Functional Polyethylene Glycol with Carboxyl-supported Platinum as an Efficient Catalysis System for the Hydrosilylation of Alkenes*
BAI Ying (白赢), PENG Jiajian (彭家建), YANG Hu (杨虎), LI Jiayun (厉嘉云), LAI Guoqiao (来国桥),** and LI Xiaonian (李小年)**
1 State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Institute of Industrial Catalysis,Zhejiang University of Technology, Hangzhou 310014, China 2 Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou 310012, China
Functional Polyethylene Glycol with Carboxyl-supported Platinum as an Efficient Catalysis System for the Hydrosilylation of Alkenes*
BAI Ying (白赢)1,2, PENG Jiajian (彭家建)2, YANG Hu (杨虎)2, LI Jiayun (厉嘉云)2, LAI Guoqiao (来国桥)1,2,** and LI Xiaonian (李小年)1,**
1State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Institute of Industrial Catalysis,Zhejiang University of Technology, Hangzhou 310014, China2Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou 310012, China
A series of carboxylated long chain polyethylene glycols (abbreviated as PEGCOOH) has been synthesized and used to support chloroplatinic acid. These supported catalysts were then tested for their efficiency in the hydrosilylation of alkenes. The factors affecting their catalytic properties, e.g. relative molecular mass of polyethylene glycol, reaction temperature, platinum content, and type of alkenes, have been studied. It was found that the activity of the platinum catalyst decreased with increasing length of the polyethylene glycol chain, and increased with reaction temperature. Moreover, these catalysts could be reused several times without a noticeable decrease in activity or selectivity. The reaction pathway leading to excellent selectivity for the β-adduct of hydrosilylation of alkenes with triethoxysilane catalyzed by this catalysis system was discussed.
polyethylene glycol, modification, platinum, hydrosilylation, alkene
1 INTRODUCTION
Hydrosilylation of unsaturated carbon-carbon bonds leading to C Si bond formation is a widely applied reaction in organosilicon chemistry [1-3]. Many catalysts have been applied for hydrosilylation reactions in the synthesis of silicone monomers, silicone rubbers, and particularly polycarbosilanes, among which platinum complexes are the most prominent.Among the efficient catalysts, Speier’s catalyst(H2PtCl6/iPrOH) [4-6] and Karstedt’s catalyst(Pt2[(η2-vinylSiMe2)2O]3) [7-9] are the most widely used. Additionally, various modified Karstedt’s catalysts have been investigated [10, 11], but none of them gave excellent selectivity. Obtaining high selectivity for the adduct in the hydrosilylation of functional olefins is a great challenge and a difficult task. A wide range of platinum complexes containing phosphine[12-15], sulfur [16, 17], or nitrogen ligands [18, 19] have been synthesized and applied as homogeneous catalysts in regio-, stereo-, and/or enantioselective hydrosilylation. In order to address the issue of recyclability, some heterogeneous catalysts have been prepared and used for hydrosilylation [20-22]. Recyclable rhodium complex/ionic liquid systems have been investigated [23-26], and the catalytic activity and selectivity were found improved by using an ionic liquid as the medium or a “soft support”.
On the other hand, soluble polymers loaded with transition metal complex catalysts offer many unique properties, and open new perspectives in the recovery of the catalyst. Specifically, PEG-supported catalysts exhibiting high catalytic activity have been extensively researched. Fan et al. [27] reported the synthesis of MeO-PEG bearing ruthenium or rhodium catalytic centers ligated by phosphine ligands such as (R)-BINAP.Application of these systems in the asymmetric hydrogenation of enamines and aromatic ketones led to high activity and high stereoselectivity. Moreover, the catalysts could be recycled several times without any obvious deactivation [28, 29]. Transition metals supported on polyethylene glycol (PEG) and functional derivatives thereof have also been successfully used as catalysts for Heck reactions [30], hydroformylation [31],asymmetric cyclopropanation [31, 32], ring-closing metathesis of alkenes [33, 34], asymmetric epoxidation[35-37], catalytic oxidation [38] and the formylation of hydrocarbons [39-44]. Herein, we focus on the application of functionalized polyethylene glycol loaded with platinum catalyst in the hydrosilylation of alkenes.
Very recently, we have reported that low relative molecular mass polyethylene glycol functionalized with maleic anhydride as a promoter for the platinum catalysis hydrosilylation of alkene [45]. In this paper,we will report more long chain polyethylene glycol functionalized with maleic anhydride as a support for platinum, and the catalytic activity of this system in the hydrosilylation of different alkenes with triethoxysilane (Fig. 1). The effect of PEG modified with maleic anhydride on the catalytic activity, selectivity,recovery performance and reaction mechanism of[Pt]/PEGCOOH catalyst is illustrated.
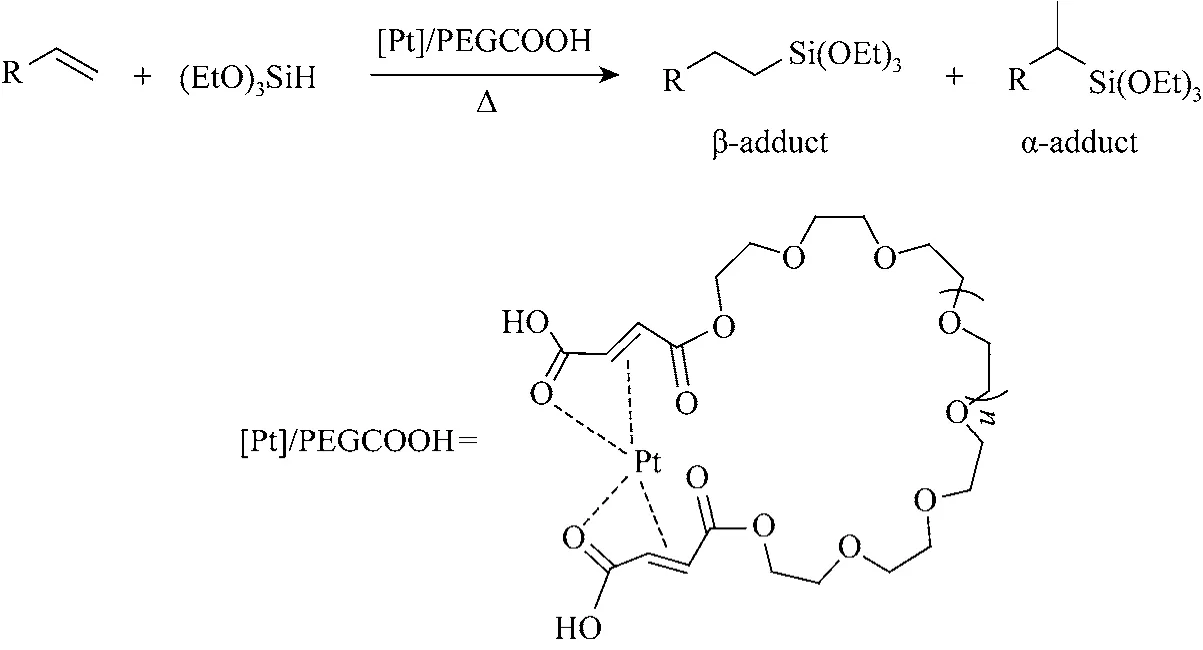
Figure 1 Hydrosilylation of alkenes with triethoxysilane catalyzed by [Pt]/PEGCOOH
2 EXPERIMENTAL
2.1 Materials
Polyethylene glycol (average molecular mass of 2000, 4000, 6000, 10000), maleic anhydride, styrene,chloroform, diethyl ether and chloroplatinic acid(H2PtCl6·6H2O) were purchased from China National Medicines Corporation Ltd.1H NMR spectra was recorded on a Bruker Advance 400 MHz spectrometer using tetramethylsilane (TMS) as an internal standard and CDCl3as solvent. IR (infra-red) spectra were recorded on a Nicolet 5700 instrument. UV (ultraviolet)absorption spectra were recorded on an Evolution-300.GC-MS (gas chromatography combined with mass spectrometry) analyses of the hydrosilylation reaction products were obtained with an Agilent 26890N/59731 equipped with a DB-5 column (30 m×2.5 mm×0.25 μm).
