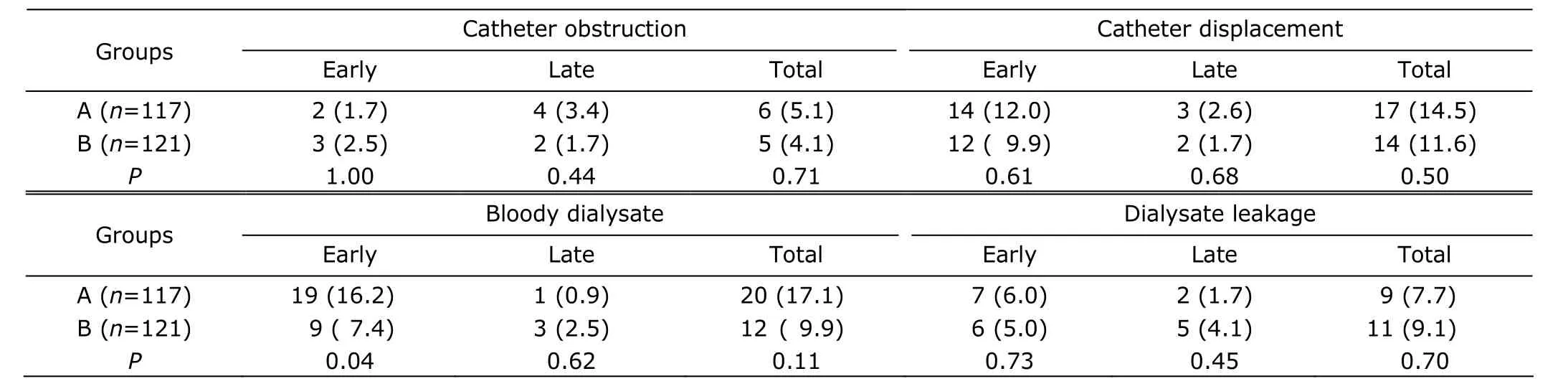
Table 2. Post-operative complications of the 2 groups [n (%)]
Overweight and post-operative complications
There were 11 overweight patients in group A and 14 in group B. No differences in post-operative complications among overweight patients were found between the 2 groups (all P>0.05, Table 3). The incidence of catheter obstruction was higher in overweight patients than in normal weight patients (16.0% vs. 3.3%, P=0.02, Table 4).
Catheter survival and patient survival
During the follow-up period, 32 (27.4%) catheters in group A and 35 (28.9%) in group B were removed. The rates of catheter loss showed no significant difference between the 2 groups (P=0.84). The main causes of catheter loss were peritoneal membrane failure (36/67, 53.7%), outflow failure (20/67, 29.9%), and peritonitis (8/67, 11.9%). The 1-, 2-, and 3-year catheter survival rates in group A were 89.8%, 81.3%, and 74.6%; in group B were 90.2%, 80.7%, and 77.5%, respectively. There was no significant difference in total catheter survival rate between the 2 groups (P=0.72). The 1-, 2-, and 3-year patient survival rates in group A were 88.2%, 76.4%, and 62.1%; in group B were 85.3%, 77.8%, 64.5%, respectively. There was also no significant difference in total patient survival rate between the 2 groups (P=0.54).
DISCUSSION
Guide wire is commonly used to aid in placing catheter into the peritoneal cavity during PDC insertion. When inserting Tenkchoff coiled catheter, guide wire is put through the catheter to make the end straight. Guide wire is therefore necessary in the insertion of Tenkchoff coiled catheter. In contrast, Tenkchoff straight catheter has a straight end, thus can be inserted directly into the peritoneal cavity. In the present study, X-ray confirmed that the inner ends of the catheters inserted without guide wire (group B) were within the lesser pelvis, suggesting that it is possible to insert Tenkchoff straight catheter to the right position without guide wire.
In addition, no significant differences were observed in catheter obstruction, catheter displacement, and dialysate leakage between group A and group B (all P>0.05). Moreover, there were significant differences in neither catheter survival rates nor patient survival rates between the 2 groups. The catheter survival rates of the patients who received PDC insertion without guide wire were similar with the results previously reported for surgical techniques.11,12Hence we conjectured that the outcomes of PDC insertion without guide wire are equal to that of PDC insertion using guide wire. Given the findings of this study, PDC insertion without guide wire can be a choice when guide wire is not available, especially in emergency PDC insertion.
In the present study, early bloody dialysate was found more common in the group using guide wire (P=0.04). Bloody dialysate is a common complication of PDC insertion, usually caused by incision bleeding or injury at abdominal organs.13Guide wire is more rigid than catheter, it may be hypothesized that omentum is more likely to be injured by guide wire. However, the bloody dialysate may also be caused by incision bleeding. Better designed studies are necessary to reach a more convincing conclusion.
Generally, PDC insertion in overweight patients is more difficult than in normal weight patients. In the present study, we successfully inserted catheter in overweight patients without guide wire, simply by expanding the incisions. Obesity is considered a risk factor for catheter-related complications. Piraino et al14reported that more catheter infections occurred in overweight patients, but the incidences of mechanical problems, such as dialysate leakage and outflow failure, were similar in overweight and normal weight patients. In the present study, overweight patients showed a higher rate of catheter obstruction compared with normal weight patients (P=0.02). We speculate thatoverweight patients usually have higher levels of serum albumin, which exudates into dialysate and become protein clots. Among all the 25 overweight patients, there was no difference in post-operative complications between the patients using guide wire and those using no guide wire, suggesting that catheter insertion without guide wire is feasible in overweight patients.