2.2 Synthesis of functionalized PEG (PEGCOOH)
PEG (Mw=2000) (12.0 g, 0.006 mol) was dissolved in toluene (100 ml) in a three-necked flask and the stirred solution was heated to reflux for 2 h to remove the water by azeotropy. The toluene was then removed by distillation under reduced pressure. Then maleic anhydride (1.3 g, 0.013 mol) was added and the reaction mixture was stirred for 6 h at 80 °C under N2atmosphere. The mixture was then cooled and hexane (200 ml) was added, whereupon a solid separated. The precipitated solid was collected by filtration and washed with hexane. The crude product was then redissolved in CH2Cl2(30 ml) and reprecipitated by adding excess dry diethyl ether to furnish a yellowish powder product. The product was purified by two fold precipitation from CH2Cl2. Various samples of PEG modified with maleic anhydride (PEGCOOH) were synthesized by the same method, and the products remained as yellowish powder.
Functionalized polyethylene glycol (Mw2000)with maleic anhydride,1H NMR (CDCl3, 400 MHz), d:6.43-6.39 (d, 2 H, J=16, CHCHCOO), 6.24-6.21 (d,2 H, J 12, CHCHCOO), 3.83-3.46 (br, 176 H,CH2CH2O ).
Functionalized polyethylene glycol (Mw4000)with maleic anhydride,1H NMR (CDCl3, 400 MHz), d:6.42-6.39 (d, 2 H, J 16, CHCHCOO), 6.25-6.22 (d,2 H, J 12, CHCHCOO), 3.74-3.65 (br, 352 H,CH2CH2O ).
Functionalized polyethylene glycol (Mw6000)with maleic anhydride,1H NMR (CDCl3, 400 MHz), d:6.41-6.38 (d, 2 H, J 12, CHCHCOO), 6.23-6.20(d, 2 H, J 12, CHCHCOO), 3.83-3.64 (br, 528 H,CH2CH2O ).
Functionalized polyethylene glycol (Mw10000)with maleic anhydride,1H NMR (CDCl3, 400 MHz), d:6.42-6.38 (d, 2 H, J 16, CHCHCOO), 6.24-6.21, (d,2 H, J 12, CHCHCOO), 3.71-3.61 (br, 890 H,CH2CH2O ).
2.3 Preparation of [Pt]/PEGCOOH catalysts
A certain amount of a solution of H2PtCl6in tetrahydrofuran (THF) (1.95×10-5mol·ml-1), according to the required loading level, was mixed with functionalized PEG (1.0 g) in a round-bottomed flask containing THF (10 ml). The mixture was stirred for 12 h.THF was then removed by distillation under reduced pressure to leave the functionalized PEG-platinum catalyst ([Pt]/PEGCOOH) as yellow waxy solid.
2.4 Catalyzed hydrosilylation reaction
All operations were performed under atmosphere.The requisite amounts of catalyst and alkene(4.0 mmol) were added to a dried 10-ml flat-bottomed tube and the reaction mixture was stirred for 5 min.Thereafter, 4.4 mmol of silane was added and the mixture was heated and stirred for appropriate time and then cooled to room temperature. The product phase was separated by decantation and the conversion of alkene and the selectivity were determined by GC/MS.
2.5 Hydrosilylation of styrene with triethoxysilane
β-Adduct [triethoxy(phenethyl)silane]:1H NMR(CDCl3, 400 MHz), d: 1.00 (t, J= 8 Hz, 2H, SiCH2),1.24 (t, J=9 Hz, 9H, CH3), 2.74 (t, J=8 Hz, 2H, CH2),3.84 (q, J=8 Hz, 6H, O CH2), 7.16-7.27 (m, 5H, Ph).
α-Adduct [triethoxy(1-phenylethyl)silane]:1H NMR(CDCl3, 400 MHz), d: 1.18 (t, J= 8 Hz, 9H, CH3),1.33 (d, J = 8 Hz, 3H, CH3), 3.65 (q, J=8 Hz, 1H,SiCH), 3.76 (q, J=8 Hz, 6H, O CH2), 7.12-7.19(m, 5H, Ph).
Ethylbenzene:1H NMR (CDCl3, 400 MHz), d:1.15 (t, J=8 Hz, 3H, CH3), 2.64 (q, J=8 Hz, 2H,CH2), 7.11-7.25 (m, 5H, Ph).
2.6 Hydrosilylation of other alkenes with triethoxysilane
β-Adduct (hexyltriethoxysilane):1H NMR (CDCl3,400 MHz), d: 0.64 (t, J= 6 Hz, 2H, SiCH2), 0.89 (t,J=8 Hz, 3H, CH3), 1.22-1.42 (m, 17H, CH2CH3),3.81 (q, J=8 Hz, 6H, O CH2).
β-Adduct [triethoxy(p-methyl-phenethyl)silane]:1H NMR (400 MHz, CDCl3), d: 1.02 (t, J= 9 Hz, 2H,SiCH2), 1.28 (t, J=7 Hz, 9H, CH3), 2.31 (s, 3H,CH3), 2.70 (t, J=9 Hz, 2H, CH2), 3.87 (q, J 7.0 Hz,6H, O CH2), 7.07-7.11 (m, 4H, Ph).
β-Adduct [triethoxy(p-chloro-phenethyl)silane]:1H NMR (400 MHz, CDCl3), d: 0.97 (t, J= 9 Hz, 2H,SiCH2), 1.25 (t, J=7 Hz, 9H, CH3), 2.70 (m, 2H,CH2), 3.87 (q, J=7 Hz, 6H, O CH2), 7.12-7.24 (m,4H, Ph).
2.7 Catalyst recycling test
When the reaction was over, the liquid reactant was withdrawn from the reaction vessel after setting aside for 1 h and centrifuging, and the catalyst left in the vessel was used for the next test.
3 RESULTS AND DISCUSSION
3.1 Preparation and characterization of PEGCOOH
PEG with various relative molecular mass modified with maleic anhydride was successfully prepared according to the method outlined in the experimental section. Fig. 2 shows typical IR spectra of the synthesized PEGCOOH, which feature bands at 2851-2920 cm-1(2CHv ), 1729 cm-1(vCO), and 1639 cm-1(vCC). These bands were characteristic of the desired compounds. The structures of the compounds were further supported by their1H NMR spectra.
Figure 3 shows the UV/Vis spectra of PEG2000,PEGCOOH, H2PtCl6, and [Pt]/PEGCOOH. No absorbance peak was found in the spectrum of PEG. The spectrum of PEGCOOH featured an absorbance peak
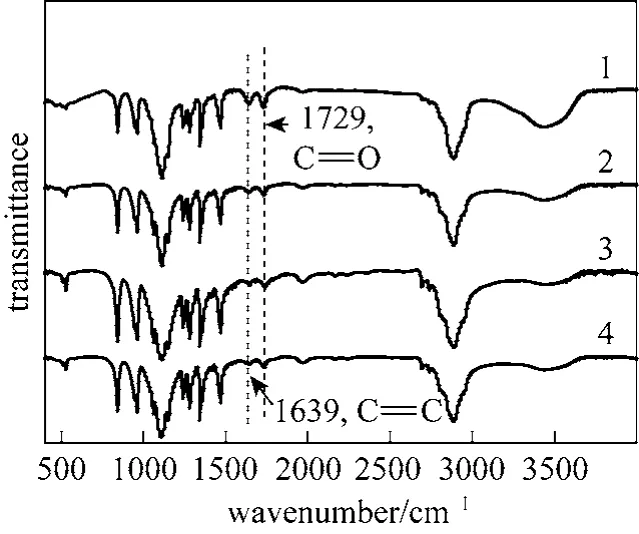
Figure 2 FTIR spectra of PEGCOOH samples of various chain lengths1—PEG(2000); 2—PEG(4000); 3—PEG(6000); 4—PEG(10000)
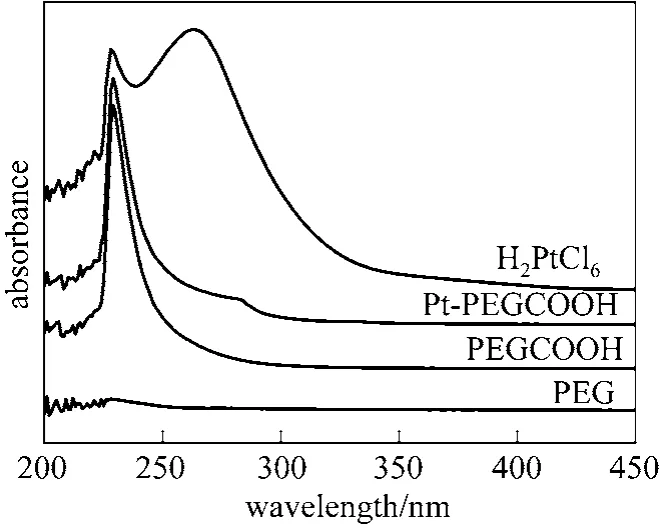
Figure 3 UV/Vis absorption spectra of the catalyst system and its components
centered at 229 nm, which could be assigned to the COOH group. An absorbance centered at 263 nm was seen for the hexachloroplatinic acid precursor, but this absorbance decreased for the [Pt]/PEGCOOH sample, and a new absorbance appeared at 281 nm.This absorbance band suggested that Pt atoms had become attached to the PEGCOOH groups.
3.2 Catalytic hydrosilylation of alkenes with triethoxysilane
3.2.1 Hydrosilylation of styrene with triethoxysilane Various platinum catalysts were used for the hydrosilylation of styrene with triethoxysilane and the reaction results are listed in Table 1. All of the homogeneous catalysts, namely aqueous H2PtCl6/THF,Speier’s catalyst, and Karstedt’s catalyst, showed activity for the reaction, but showed low selectivity for the β-adduct. On the other hand, PEG-supported H2PtCl6was also effective in catalyzing the reaction,and higher selectivity for the β-adduct in comparison with the other three catalysts. Furthermore, with functionalized polyethylene glycol-supported platinum,high conversion of styrene was attained. Encouragingly, the selectivity for the β-adduct was remarkably promoted from 59.3% to about 94%. While some heterogeneous catalysts were reported for this reaction,for example, under the condition of n(Pt)/n(styrene)1/1000, 63% β-adduct was obtained by using MCM-41-supported mercapto platinum complex as catalyst at 110 °C for 80 min [22]; Under the condition of n(Pt)/n(styrene)= 1/4000, 90% conversion was obtained by using SiO2-PEG-Pt as catalyst at 50 °C for 100 min [46].