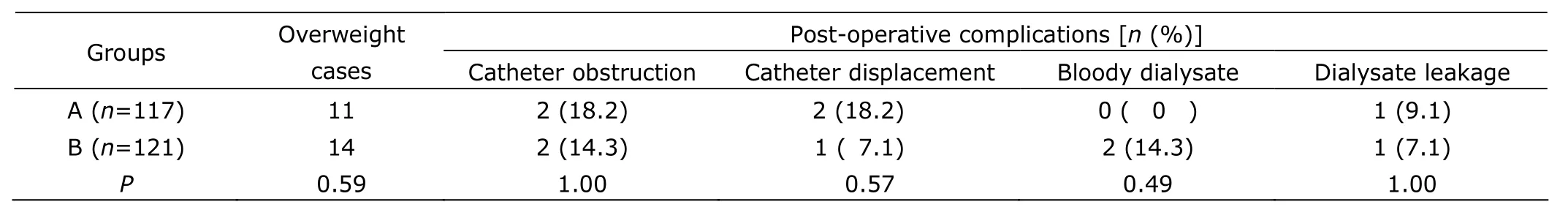
Table 3. Post-operative complications of overweight patients in the 2 groups
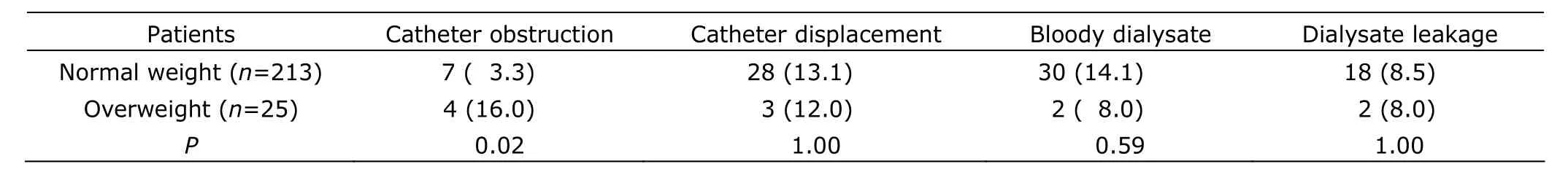
Table 4. Post-operative complications in overweight and normal weight patients [n (%)]
This study has several limitations: firstly, it was an unrandomized retrospective study; secondly, the study sample was small and follow-up time was not long enough. However, the 2 groups in the present study had comparable baseline characteristics. According to the findings of this study, PDC insertion with no aid of guide wire may be an alternative option when guide wire is not available.
We thank all the research staff involved, and Kai-jie Wu for kindly editing the manuscript.
1. Chow KM, Szeto CC. Open surgical insertion of tenckhoff catheters for peritoneal dialysis. Perit Dial Int 2010; 30:502-3.
2. Zaman F. Peritoneal dialysis catheter placement by nephrologists. Perit Dial Int 2008; 28:138-41.
3. Maio R, Figueiredo N, Costa P. Laparoscopic placement of Tenckhoff catheters for peritoneal dialysis: a safe, effective and reproducible procedure. Perit Dial Int 2008; 28:170-3.
4. Li PK, Chow KM. Importance of peritoneal dialysis catheter insertion by nephrologists: practice makes perfect. Nephrol Dial Transplant 2009; 24:3274-6.
5. Ash SR. Chronic peritoneal dialysis catheters: overview of design, placement, and removal procedures. Semin Dial 2003; 16:323-34.
6. Dombros N, Dratwa M, Feriani M, et al. European best practice guidelines for peritoneal dialysis. 3 peritoneal access. Nephrol Dial Transplant 2005; 20:ix8-12.
7. Shahbazi, N, McCormick BB. Peritoneal dialysis catheter insertion strategies and maintenance of catheter function. Semin Nephrol 2011; 31:138-51.
8. Flanigan M, Gokal R. Peritoneal catheters and exit-site practices toward optimum peritoneal access: a review of current developments. Perit Dial Int 2005; 25:132-9.
9. Peppelenbosch A, van Kuijk WHM, Bouvy ND, et al. Peritoneal dialysis catheter placement technique and complications. NDT Plus 2008; 1:iv23-8.
10. Holes-Lewis KA, Malcolm R, O'Neil PM. Pharmacotherapy of obesity: clinical treatments and considerations. Am J Med Sci 2013; 345:284-8.
11. Balaskas EV, Ikonomopoulos D, Sioulis A, et al. Survival and complications of 225 catheters used in continuous ambulatory peritoneal dialysis: one-centre experience in Northern Greece. Perit Dial Int 1999; 19:S167-71.
12. Weber J, Mettang T, Hübel E, et al. Survival of 138 surgically placed straight double-cuff Tenckhoff catheters in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 1993; 13:224-7.
13. Ikee R, Branch J, Honda K, et al. Recurrence of severe hemoperitoneum in a patient on peritoneal dialysis. Perit Dial Int 2009; 29:583-5.
14. Piraino B, Bernardini J, Centa PK, et al. The effect of body weight on CAPD related infections and catheter loss. Perit Dial Int 1991; 11:64-8.
 Chinese Medical Sciences Journal2013年2期
Chinese Medical Sciences Journal2013年2期
- Chinese Medical Sciences Journal的其它文章
- Up-regulation of Fas Ligand Expression by Sirtuin 1 in both Flow-restricted Vessels and Serum-stimulated Vascular Smooth Muscle Cells△
- Lysine-specific Demethylase 1 Represses THP-1 Monocyte-to-macrophage Differentiation△
- Standard Versus Extended Pancreaticoduodenectomy in Treating Adenocarcinoma of the Head of the Pancreas
- Safety and Efficacy of Frameless Stereotactic Brain Biopsy Techniques
- Chinese Herbal Medicine in Treatment of Polyhydramnios: a Meta-analysis and Systematic Review△
- Notice of Retraction