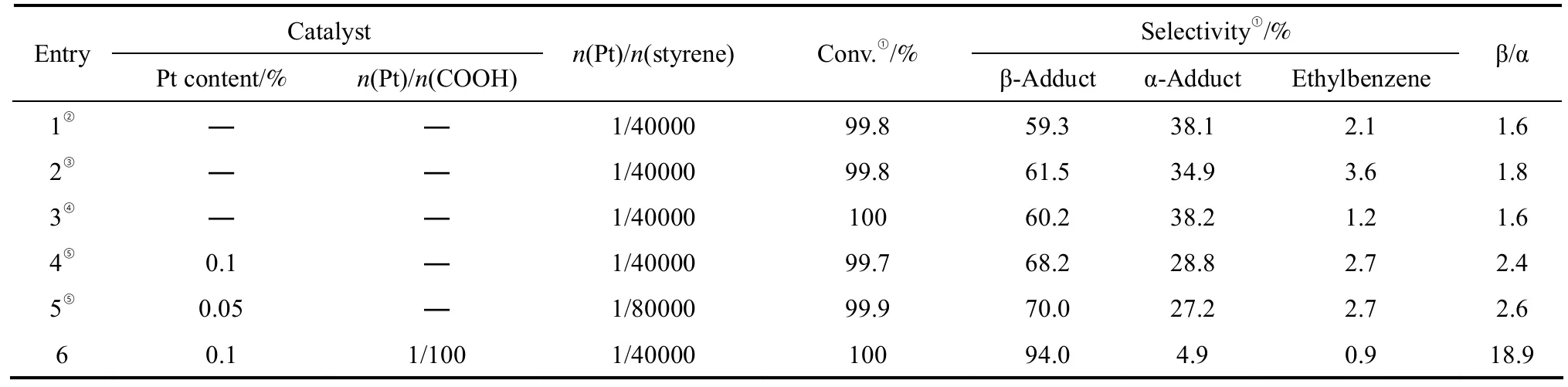
Table 1 Hydrosilylation of styrene with triethoxysilane catalyzed by various Pt catalysts
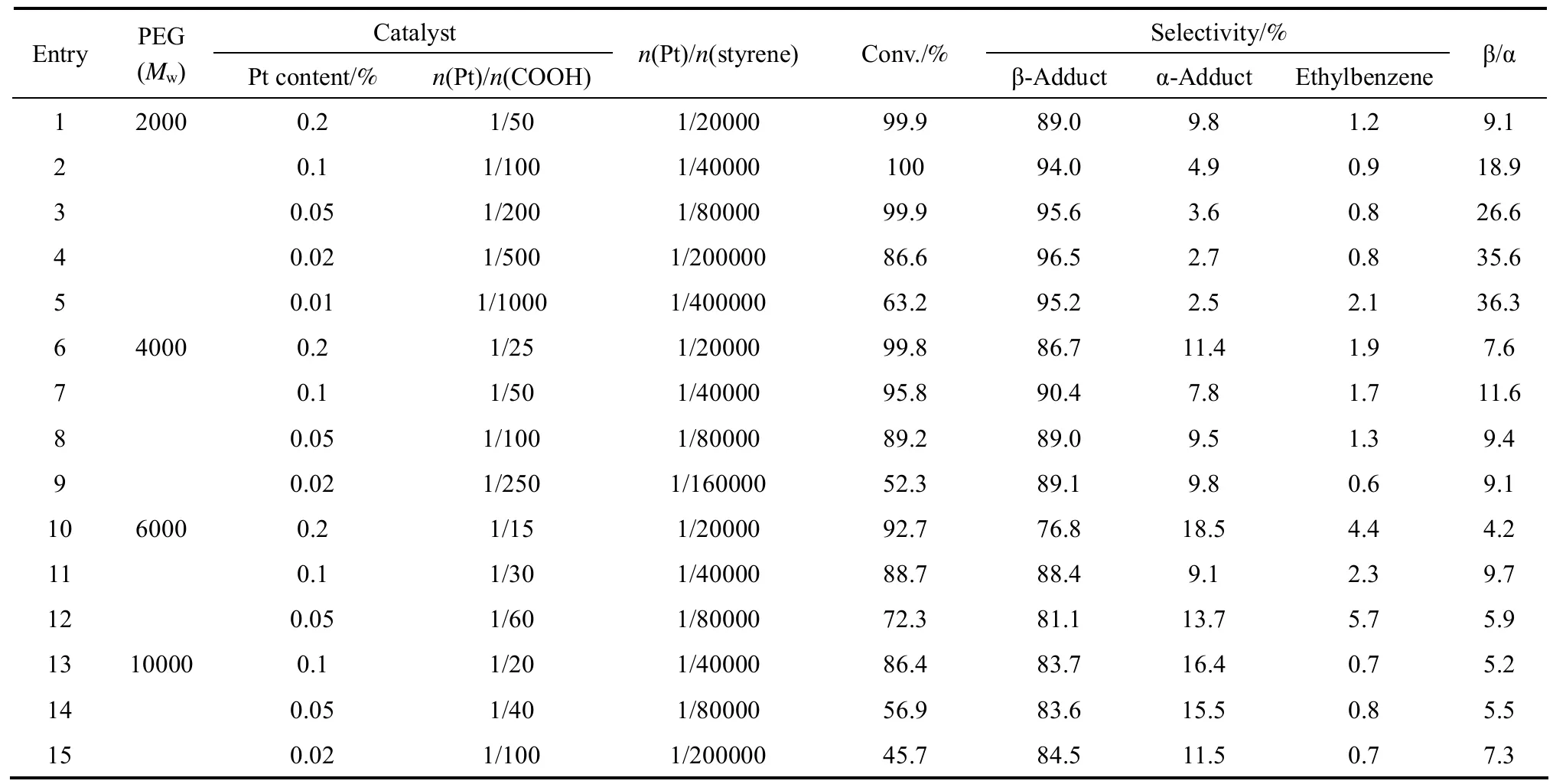
Table 2 Effects of the relative molecular mass of polyethylene glycol and the Pt content on the hydrosilylation
3.2.2 Effects of average molecular mass of polyethylene glycol and the amount of Pt loading on the hydrosilylation reaction
Table 2 shows the results of hydrosilylation reactions catalyzed with platinum complex supported on polyethylene glycol of various relative molecular mass modified with maleic anhydride. The results indicate that polyethylene glycol length affected adversely the catalytic activity and selectivity for the β-adduct. The effect of chain length of the polyethylene glycol on the catalytic activity is illustrated in Fig. 4. It may be speculated that longer-chain PEG will enclose the Pt ion more completely, thereby preventing approach of the substrates to the catalytic center.
The compositions of the catalysts and their properties affect the catalytic performance in the hydrosilylation reaction. The n(Pt)/n(COOH) molar ratio and the amount of platinum loading on the functionalized PEG affect the selectivity for the β-adduct. A low n(Pt)/n(COOH) molar ratio leads to higher selectivity.
3.2.3 Effects of the amount of Pt and the reaction temperature on the hydrosilylation

Figure 4 Plots of conversion versus time for catalytic hydrosilylations using [Pt]/PEGCOOH of various relative molecular mass [reaction conditions: n(Pt)/n(styrene)1/80000, 90 °C]Mw of [Pt]/PEGCOOH: ■ 2000; ● 4000; ▲ 6000; ★ 10000
The effect of the amount of platinum on the conversion of styrene is illustrated in Fig. 5. It can be seen that higher conversion was obtained when a greater amount of platinum was used for the same reaction time, although the conversion of styrene could also reach 100% by prolonging the reaction time in the presence of a lower amount of catalyst.
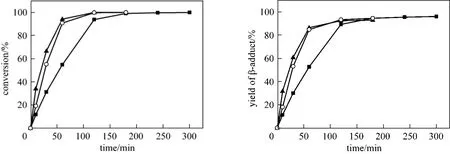
Figure 5 Effect of the amount of catalyst on the hydrosilylation of styrene [reaction conditions: [Pt]/PEGCOOH (2000),n(Pt)/n(COOH) = 1/200, 90 °C]catalyst amount: ■ 0.01 g; ○ 0.02 g; ▲ 0.04 g
The effect of reaction temperature on the catalytic activity was illustrated in Fig. 6, indicating that there was no remarkable induction period at different temperature, and that the activity of the platinum catalyst increased with increasing reaction temperature. Using the same amount of catalyst, 87.7% conversion was obtained at 70 °C for 2.5 h, and 93.6%conversion was obtained after 7 h. When the temperature exceeded 90 °C, 100% conversion of styrene was obtained within 2 h. At 110 °C, the conversion of styrene reached 100% within 1.5 h. However, excessive reaction temperature can reduce the selectivity for the β-adduct.
3.2.4 Effect of different alkenes on the hydrosilylation
We next selected the [Pt]/PEGCOOH catalyst system based on PEG with relative molecular mass 2000 to evaluate the scope of this hydrosilylation with a variety of terminal alkenes. As can be seen from Table 3, various alkenes, including aliphatic and aryl alkylenes, could react with (EtO)3SiH to give the corresponding adducts. Additionally, the reactions showed a remarkable selectivity for the β-adduct. For 1-octene, 100% conversion along with 98.5% selectivity of β-adduct was obtained. While it was reported that 96.5% yield of 1-octyltriethoxysilane was obtained with nitro-platinum complex supported on the silica as catalyst at 110 °C for 2.5 h [47].
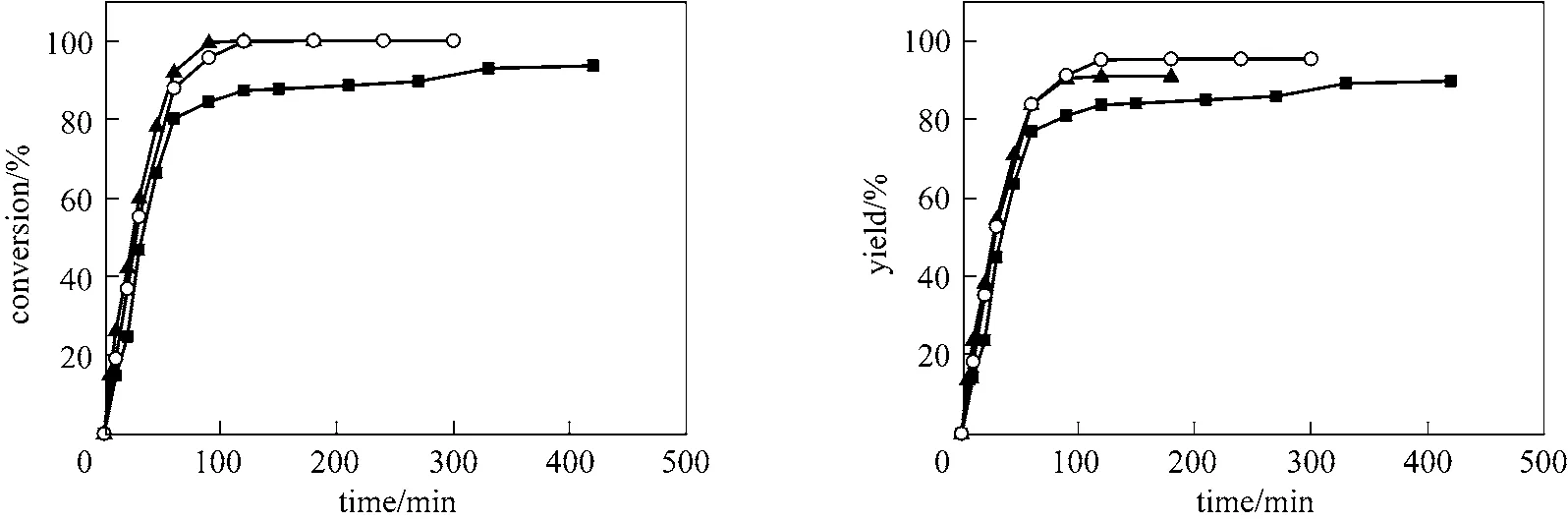
Figure 6 Effect of reaction temperature on the hydrosilylation of styrene [reaction conditions: [Pt]/PEGCOOH (2000),n(Pt)/n(styrene)=1/80000, n(Pt)/n(COOH) = 1/200]■ 70 °C; ○ 90 °C; ▲ 110 °C
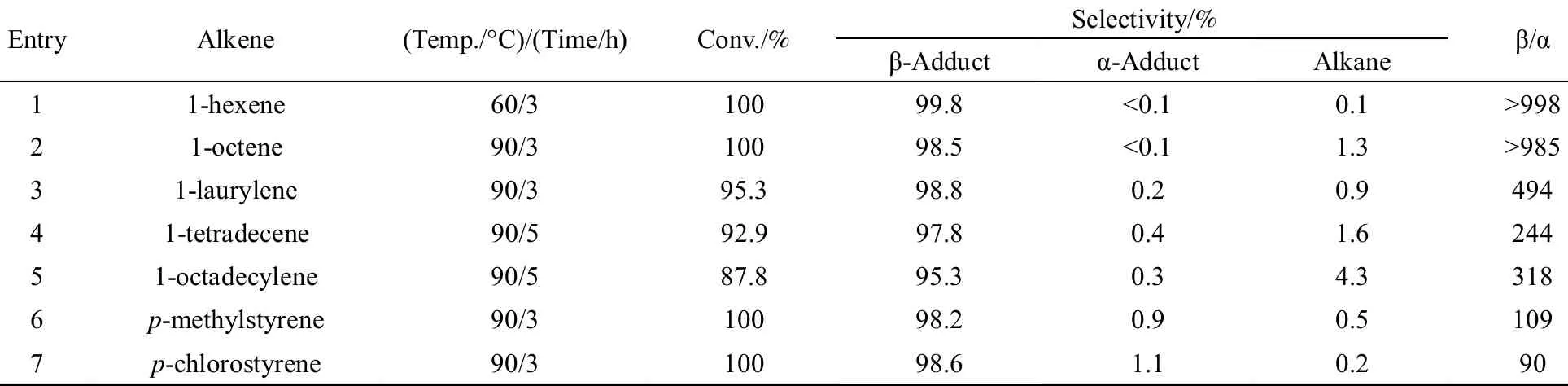
Table 3 Hydrosilylation of various alkenes with triethoxysilane
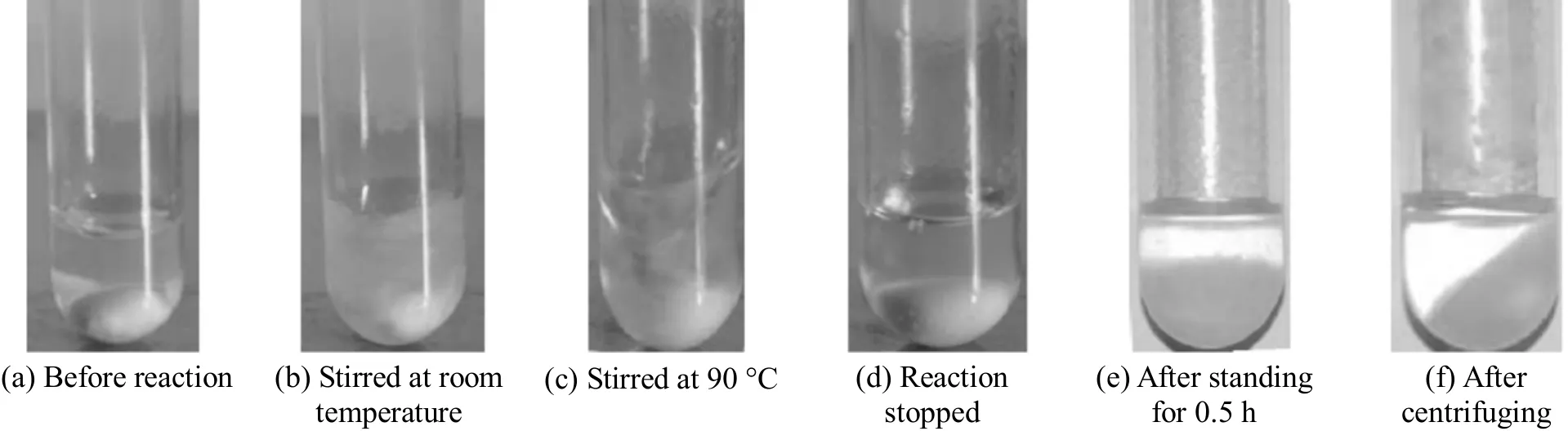
Figure 7 View of catalytic hydrosilylation by [Pt]/PEGCOOH [Magnetic stirrer is visible at the bottom in (a)-(d)]
3.2.5 Catalyst recycling experiment
The catalyst recycling performance in the hydrosilylation of 1-octene with triethoxysilane was also tested. As can be seen from Fig. 7, the catalyst system showed good settling performance from the resulting mixture. In general, the catalyst system of[Pt]/PEGCOOH shows good stability for the hydrosilylation of 1-octene and triethoxysilane. From Fig. 8, it is evident that the activity of [Pt]/PEGCOOH showed no noticeable decrease after eight runs.
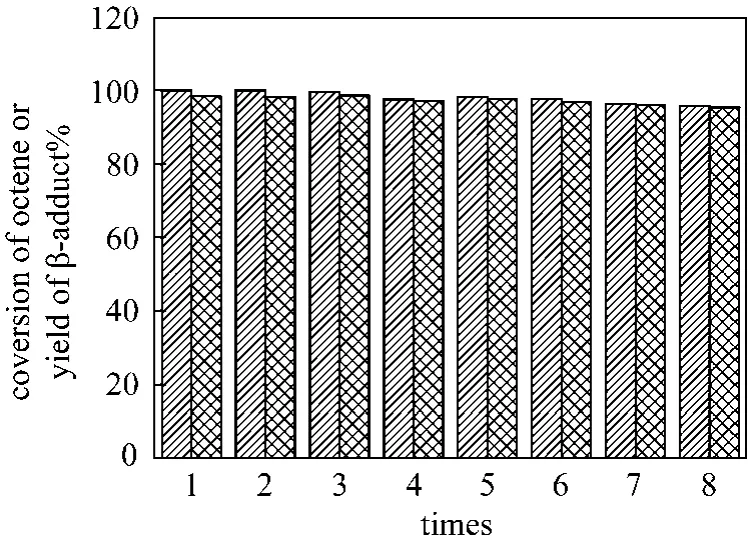
Figure 8 Recyclability of the catalyst in the hydrosilylation of 1-octene [reaction conditions: [Pt]/PEGCOOH(2000),n(Pt)/n(alkene)=1/80000, n(Pt)/n(COOH)=1/200]conversion of octene; yield of β-adduct
3.2.6 Catalytic pathway of the hydrosilylation of alkenes by [Pt]/PEGCOOH
On the basis of the results of hydrosilylations of alkenes with triethoxysilane catalyzed by [Pt]/PEGCOOH, the reaction is proposed to proceed according to the modified Chalk-Harrod mechanism similar to many references [48-50]. Using hydrosilylation of styrene with triethoxysilane as example, the postulated pathway is illustrated. First, (EtO)3SiH was activated by platinum site and formed intermediate 1,then complexed with alkene to form 2. Second, intermediate 2 was formed by cooperation of styrene with intermediate 1. Third, intermediate 3 and 4 have completed on the platinum. At last, two kinds of adducts were obtained and platinum run into next catalytic cycle. Because of steric effect of PEGCOOH, the formation of intermediate 3 is preferred to 4, thus β-adduct was dominant product. In addition, we speculate that the PEGCOOH plays the important role of heterolysis of the C C double bond in this catalysis system, which can lead to better selectivity for the β-adduct of hydrosilylation.
4 CONCLUSIONS
In summary, [Pt]/PEGCOOH can be used in the hydrosilylation of alkenes with triethoxysilane. The PEGCOOH groups are especially effective for promoting the catalytic activity and selectivity of the platinum for the β-adduct. The chain length of the polyethylene glycol has an impact on the catalytic process; the catalytic activities of [Pt]/PEGCOOH decreased with increasing length of the polyethylene glycol chain,while the β/α ratio increased slightly. The catalytic system showed higher activity and selectivity when using linear-chain alkenes as substrates. In addition,the catalytic system showed good recyclability, being reusable for at least eight runs. The results indicate a reaction pathway in which PEGCOOH plays an important role of heterolysis of the C C double bond in this catalysis system, which can lead to better selectivity for the β-adduct of hydrosilylation.

Figure 9 Postulated pathway for the hydrosilylation of alkenes catalyzed by [Pt]/PEGCOOH
1 Marciniec, B., “Catalysis by transition metal complexes of alkene silylation—Recent progress and mechanistic implications”, Coord.Chem. Rev., 249 (21-22), 2374-2390 (2005).
2 Marciniec, B., Comprehensive Handbook on Hydrosilylation, Pergamon Press, Oxford, England (1992).
3 Roy, A.K., “A review of recent progress in catalyzed homogeneous hydrosilation (hydrosilylation)”, Adv. Organomet. Chem., 55, 1-59(2007).
4 Speier, J.L., Webster, J.A., Barnes, G.H., “The addition of silicon hydrides to olefinic double bonds. Part II. The use of group VIII metal catalysts”, J. Am. Chem. Soc., 79 (4), 974-979 (1957).
5 Saam, J.C., Speier, J.L., “The addition of silicon hydrides to olefinic double bonds. Part III. The addition to non-terminal olefins in the presence of chloroplatinic acid”, J. Am. Chem. Soc., 80 (15),4104-4106 (1958).
6 Speier, J.L., “Homogeneous catalysis of hydrosilylation by transition metals”, Adv. Organomet. Chem., 17, 407-447 (1979).
7 Karstedt, B.D., Scotia, N.Y., “Platinum complexes of unsaturated siloxanes and platinum containing organopolysiloxanes”, U.S. Pat.,3775452 (1973).
8 Chandra, G., Lo, P.Y., Hitchcock, P.B., Lappert, M.F., “A convenient and novel route to bis(.eta.-alkyne) platinum(0) and other platinum(0)complexes from Speier’s hydrosilylation catalyst H2[PtCl6]·xH2O.X-ray structure of [Pt{(.eta. CH2CHSiMe2)2O}(P-t-Bu3)]”,Organometallics, 6 (1), 191-192 (1987).
9 Lewis, L.N., Colborn, R.E., Grade, H., Bryant, G.L., Sumpter, C.A.,Scott, R.A., “Mechanism of formation of platinum(0) complexes containing silicon-vinyl ligands”, Organometallics, 14 (5), 2202-2213(1995).
10 Sprengers, J.W., Mars, M.J., Duin, M.A., Cavell, K.J., Elsevier, C.J.,“Selective hydrosilylation of styrene using an in situ formed platinum(1,3-dimesityl-dihydroimidazol-2-ylidene) catalyst”, J. Organomet.Chem., 679 (2), 149-152 (2003).
11 Buisine, O., Berthon-Gelloz, G., Brière, J.F., Stérin, S., Mignani, G.,Branlard, P., Tinant, B., Declercq, J.P., Marko, I.E., “Second generation N-heterocyclic carbene-Pt(0) complexes as efficient catalysts for the hydrosilylation of alkenes”, Chem. Commun., (30), 3856-3858(2005).
12 Prignano, A.L., Trogler, W.C., “Silica-supported bis(trialkylphosphine)platinum oxalates. Photogenerated catalysts for hydrosilylation of olefins”, J. Am. Chem. Soc., 109 (12), 3586-3595 (1987).
13 Liedtke, J., Loss, S., Alcaraz, G., Gramlich, V., Grutzmacher, H.,“Very stable phosphiranes”, Angew. Chem. Int. Ed., 38 (11),1623-1626 (1999).
14 Liedtke, J., Loss, S., Widauer, C., Grutzmacher, H., “Phosphiranes as ligands for platinum catalyzed hydrosilylations”, Tetrahedron, 56 (1),143-156 (2000).
15 Murphy, P.J., Spencer, J.L., Procter, G., “Allylsilanes in organic synthesis; convenient preparation of synthetic intermediates by catalytic hydrosilation of acetylenic alcohols”, Tetrahedron Lett., 31 (7),1051-1054 (1990).
16 Hu, C.Y., Han, X.M., Jiang, Y.Y., “Hydrosilylation of acetylene catalyzed by a sulfur-containing polysiloxane platinum complex”, J.Mol. Catal., 35 (3), 329-333 (1986).
17 Chen, Y.M., Lu, X.R., Zhong, Z.L., “Synthesis of poly-4,7-dithianonylsesquisiloxane platinum complex and its catalytic activity on hydrosilylation of olefins with triethoxysilane”, Appl. Chem., 9(3), 26-30 (1992). (in Chinese)
18 Madine, J.W., Wang, X., Widenhoefer, R., “Cyclization/hydrosilylation of functionalized diynes catalyzed by a cationic platinum phenanthroline complex”, Org. Lett., 3 (3), 385-388 (2001).
19 Wang, X., Chakrapani, H., Madine, J.W., Keyerleber, M.A., Widenhoefer, R.A., “Cyclization/hydrosilylation of functionalized 1,6-diynes catalyzed by cationic platinum complexes containing bidentate nitrogen ligands”, J. Org. Chem., 67 (9), 2778-2788 (2002).
20 Drake, R., Dunn, R., Sherrington, D.C., Thomson, S.J., “Remarkable activity, selectivity and stability of polymer-supported Pt catalysts in room temperature, solvent-less, alkene hydrosilylations”, Chem.Commun., (19), 1931-1932 (2000).
21 Drake, R., Dunn, R., Sherrington, D.C., Thomson, S.J., “Polymethacrylate and polystyrene-based resin-supported Pt catalysts in room temperature, solvent-less, l-octene hydrosilylations using trichlorosilane and methyldichlorosilane”, J. Mol. Catal. A: Chem.,177 (1), 49-69 (2001).
22 Hu, R.H., Zha, L.F., Cai, M.Z., “MCM-41-supported mercapto platinum complex as a highly efficient catalyst for the hydrosilylation of olefins with triethoxysilane”, Catal. Commun., 11 (6),563-566 (2010).
23 Geldbach, T.J., Zhao, D.B., Castillo, N.C., Laurenczy, G., Weyershausen, B., Dyson, P.J., “Biphasic hydrosilylation in ionic liquids: A process set for industrial implementation”, J. Am. Chem. Soc., 128(30), 9773-9780 (2006).
24 Peng, J.J., Li, J.Y., Bai, Y., Gao, W.H., Qiu, H.Y., Wu, H., Deng, Y.,Lai, G.Q., “Rh(PPh3)3Cl/ionic liquid (molten salt) as a thermoregulated and recyclable catalytic system for hydrosilylation”, J. Mol.Catal. A: Chem., 278 (1-2), 97-101 (2007).
25 Peng, J.J., Li, J.Y., Bai, Y., Qiu, H.Y., Jiang, K.Z., Jiang, J.X., Lai,G.Q., “Ionic liquid (molten salt): Thermoregulated catalyst support for catalytic hydrosilylation process”, Catal. Commun., 9 (13),2236-2238 (2008).
26 Hofmann, N., Bauer, A., Frey, T., Auer, M., Stanjek, V., Schulz, P.S.,Taccardi, N., Wasserscheid, P., “Liquid-liquid biphasic, platinum-catalyzed hydrosilylation of allyl chloride with trichlorosilane using an ionic liquid catalyst phase in a continuous loop reactor”,Adv. Synth. Catal., 350 (16), 2599-2609 (2008).
27 Fan, Q.H., Deng, G.J., Lin, C.C., Chan, A.S.C., “Preparation and use of MeO-PEG-supported chiral diphosphine ligands: Soluble polymer-supported catalysts for asymmetric hydrogenation”, Tetrahedron:Asymmetry, 12 (8), 1241-1248 (2001).
28 Guerreiro, P., Ratovelomanana-Vidal, V., Genêt, J.P., Dellis, P.,“Recyclable diguanidinium-BINAP and PEG-BINAP supported catalysts: Syntheses and use in Rh(I) and Ru(II) asymmetric hydrogenation reactions”, Tetrahedron Lett., 42 (20), 3423-3426 (2001).
29 Chen, W., Hems, W., King, F., Xiao, J., “Asymmetric hydrogenation of ketones with polymer-supported chiral 1,2-diphenylethylenediamine”,Org. Lett., 5 (24), 4559-4561 (2003).
30 Bergbreiter, D.E., Osburn, P.L., Liu, Y.S., “Tridentate SCS palladium(II) complexes: New, highly stable, recyclable catalysts for the Reck reaction”, J. Am. Chem. Soc., 121 (41), 9531-9538 (1999).
31 Panda, A.G., Tambade, P.J., Patil, Y.P., Bhanage, B.M., “Hydroformylation of allyl acetate using rhodium polyether diphosphinite catalyst”, React. Kinet. Mech. Cat., 99 (1), 143-148 (2010).
32 Glos, M., Reiser, O., “Aza-bis(oxazolines): New chiral ligands for asymmetriccatalysis”, Org. Lett., 2 (14), 2045-2048 (2000).
33 Yao, Q., “A soluble polymer-bound ruthenium carbene complex: A robust and reusable catalyst for ring-closing olefin metathesis”,Angew. Chem. Int. Ed., 39 (21), 3896-3898 (2000).
34 Varray, S., Lazaro, R., Martinez, J., Lamaty F., “New soluble-polymer bound ruthenium carbene catalysts: Synthesis, characterization, and application to ring-closing metathesis”, Organometallics, 22 (12), 2426-2435 (2003).
35 Reger, T.S., Janda, K.D., “Polymer-supported (salen)Mn catalysts for asymmetric epoxidation: A comparison between soluble and insoluble matrices”, J. Am. Chem. Soc., 122 (29), 6929-6934 (2000).
36 Zhang, J.L., Che, C.M., “Soluble polymer-supported ruthenium porphyrin catalysts for epoxidation, cyclopropanation, and aziridination of alkenes”, Org. Lett., 4 (11), 1911-1914 (2002).
37 Guo, H., Shi, X., Qiao, Z., Hou, S., Wang, M., “Efficient soluble polymer-supported sharpless alkene epoxidation catalysts”, Chem.Commun., 3 (2), 118-119 (2002).
38 Cohen, M., Neumann, R., “Silica tethered with poly(ethylene and/propylene) oxide as supports for polyoxometalates in catalytic oxidation”, J. Mol. Catal. A: Chem., 146 (1-2), 291-298 (1999).
39 Chen, R.F., Liu, X.Z., Jin, Z.L., “Thermoregulated phase-transfer ligands and catalysis. Part VI. Two-phase hydroformylation of styrene catalyzed by the thermoregulated phase-transfer catalyst OPGPP/Rh”, J. Organomet. Chem., 571 (2), 201-204 (1998).
40 Jiang, J.Y., Wang, Y.H., Liu, C., Xiao, Q.M., Jin, Z.L., “Thermoregulated phase transfer ligands and catalysis XIV: Synthesis of N,N-dipolyoxyethylene-substituted-4-(diphenylphosphino) benzenesulfonamide (PEO-DPPSA) and the catalytic activity of its rhodium complex in hydroformylation of 1-decene”, J. Mol. Catal. A:Chem., 171 (1-2), 85-89 (2001).
41 Liu, C., Jiang, J.Y., Wang, Y.H., Cheng, F., Jin, Z.L., “Thermoregulated phase transfer ligands and catalysis XVIII: Synthesis of N,N-dipolyoxyethylene-substituted-2-(diphenylphosphino)phenylam ine (PEO-DPPPA) and the catalytic activity of its rhodium complex in the aqueous-organic biphasic hydroformylation of 1-decene”, J.Mol. Catal. A: Chem., 198 (1-2), 23-27 (2003).
42 Chen, R.F., Jiang, J.Y., Wang, Y.H., Jin, Z.L., “Thermoregulated phase-transfer ligands and catalysis: VIII. Two-phase hydroformylation of 4-isobutylstyrene catalyzed by thermoregulated phase-transfer catalyst OPGPP/Rh”, J. Mol. Catal. A: Chem., 149 (1-2), 113-117 (1999).
43 Zheng, X.L., Jiang, J.Y., Liu, X.Z., Jin, Z.L., “Thermoregulated phase transfer ligands and catalysis. III. Aqueous/organic two-phase hydroformylation of higher olefins by thermoregulated phase-transfer catalysis”, Catal. Today, 44 (1-4), 175-182 (1998).
44 Wang, Y.H., Jiang, J.Y., Miao, Q., Wu, X.W., Jin, Z.L., “Thermoregulated phase transfer ligands and catalysis: Part XIII. Use of nonionic water-soluble phosphine ligands to effect homogeneous catalyst separation and recycling”, Catal. Today, 74 (1-2), 85-90(2002).
45 Bai, Y., Peng, J.J., Li, J.Y., Lai, G.Q., “Use of carboxylated polyethylene glycol as promoter for platinum-catalyzed hydrosilylation of alkenes”, Appl. Organomet. Chem., 25 (5), 400-405 (2011).
46 Luo, Y., Dai, Y.F., Zhu, W.B., Fu, M., “Silica gel supported platinum and PEG complex catalyst for hydrosilylation of styrene”, New Chem. Mater., 35 (10), 17-29 (2007). (in Chinese)
47 Li, J., Yang, C.H., Zhang, L., Ma, T.L., “A novel fumed silica-supported nitrogenous platinum complex as a highly efficient catalyst for the hydrosilylation of olefins with triethoxysilane”, J.Organomet. Chem., 696 (9), 1845-1849 (2011).
48 Sakaki, S., Mizoe, N., Sugimoto, M., “Theoretical study of platinum(0)-catalyzed hydrosilylation of ethylene. Chalk-Harrod mechanism or modified Chalk-Harrod mechanism”, Organometallics, 17(12), 2510-2525 (1998).
49 Sakaki, S., Ogawa, M., Musashi, Y., “C2H4insertion into Pt(II) SiH3and Pt(II) H bonds. An ab initio MO/MP4study”, J.Am. Chem. Soc., 116 (16), 7258-7265 (1994).
50 Sakaki, S., Sumimoto, M., Fukuhara, M., Sugimoto, M., Fujimoto,H., Matsuzaki, S., “Why does the rhodium-catalyzed hydrosilylation of alkenes take place through a modified Chalk-Harrod mechanism?A theoretical study”, Organometallics, 21 (18), 3788-3802 (2002).
2011-05-16, accepted 2012-02-19.
* Supported by the Educational Commission of Zhejiang Province of China (Y201017419) and Zhejiang Province Program(2008C14041).
** To whom correspondence should be addressed. E-mail: gqlai@hznu.edu.cn; xnli@zjut.edu.cn
 Chinese Journal of Chemical Engineering2012年2期
Chinese Journal of Chemical Engineering2012年2期
- Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- Application of Choline Chloride·xZnCl2 Ionic Liquids for Preparation of Biodiesel*
- Inhibiting Effect of Ciprofloxacin, Norfloxacin and Ofloxacin on Corrosion of Mild Steel in Hydrochloric Acid*
- Experimental and Numerical Study on Heat Transfer Enhancement of a Rectangular Channel with Discontinuous Crossed Ribs and Grooves*
- Effect of the Interference Instant of Zeolite HY Catalyst on the Pyrolysis of Pubescens*
- Translocation of Polymer Through a Nanopore Studied by Langevin Dynamics: Effect of the Friction Coefficient*
